Abstract
Objectives:
To investigate the protective efficacy of aqueous extract of Hippophae rhamnoides against chronic hypoxic injury using primary rat hepatocytes.
Materials and Methods:
The extract was prepared using maceration method and characterized by its phenolic and flavonoid content and chemical antioxidant capacity using ferric reducing antioxidant power assay. Hepatocytes were maintained in hypoxia chamber (3% and 1% oxygen) for 72 h. The cells kept under normoxic condition served as control. The cells were treated with the extract and flavonoids; isorhamentin, kaempferol or qurecetin-3-galactoside. After the end of exposure period; cell survival, reactive oxygen species (ROS), leakage of lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), reduced glutathione (GSH), glutathione peroxidase (GPx), and superoxide dismutase (SOD) levels were measured.
Results:
The extract showed presence of high phenolic and flavonoid content with significant antioxidant activity in chemical assay. The cell exposed to hypoxia showed concentration dependent cell death and harbored higher reactive oxygen species. In addition, these cells showed significant leakage of intracellular LDH, ALT, and AST accompanied by the diminished levels/activities of GSH, GPx, and SOD. The treatment of cells with aqueous extract of H. rhamnoides reduced hypoxia-induced cell death and prevented increase in ROS levels and leakage of intracellular LDH, ALT, and AST from cells. Moreover, these cells maintained better levels/activities of GSH, GPx, and SOD in comparison to the respective controls. The major flavonoids present in aqueous extract of H. rhamnoides; quercetin-3-galactoside, kaempferol, and isorhamentin also prevented hypoxia induced cell injury individually or in combination, however, the protection offered by these compounds taken together could not match to that of the extract.
Conclusions:
Overall the findings reveal significance of aqueous extract of H. rhamnoides in controlling ROS-meditated hypoxic injury in cells and can be useful in many hepatic complications.
KEY WORDS: chronic, Hepatocytes, Hippophae rhamnoides, Hypoxia
Introduction
A limited oxygen supply leads to hepato-cellular damage and death[1] and prolonged hypoxic injury has been implicated in progression of many hepatic disorders. It is reported that isolated hepatocytes from rat liver exposed to hypoxia resulted in wide variety of structural and metabolic changes leading to loss of cell viability[2,3] Hypoxia-evoked reactive oxygen species (ROS)-driven oxidative stress is an underlying mechanism of hepatic cell death and unchecked production of these oxidants for longer period reduces efficacy of antioxidant system resulting in disintegration of cellular architecture[4] It is documented that various antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase are reported for their beneficial effects in protecting tissues against oxidants including hypoxia[4,5,6] Further, exogenous supplementation of biologically active extracts derived from herbal plants reduces oxidant load.[7,8,9]
Hippophae rhamnoides L. commonly known as seabuckthorn belongs to family Elaeagnaceae and genus Hippophae. It is a wild thorny deciduous shrub, grows at high altitude (2500-4000 m) in adverse climatic conditions and is native to Europe and Asia.[10] In India, it grows naturally in Ladakh, Uttarkhand, Himachal Pradesh, Jammu and Kashmir, Sikkim and Arunachal Pradesh regions.[11] There are six species of seabuckthorn found in the world, of which three species are reported in India viz. H. rhamnoides subspecies Turkestania Rousi, H. salicifolia and H. tibitana. Most of the scientific studies available on the species H. rhamnoides were published by Russian and Chinese scientists.[12] These plants are valuable for their nutritional and medicinal products; all parts of plant are a good source of bioactive substances.[13] In India, Central Himalayan local residents utilize these plants for various purposes, as food, medicine, veterinary purposes, fuel, etc.,[14] Numerous medicinal effects of seabuckthorn are well-known and include, beneficial effects in cardiovascular diseases, mucosal injuries, skin disorders and adaptation to the effects of stress.[8,9,13,15]
The medicinal properties to extracts of H. rhamnoides leaves are attributed due to the presence of high content of flavonoids, tannins, and triterpenes.[16] The major flavonoids found in the leaves of seabuckthorn include quercetin, kaempferol, and isorhamentin, catechin, and rutin.[17] The alcoholic extract of seabuckthorn leaves are reported to possess anti-oxidant, anti-stress, immunomodulatory, and anti-inflammatory properties, and could prevent sodium nitroprusside cytotoxicity in murine macrophages.[7-8] Further alcoholic extract has been documented for preventing hypoxia induced pulmonary vascular leakage[18] and oxidative stress.[19] The pharmacological properties of aqueous extract of seabuckthorn leaves such as cytoprotective and anti-oxidative,[19] adaptogenic,[8] wound healing,[20] and anti-dengue activity[21] have been documented. The adverse cellular effect linked to hypoxia exposures are mediated via generation of ROS and is underlying cause for many clinical disorders including hepatic cell death. We hypothesize here that H. rhamnoides rich in flavonoids and having anti-oxidative properties may exert anti-hypoxic activity too.
