Abstract
Aim:
The present study was designed to investigate the anxiolytic activity of 6g, a novel serotonin type-3 receptor (5-HT3) receptor antagonist in experimental mouse models of anxiety.
Materials and Methods:
The anxiolytic activity of “6g” (1 and 2 mg/kg, intraperitoneally [i.p.]) was evaluated in mice by using a battery of behavioral tests of anxiety such as elevated plus maze (EPM), light-dark (L&D) box, hole board (HB), and open field test (OFT) with diazepam (2 mg/kg, i.p.) as standard anxiolytic. None of the tested dose of “6g” affects the base line locomotion.
Results:
The new chemical entity “6g” (2 mg/kg, i.p.) and diazepam (2 mg/kg, i.p.) significantly (P < 0.05) increased the percentage of time spent and number of entries in open arm in the EPM test. In the L&D test compound “6g” (2 mg/kg, i.p.) and diazepam (2 mg/kg, i.p.) significantly (P < 0.05) increased the total time spent in light compartment as well as number of transitions from one compartment to other. Compound “6g” (1 and 2 mg/kg, i.p.) and diazepam (2 mg/kg, i.p.) also significantly (P < 0.05) increased number of head dips, whereas significantly (P < 0.05) decreased the head dipping latency in HB test as compared to vehicle control group. In addition, 6g (2 mg/kg, i.p.) and diazepam (2 mg/kg, i.p.) significantly (P < 0.05) increased the ambulation scores (square crossed) in OFT and there was no significant effect of 6g (1 and 2 mg/kg, i.p.) and diazepam (2 mg/kg, i.p.) on rearing scores.
Conclusion:
In conclusion, these findings indicated that compound “6g” exhibited an anxiolytic-like effect in animal models of anxiety.
KEY WORDS: Anxiety, elevated plus maze, hole-board, 5-HT3 receptor antagonist, open field test
Introduction
Anxiety, an emotional state is one of the most frequently occurring psychiatric disorder globally.[1] Moreover, anxiety disorders associated with significant disability, has a negative impact on the quality of life; and an incidence of 18.1% and prevalence of 28.8%.[2] Despite a steady increase in the development of anxiolytic drugs, the prevalence of the disorder remains stable that could be attributed to the unclear neurobiological understanding of pathophysiology or the inconsistent efficacy of current pharmacological treatment.[3]
Benzodiazepines are the major class of compounds used in anxiety and they remain the most commonly prescribed treatment for anxiety.[4] However, the realization that benzodiazepines have a narrow safety margin have prompted many researchers to evaluate new compounds in the hope of identifying other potent anxiolytic drugs with a novel mechanism and larger safety margin.
Considerable research has shown that serotonin is a major neurotransmitter involved in the pathophysiology of anxiety disorders.[5] Moreover, serotonergic neurotransmission in prefrontal cortex plays a key role in regulating emotion and cognition under normal and pathological conditions. Seven super families of serotonin receptors are identified in that, 5-HT3 receptors are pentameric ligand gated ion channels, belonging to the superfamily of Cys-loop receptors. Previous studies have been shown that 5-HT3 receptors are known to be expressed in the brain areas, involved in the vomiting reflex, processing of pain, cognition, depression, and anxiety control.[5,6] The preferential localization on nerve endings is consistent with a physiological role of 5-HT3 receptors in the control of neurotransmitter release such as dopamine, cholecystokinin, glutamate, acetylcholine, γ-amino butyric acid (GABA), substance P, or serotonin itself. 5-HT3 receptor agonists cause unpleasant effects like nausea and anxiety, and no clinical use has been considered. In contrast, the introduction of 5-HT3 receptor antagonists for chemotherapy-induced vomiting was extremely successful. The involvement of 5-HT3 receptors in anxiety is complemented by studies of 5-HT3 knockout mice, which revealed the regulation of 5-HT3 (3A subtype) in anxiety-related behaviors.[7] Increased availability of 5-HT on 5-HT2 and 5-HT3 receptors increases anxiety and is probably at least in part responsible for anxiogenic-like effects of antidepressants, while 5-HT3 receptor blockade has anxiolytic effects.[8] Both hippocampal and accumbens 5-HT3 receptors seem to contribute to the anxiolytic-like effects of 5-HT3 receptor antagonists. It also appears that this effect of 5-HT3 receptor antagonists is related to their action on postsynaptic 5-HT3 receptors within the nucleus accumbens, and depends on the functional state of the 5-HT innervations ascending from the raphe nuclei.[9] The proposed mechanism for anxiolytic and antidepressant effect of “6g” is postsynaptic 5-HT3 receptor antagonism in serotonergic neurons can facilitate specific binding of 5-HT to other postsynaptic receptors such as 5-HT1B, 5-HT2A, and 5-HT2C, thereby aiding in serotonergic transmission.[10]
Despite increased interest among the clinical neurosciences, information regarding the anxiolytic activity of 5-HT3 modulators is lacking. Thus, selecting the tests sensitive to anxiolytic drugs, the present study was designed to investigate the anxiolytic potential of 6g in rodent models of anxiety.
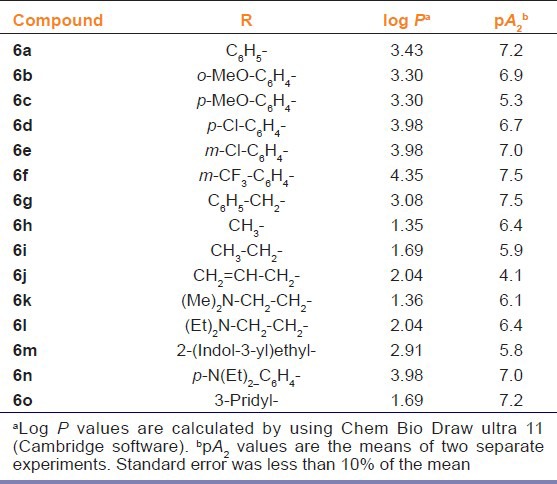
In the present study, compound 6g (4-benzylpiperazin-1-yl)(3-methoxyquinoxalin-2-yl) methanone (synthesized in our lab) which exhibited good log P (3.08) and pA2 value (7.5) greater than the standard 5-HT3 receptor antagonist, ondansetron (OND) (pA2“6.9) was selected for the preliminary anxiolytic screening in the standard rodent models of anxiety as mentioned above [Table 1].[11]
Table 1.
Log P and pA2 values of 3-methoxyquinoxalin-2-carboxamides
Materials and Methods
Animals
Behavioral based experiments were carried out using male Swiss Albino mice (20-25 g), procured from Chaudhary Charan Singh Agricultural University, Hisar, Haryana, India. Animals were kept in polypropylene boxes under standard laboratory conditions (temperature 23 ± 2°C and room humidity 60 ± 10%), maintained on 12:12 h light-dark cycle. Standard diet and filtered water were given ad libitum. All the experiments were carried out between 09.00 a.m. and 02.00 p.m. in accordance with the Institutional Animal Ethics Committee of Birla Institute of Technology & Science, Pilani, India (Protocol No. IAEC/RES/14/04).
Drugs and treatments
Diazepam was purchased from Cipla Ltd. India. “6g” and diazepam were prepared freshly before use in distilled water. “6g” (1 and 2 mg/kg, intraperitoneally [i.p.]) and diazepam (2 mg/kg, i.p.) were administered i.p. to respective groups, 30 min prior to behavioral observation in each test.
Elevated plus maze (EPM)
The elevated plus maze (EPM) test was first evaluated for rats[12] and later adapted for mice.[13] In brief, the apparatus consisted of a wooden maze with two enclosed arm (30 cm × 5 cm × 15 cm) and two open arms (30 cm × 5 cm × 0.25 cm) that extended from a central platform (5 cm × 5 cm) to form a plus sign. The plus-maze apparatus was elevated to a height of 45 cm and placed inside a sound-attenuated room. The trial was started by placing a mouse on the central platform of the maze facing its head towards an open arm. The behavioral performances recorded during a 5 min test period were; percentage open arm entries and percentage time spent in open arm.[12] Entry into an arm was considered valid only when all four paws of the mouse were inside that arm.[13] The animal activities were tracked and recorded via an overhead video camera linked to a monitor with computer software Smart Version 2.5 (Panlab Co., USA). The apparatus was thoroughly cleaned with 70% ethanol after each trial.
Light-dark (L&D) aversion test
The light-dark (L&D) apparatus comprised of a box divided into two separate compartments, occupying two-third and one-third of the total size, respectively. The larger compartment (light compartment) was illuminated by a 60-W bulb, while the smaller (dark compartment) was entirely black and enclosed under a dark cover. The L&D compartments were separated by a partition with a tunnel to allow passage from one compartment to the other.[14] At the beginning of the test, the mouse was placed individually at the center of the light compartment facing towards the tunnel and was allowed to explore the entire apparatus for 5 min. The behavioral parameters such total time spent in the light compartment and number of transitions between the L&D compartments were tracked and recorded using computer software Smart Version 2.5 (Panlab Co., USA). A compartment entry was considered valid when the animal's all four paws were inside that chamber. The apparatus was thoroughly cleaned with 70% ethanol after each trial.