Further, it is reported that the pharmacological effects of the aqueous extract of H. rhamnoides leaf prepared by the maceration method might be due to the presence of major flavonoids such as quercetin-3-galactoside, kaempferol, and isorhamentin.[22] Therefore, we sought whether any of this major flavonoids alone or in combination could reserve adverse changes associated with hypoxia and whether protection offered by these bio-active flavonoids equalizes with that of the extract.
Therefore, this study was designed to investigate whether hypoxia evoked the production of ROS in cultured rat hepatocytes could be attenuated by the supplementation of aqueous leaf extract of H. rhamnoides and its major bio-active flavonoids.
Materials and Methods
Plant material
H. rhamnoides leaves were collected in the month of September from hilly regions of western Himalayas, India, where the plant grows widely under natural conditions. The Field Research Laboratory, Leh, India, where the voucher specimen of the plant material is preserved in the herbarium, carried out the ethnobotanical identification of the plant. Fresh leaves of seabuckthorn were cleaned and washed thoroughly with water and re-washed with distilled water. Washed fresh leaves were dried under shade in a clean, dust free environment and crushed using laboratory blender.
Extract preparation
Aqueous lyophilized extract of H. rhamnoides dried leaves was prepared by maceration method as described earlier.[8] One gram of dried H. rhamnoides leaves produced 0.12 g of lyophilized seabuckthorn aqueous extract powder. The extract has been characterized by its phenolic and flavonoid content and chemical antioxidant capacity by ferric reducing antioxidant power assay.
Determination of total phenol content
The total phenolic content in extract was estimated using Folin – Ciocalteu reagent (FCR) based assay.[23] The following were added to a tube; 20 μl of stock solution (1 mg/ml) of the extract, 80 ml of water, and 500 ml of FCR. After 5 min incubation in the dark at room temperature, 400 ml of 7.5% sodium carbonate solution was added. The mixture was incubated in the dark for 30 min at room temperature and absorbance of the color developed was read at 765 nm. Total phenols (mg/g) in the extract were expressed as gallic acid equivalent (GAE), using standard curve prepared from gallic acid (0.1 mg/ml) solution.
Determination of total flavonoid content
Total flavonoid content was estimated by the aluminum chloride colorimetric assay as described elsewhere[24] using rutin as a standard. 1 ml of extract was added to 4 ml distilled water and subsequently 0.3 ml of 5% NaNO2 solution was added. After 5 min, 0.3 ml of 10% AlCl3 solution was added and allowed to stand for 5 min, then 0.2 ml of 4% NaOH solution was added to the mixture and the volume was adjusted up to 10 ml with distilled water. Absorbance of the mixture was read at 510 nm. Total flavonoid content (mg/g) in the extract was expressed as rutin equivalent.
FRAP assay
The FRAP assay was estimated using method described elsewhere.[25] The fresh FRAP working solution was prepared by mixing 25 ml of 300 mM acetate buffer (3.1 g C2H3NaO2 3H2O and 16 ml C2H4O2, pH 3.6), 2.5 ml of 10 mM TPTZ (2, 4, 6-tripyridyl-s-triazine in 40 mM HCl), and 2.5 ml of 20 mM FeCl3.6H2O solution, which was warmed at 37°C prior to use. 150 μL of extract (1 mg/ml) was allowed to react with 2850 μl of the FRAP solution for 30 min in dark and the absorbance of the color developed was read at 593 nm. Results are expressed mg Trolox equivalent/g of extract, using standard curve prepared from Trolox solution.
Isolation of rat hepatocytes
Hepatocytes were isolated from male Sprague-Dawley rats following the two step perfusion method described elsewhere.[26] Briefly, the liver was perfused retrogradely with 250 ml of 135 mM NaCl, 7 mM KCl, 12 mM glucose, and 10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), pH 7.4, followed by 250 ml of the same medium supplemented with collagenase (150 U/ml) and 1 mM CaCl2. Hepatocytes were diluted into William's E medium supplemented with 10% fetal calf serum, 50 mg/ml penicillin-streptomycin, 5 mg/ml insulin and 4 ng/ml dexamethasone, 10 mM HEPES and 1 mM CaCl2. Cells were then plated in a density of 0.5 × 105 cells/ml in 96 well plates or 3 × 106 cells/ml in 60 mM plates. Viability of the cells was determined by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay. Cells were exposed to 3% or 1% oxygen for 72 h in presence or absence of H. rhamnoides extract.
Mitochondrial integrity (MTT assay)
Mitochondrial integrity was measured by the reduction of mitochondrial enzyme succinate dehydrogenase using MTT assay. The MTT reduction was carried out by the method of Mosman 1983.[27] Briefly, MTT was dissolved in culture medium at a concentration of 0.5 mg/ml and filtered to remove a small amount of insoluble residue. MTT containing medium was added to each well in a volume of 0.2 ml and incubated for 3 h. Thereafter, the supernatant was removed and a solution of 0.4 N HCl-isopropanol (1:24 v/v) was added to extract and solubilize the formazon. After 30 min of incubation at room temperature, the absorbance of the formazon was read at a wavelength of 570 nm on a plate reader (Power Wave XS2, BioTek, USA).
Leakage of lactate dehydrogenase (LDH), alanine aminotransferase (ALT), and aspartate aminotransferase (AST)
Aliquots of the cell suspension were drawn at different time intervals. Extracellular and total LDH, ALT, and AST were estimated by a commercial diagnostic kit at a wavelength of 340 nm. Leakage of intracellular enzymes was expressed as percent leakage.
Assay for intracellular redox state
Intracellular redox state levels were measured using the fluorescent dye, 2,7-dichlorofluorescein diacetate (H2-DCFH-DA). Briefly, cells were washed once with Hank's Balanced Salt Solution (HBSS) and incubated in the same buffer containing 5-10 μg of DCFH-DA for 30 min at 37°C. Intracellular fluorescence was detected with excitation at 485 nm and emission at 530 nm (Cary Eclipse, Varian, USA).
Bioassays
At the end of exposure period, the cultured hepatocytes were suspended in 0.1 M phosphate buffer (pH 7.4) and sonicated. The homogenate was then centrifuged at 10000 rpm for 10 min and supernatant was collected for analysis of GSH, GPx, and SOD. Cellular GSH content was measured by the method of Beutler et al., 1963.[28] The activities of GPx and SOD were measured by commercial diagnostic kits. The values were expressed as percent change over controls.
Statistical analysis
Results are expressed as mean ± SE 0 The data was analyzed by one-way ANOVA followed by Dunnet's test for comparing control and the various groups, using Graph-Pad software. Statistical significance was estimated at the 5% level.
Results
Chemical Standardization of Seabuckthorn Leaf Extract
Figure 1 depicts total phenols, flavonoid content, and antioxidant activity of aqueous leaf of H. rhamnoides. The total phenolic and flavonoid content in the H. rhamnoides aqueous leaf extract was found to be 119.6 mg/g extract and 96.7 mg/g extract, respectively in terms of GAE. Further the extract showed higher antioxidant activity of 309.2 mg/g extract as evident by FRAP assay.
Figure 1.