Hole-board (HB) test
The hole-board (HB) apparatus consisted of a grey Plexiglas platform (40 cm × 40 cm) raised to a height of 15 cm from the floor of a gray wooden box (40 cm × 40 cm × 40 cm). The grey Plexiglas platform consisted of 16 equivalent square compartments (12 peripheral and 4 central), each featuring a central circular hole (3 cm diameter). Test session was started by placing each animal in the center of the HB and allowed to freely explore on the apparatus for 5 min. The behavioral performances such as number of head dipping and latency to the first head dipping[15] were tracked and recorded using computer software Smart Version 2.5 (Panlab Co., USA).
Open field test (OFT)
The apparatus consisted of a wooden box (60 cm × 60 cm × 30 cm) with the floor divided into 16 squares (15 cm × 15 cm) squares by black parallel and intersecting lines. The apparatus was illuminated with 60 W bulb suspended 100 cm above. At the beginning of the test, the mouse was placed individually at the center of the square arena. The ambulation scores (number of square crossed) and rearing number (standing upright on the hind legs) were recorded using computer software Smart Version 2.5 (Panlab Co., USA) for 5 min period. After each individual test session the floor was thoroughly cleaned with 70% ethanol.[16]
Statistical analysis
All values were expressed as mean ± SEM. The data obtained from various groups were statistically analyzed using one-way analysis of variance followed by the post-hoc Dunnett's test in Graph-pad prism 3. The P value <0.05 was considered to be statistically significant.
Results
EPM
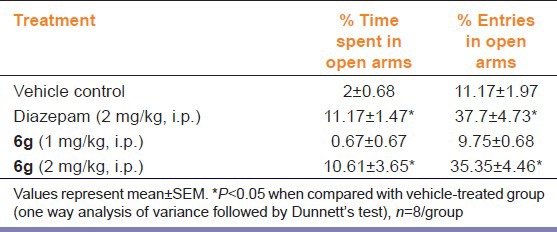
The effects of “6g” and diazepam on the behavior of mice in the EPM are summarized in Table 1. Acute treatment with “6g” (2 mg/kg, i.p.) and diazepam (2 mg/kg, i.p.) significantly (P < 0.05) increased the percentage of both open arm entries and time spent in open arm as compared to vehicle control group [Table 2], while “6g” (1 mg/kg, i.p.) did not produce any significant effect on the behaviors of mice in EPM test.
Table 2.
Effect of “6g” on behavior of mice in elevated plus maze test
L&D test
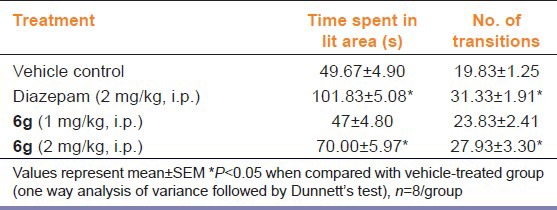
“6g” (2 mg/kg, i.p.) and diazepam (2 mg/kg, i.p.) treatment significantly at P < 0.05 increased the number of entries from one compartment to other, as well as increase the total time spent in lit area [Table 3]. Lower dose of “6g” (1 mg/kg, i.p.) did not produce significant change in any of the parameters [Table 3].
Table 3.
Effect of “6g” on behavior of mice in light-dark test
HB test
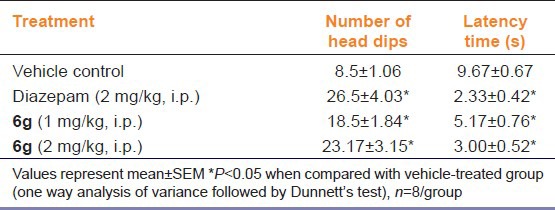
The results of the HB test are shown in Table 3. Here, “6g” (1 & 2 mg/kg, i.p.) and diazepam (2 mg/kg, i.p.) treatment significantly (P < 0.05) decreased the head dipping latency and increased the number of head dips [Table 4].
Table 4.
Effect of “6g” on behavior of mice in hole board test
OFT
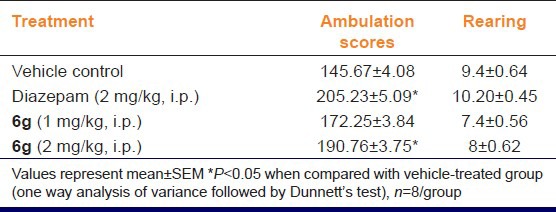
The results of the OFT test are shown in Table 5, Here, “6g” (2 mg/kg, i.p.) and diazepam (2 mg/kg, i.p.) treatment significantly (P < 0.05) increased the number of square crossed as compared to vehicle treatment group. While the lower dose of “6g” (1 mg/kg, i.p.) did not produce any significant change in ambulation scores. None of the tested dose of diazepam and “6g” significantly affect the rearing score [Table 5].
Table 5.
Effect of “6g” on behavior of mice in open field test
Discussion
In present study, the anxiolytic effects of “6g” was examined in animal models of anxiety such as the EPM, L&D test, HB test, and OFT.[15] The results of the present study, verified the designed hypothesis that 5-HT3 receptor antagonists play an important role in pathogenesis of anxiety disorder via increasing the availability of serotonin at postsynaptic receptors. Although, it is uncertain that any single animal model captures all of the components of the complex expression of anxiety, thus a battery of tests have been used to evaluate the potential anxiolytic effect of “6g” in the present study.
The EPM test is most popular test for evaluation of anxiolytic compounds. The EPM test used to evaluate the psychomotor performance and emotional aspects of rodents. EPM is considered as one of the well-established model for unconditioned anxiety to detect anxiolytic/anxiogenic-like activity by investigating aspects of physiological and pharmacological behavior. In the EPM test increase number of entries and time spent into the open arms arm the most obvious index/reliable indicators of decreased anxiety or indicating the anxiolytic-like activity of a compound, while anxiogenic substances have the opposite effect.[12,13,16] In our study, treatment with “6g” produced anxiolytic-like effects in the EPM test at higher dose, as evidenced by increased percentages of both open arm entries and time spent in open arms.[12] In addition, diazepam used as reference anxiolytic also showed the potential anxiolytic effects in EPM.
The L&D test is another widely used animal model for screening anxiolytic or anxiogenic drugs[14] by utilizing the animal's natural preference for dark spaces. The L&D paradigm is based on natural aversion of mice to brightly lit place. Anxiolytic drugs reduce the natural aversion to light and increase the time spent in light area and number of transition from one compartment to other showed anxiolytic potential of compound.[16] In the present study, we found that “6g” treatment significantly increased the time spent in lit compartment as well as number of transitions. Some studies have reported that an anxiolytic drug increased the transitions between the two compartments.[14]
The anxiolytic-like effects of “6g” were further confirmed using the HB test. Recently, HB test has been popular as a model of anxiety and offers a simple method for measuring the behavioral response of rodents to an unfamiliar environment.[17] The head dipping behavior of a rodent in HB is sensitive to change in emotional state of the animal.[18] The present study results revealed that “6g” treatment significantly increased the number of head dipping and latency of head dipping reflecting the anxiolytic activity of compound. This effect is in agreement with previous studies which suggest that increase in the head dipping number and decrease latency of head dipping reflected the anxiolytic like activity of a compound.[17,19]
The OFT is also widely used for the screening of anxiolytic/anxiogenic drugs. Normal aversion of a rodent to the brightly lit area produces the anxiety and fear, which is characterized by alteration in the behavioral parameters of animal in open field. Previous reports suggested that anxiolytic compound have a tendency to reduce the fearful behaviors of rodents in open field.[20] “6g” treatment increased the ambulation scores in OFT indicating the anxiolytic effect of “6g”.