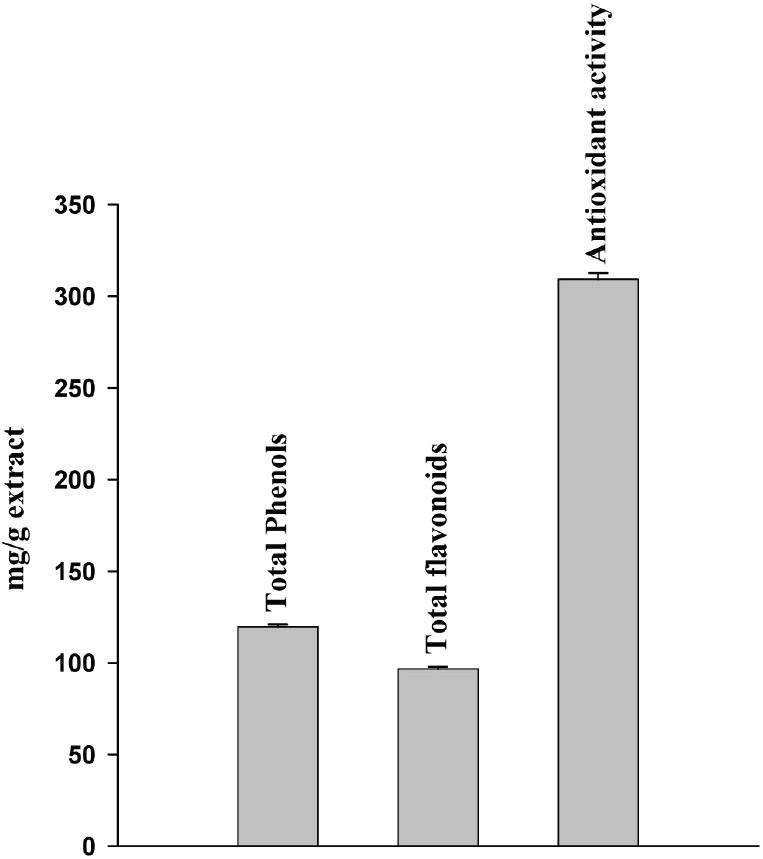
Total phenolic and flavonoid content of aqueous leaf extract of Hipphophae rhamnoides and its antioxidant activity in ferric reducing antioxidant power chemical assay
Cytotoxicity Induced by H. rhamnoides per se in Hepatocytes
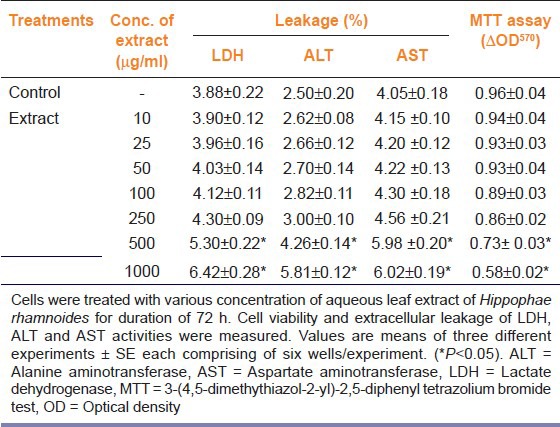
Table 1 shows effect of aqueous leaf of H. rhamnoides on LDH, ALT, and AST leakage and mitochondrial succinate dehydrogenase activity (MTT assay) in rat hepatocytes. When hepatocytes were treated with various doses (0-1000 μg/ml) of aqueous leaf extract of H. rhamnoides for 72 h of duration, the extract did not show any cytotoxicity per se up to a concentration of 250 μg/ml. However, cells treated with 250 μg/ml extract showed marginal increased leakage of LDH into the culture medium. Furthermore, treatment of cells with 500 or 1000 μg/ml of extract revealed intracellular leakage of LDH, ALT and AST to the medium. In addition, at the doses of 500 or 1000 μg/ml of extract decrease in cell survival was also evident. Therefore, all the doses of extract above 250 μg/ml were excluded from the further studies.
Table 1.