In summary, the results of present study suggest the anxiolytic activity of “6g” in animal models of anxiety. However, further some studies such as acute toxicity, chronic dose studies and some mechanistic studies are required in order to better evaluate the possible mechanisms, underlying the anxiolytic-like effects of “6g”.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Rouillon F. Anxiety with depression: A treatment need. Eur Neuropsychopharmacol. 1999;9:S87–92. doi: 10.1016/s0924-977x(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rickels K, Garcia-Espana F, Mandos LA, Case GW. Physician Withdrawal Checklist (PWC-20) J Clin Psychopharmacol. 2008;28:447–51. doi: 10.1097/JCP.0b013e31817efbac. [DOI] [PubMed] [Google Scholar]
- 4.Grundmann O, Nakajima J, Seo S, Butterweck V. Anti-anxiety effects of Apocynum venetum L. in the elevated plus maze test. J Ethnopharmacol. 2007;110:406–11. doi: 10.1016/j.jep.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 5.Haus U, Späth M, Färber L. Spectrum of use and tolerability of 5-HT3 receptor antagonists. Scand J Rheumatol Suppl. 2004;119:12–8. [PubMed] [Google Scholar]
- 6.Mahesh R, Perumal RV, Pandi PV. Cancer chemotherapy-induced nausea and vomiting: Role of mediators, development of drugs and treatment methods. Pharmazie. 2005;60:83–96. [PubMed] [Google Scholar]
- 7.Kelley SP, Bratt AM, Hodge CW. Targeted gene deletion of the 5-HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur J Pharmacol. 2003;461:19–25. doi: 10.1016/s0014-2999(02)02960-6. [DOI] [PubMed] [Google Scholar]
- 8.Bell R, Gilmore PE. Effects of the 5-HT3 receptor agonist SR 57227A and antagonist Y-25130 on the behaviour of rats in the elevated zero-maze model of anxiety. J Psychopharmacol. 2003;17:A30. [Google Scholar]
- 9.Lecrubier Y, Puech AJ, Azcona A, Bailey PE, Lataste X. A randomized double-blind placebo-controlled study of tropisetron in the treatment of outpatients with generalized anxiety disorder. Psychopharmacology (Berl) 1993;112:129–33. doi: 10.1007/BF02247373. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar R, Mahesh R. The auspicious role of the 5-HT3 receptor in depression: A probable neuronal target? J Psychopharmacol. 2010;24:455–69. doi: 10.1177/0269881109348161. [DOI] [PubMed] [Google Scholar]
- 11.Mahesh R, Devadoss T, Dhar AK, Venkatesh SM, Mundra S, Pandey DK, et al. Ligand-based design, synthesis, and pharmacological evaluation of 3-Methoxyquinoxalin-2-carboxamides as structurally novel serotonin type-3 receptor antagonists. Arch Pharm (Weinheim) 2012;345:687–94. doi: 10.1002/ardp.201200038. [DOI] [PubMed] [Google Scholar]
- 12.Klodzinska A, Tatarczyñska E, Chojnacka-Wójcik E, Nowak G, Cosford ND, Pilc A. Anxiolytic-like effects of MTEP, a potent and selective mGlu5 receptor agonist does not involve GABA(A) signaling. Neuropharmacology. 2004;47:342–50. doi: 10.1016/j.neuropharm.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Biala G, Kruk M. Calcium channel antagonists suppress cross-tolerance to the anxiogenic effects of D-amphetamine and nicotine in the mouse elevated plus maze test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:54–61. doi: 10.1016/j.pnpbp.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Mi XJ, Chen SW, Wang WJ, Wang R, Zhang YJ, Li WJ, et al. Anxiolytic-like effect of paeonol in mice. Pharmacol Biochem Behav. 2005;81:683–7. doi: 10.1016/j.pbb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Silva MI, de Aquino Neto MR, Teixeira Neto PF, Moura BA, do Amaral JF, de Sousa DP, et al. Central nervous system activity of acute administration of isopulegol in mice. Pharmacol Biochem Behav. 2007;88:141–7. doi: 10.1016/j.pbb.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Yadav AV, Kawale LA, Nade VS. Effect of Morus alba L. (mulberry) leaves on anxiety in mice. Indian J Pharmacol. 2008;40:32–6. doi: 10.4103/0253-7613.40487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda H, Tsuji M, Matsumiya T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol. 1998;350:21–9. doi: 10.1016/s0014-2999(98)00223-4. [DOI] [PubMed] [Google Scholar]
- 18.Nolan NA, Parkes MW. The effects of benzodiazepines on the behaviour of mice on a hole-board. Psychopharmacologia. 1973;29:277–86. doi: 10.1007/BF00414043. [DOI] [PubMed] [Google Scholar]
- 19.Hranilovic D, Cicin-Sain L, Bordukalo-Niksic T, Jernej B. Rats with constitutionally upregulated/downregulated platelet 5HT transporter: Differences in anxiety-related behavior. Behav Brain Res. 2005;165:271–7. doi: 10.1016/j.bbr.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Mechan AO, Moran PM, Elliott M, Young AJ, Joseph MH, Green R. A comparison between Dark Agouti and Sprague-Dawley rats in their behaviour on the elevated plus-maze, open-field apparatus and activity meters, and their response to diazepam. Psychopharmacology (Berl) 2002;159:188–95. doi: 10.1007/s002130100902. [DOI] [PubMed] [Google Scholar]