Effect of various concentrations of aqueous leaf extract of Hippophae rhamnoides on the cell viability and enzyme leakage of primary rat hepatocytes
Protective efficacy of aqueous leaf extract of H. rhamnoides on Hypoxia induced cell death, ROS levels and enzyme leakage
Table 2 depicts effect of hypoxia on LDH, ALT, and AST leakage and mitochondrial succinate dehydrogenase activity (MTT assay) in presence or absence H. rhamnoides extract. When hepatocytes were exposed to 3% or 1% oxygen for 72 h a concentration dependent decrease in cell survival was evident, which was accompanied by significant leakage of intracellular LDH, ALT, and AST into the culture medium from the cells. Exposure of cells to 3% and 1% O2 resulted in ~24% and ~45% cell death, respectively [Table 2]. Cells exposed to 3% O2 showed 2.37, 2.67 and 3.31 fold increases in LDH, ALT, and AST leakage to the culture medium over the controls, respectively. Whereas a 6.05, 5.88 and 7.20 fold increase in LDH, ALT, and AST leakage to the culture medium, respectively, was observed in cells after exposure to 1% O2 for 72 h in comparison to unexposed cells [Table 2]. Concomitantly to the observed cell death to hypoxia, there were significant elevated levels of ROS after exposure either 3% or 1% O2 [Table 3]. Cells exposed 3% or 1% O2 in presence of H. rhamnoides extract showed reduced levels of ROS than the hypoxia exposed cells with no extract [Table 3; black bar]. Cell death was reduced significantly as observed in these cells treated with various concentrations of the extract compared to controls [Table 2]. The treatment of extract to cells attenuated leakage of LDH, ALT, and AST in cells exposed to hypoxia in a dose dependent manner. Taken together, the results demonstrated that extract protects against hypoxia-evoked oxidative stress mediated cell death by optimizing ROS levels.
Table 2.
Efficacy of aqueous leaf extract of Hippophae rhamnoides against hypoxia-induced cell damage and enzyme leakage in rat hepatocytes
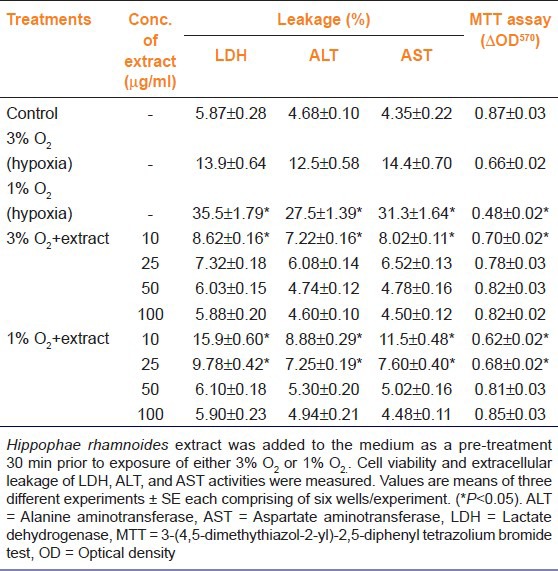
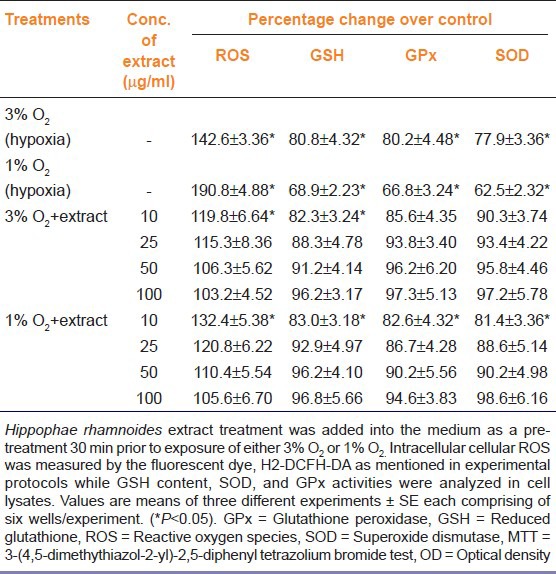
Table 3.
Efficacy of aqueous leaf extract of Hippophae rhamnoides on reactive oxygen species, reduced glutathione, glutathione peroxidase and superoxide dismutase levels in hepatocytes exposed to hypoxia
Protective efficacy of aqueous leaf of H. rhamnoides on hypoxia induced cell stress
Table 3 presents effect of hypoxia on glutathione level and activities of glutathione peroxidase, and superoxide dismutase in presence or absence of aqueous leaf extract of H. rhamnoides. In further studies, cells exposed to 3% oxygen showed 19.2, 19.8, and 22.1% decrease in GSH, GPx, and SOD levels over the controls, respectively. Whereas 31.1, 33.2, and 37.5% decrease in GSH, GPx, and SOD levels over the controls, respectively, were seen in cells exposed to 1% O2 for 72 h [Table 2]. The treatments of various doses of extract normalized all the above mentioned changes induced by hypoxia in dose dependent manner.
Protective efficacy of quercetin-3-galactoside, kaempferol and isorhamnetin aqueous leaf extract of H. rhamnoides on Hypoxia induced cell death and their comparison with the protection accorded by extract
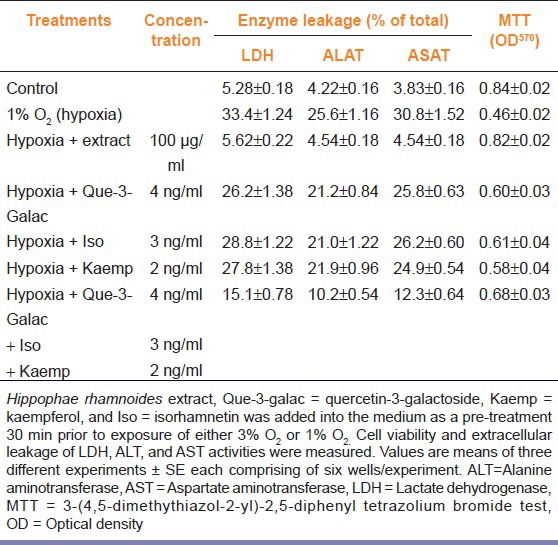
Table 4 shows effect of quercetin-3-galactoside, kaempferol, and isorhamnetin alone or together on hypoxia induced LDH, ALT, and AST leakage and mitochondrial succinate dehydrogenase activity (MTT assay). Hepatocytes pretreated (−30 min) individually with equalized amount of quercetin-3-galactoside, kaempferol, and isorhamnetin to the extract prevented LDH leakage by 7.2%, 5.6%, and 4.6%, ALT leakage by 4.4%, 3.7%, and 4.6%, and AST leakage by 5.0%, 5.9%, and 4.6%, respectively, in comparison to hypoxic cells. Further cells treated with the above mentioned compounds revealed more cell survival by 14.7%, 12.3%, and 15.9% than the cells exposed to hypoxia. Cells treated with all these compounds together showed 48.4% more cell survival with lesser leakage of LDH, ALT and AST by 18.3%, 15.4%, and 18.5%, respectively, than the hypoxic cells. In contrast, cell treated with extract had 78.9% more cell survival with lesser LDH (83.8%), ALT (82.3%), and AST (85.3%) leakage as compared to the hypoxic cells. Overall, the cell treated with extract was far better than the cells treated with quercetin-3-galactoside, kaempferol, and isorhamnetin alone or together against the hypoxic stress.
Table 4.
Comparison of protective effects of pretreatment of extract, quercetin-3-galactoside, kaempferol, and isorhamnetin in rat hepatocytes exposed to hypoxia
Discussion
Changes in oxidant and antioxidant balance may alter cellular homeostasis; cells survival/death depends upon the expression of ROS and antioxidant defense potency. Hypoxia is one such stress, which leads to disturbance in redox state of cell causing oxidative stress. Hepatocytes have been widely utilized cell model for the evaluation of deleterious effects of hypoxia as well as oxidative stress.[2,29,30]
During stressful situations supplementation of various nutrients and herbal preparation have been shown to increase stress tolerance.[8] All parts of the H. rhamnoides are considered good source of bioactive compounds and its medicinal properties are attributed due to the presence of high content of flavonoids, tannins, and triterpenes.[16] The major flavonoids found in the leaves of H. rhamnoides include, catechin, rutin, quercetin, kaempferol, and isorhamentin.[17]
Hypoxia induces cell injury which may lead to compromised cell membrane integrity and ultimately cause cell damage[2] and has been implicated in progressive hepato-cellular damage. Using hepatocytes as cell culture system, we found that cells exposed to 3% or 1% oxygen display ~24-45% death [Table 2]. Concomitantly with the death of cell, we observed that there was significant leakage of intracellular LDH, ALT, and AST in a concentration dependent manner. These data are consistent with previous studies, which observed membrane disintegrates with leakage of intracellular enzymes and loss of viability of isolated cultured hepatocytes from rat liver subjected to hypoxia.[1,2,3] In the present study, we found that hepatocytes exposed to hypoxia displayed elevated expression of ROS [Figure 1]. These results are in agreement with previous reports that the higher levels of ROS are a major damaging factor to hepatocytes.[3] It is reported that supplementation of antioxidants or biologically active extracts derived from herbal plants reduces oxidant load.[7,8,9] Here, we found that hepatocytes supplemented with extract showed resistance against hypoxic stress, reduced ROS expression, prevented leakage of intracellular enzymes (LDH, ALT, AST) and improved survival. The leaves extract of H. rhamnoides are reported to be rich in flavonoids[16] and is potent ROS scavenger.[7] Here, we believe that supplementation of extract to hepatocytes reduced oxidative stress against hypoxia exposure, thereby preventing cell death.
In response to hypoxia levels of ROS rises[3] and GSH is a major cellular antioxidant which balance these oxidant surge. In this study, we found that hepatocytes exposed to hypoxia has remarkably reduced levels of GSH which was well in agreement with earlier published reports.[3,31,32] Treatment of hepatocytes with extract prevented this decrease in GSH levels indicating antioxidant potential of leaf extract of H. rhamnoides. It is documented that aqueous leaf extract of H. rhamnoides possess anti-oxidative, and anti-stress properties.[8,19] Furthermore, in the present study, we found that activities of antioxidant enzymes such as GPx and SOD were significantly decreased in hypoxia injured hepatocytes. Nakanishi and authors (1995) suggested that the liver is susceptible to oxidative stress similar to other physiological active organs during hypoxia and has a compromised antioxidant status during such exposures. Hypoxic cells supplemented with extract showed comparable GSH levels to the controls and had maintained activities of GPx and SOD as that of unexposed cells. Here, we believe that optimization of ROS levels by the supplementation of extract, which is rich in flavonoid content, prevented decrease in GSH, GPx, and SOD levels in hypoxia injured cells.
The extraction of H. rhamnoides leaves by maceration method, the method by which the extract for the present study was prepared, showed the presence of major flavonoids such as quercetin-3-galactoside (~40 μg/g of extract), kaempferol, (~20 μg/g of extract), and isorhamnetin (~30 μg/g of extract) when analyzed by Reversed Phase-High performance liquid chromatography RP-HPLC.[22] Therefore, we sought whether any of this major flavonoid alone could reserve adverse changes induced by the hypoxia. Here, we show that hepatocytes pretreated with equalized amount of quercetin-3-galactoside, kaempferol, and isorhamnetin to the extract resulted in more cell survival than the hypoxic cells and survival increased further when all these three compounds were administered together. Moreover, all the three compounds could prevent leakage of intracellular enzymes such as LDH, ALT, and AST to certain extent when compared to hypoxic cells. However, the protection offered by these compounds alone or together was partial in contrast to the protection offered by the extract. These data suggests many other compounds present in the extract are required together for protecting cellular injury associated with hypoxia.
Overall findings of this study indicate that treatment of aqueous extract of H. rhamnoides attenuated hypoxia-induced cellular damage mediated via ROS. The protection offered by the major flavonoids present in extract quercetin-3-galactoside, kaempferol, and isorhamentin was partial in compared to the efficacy offered by the extract alone.
Acknowledgments
Author is thankful to Director, DIPAS, Delhi for her constant support and constructive suggestions and Director, DIHAR, Leh, India for providing study material.
Footnotes
Source of Support: This work was supported by the Defence Research and Development Organisation (DRDO), Ministry of Defence, India.
Conflict of Interest: None declared
References
- 1.Brecht M, Groot H. Protection of hypoxic injury in cultured hepatocytes by glycine, alanine and serine. Amino Acids. 1994;6:25–35. doi: 10.1007/BF00808120. [DOI] [PubMed] [Google Scholar]
- 2.Lefebvre VH, Van Steenbrugge M, Beckers V, Roberfroid M, Buc-Calderon P. Adenine nucleotides and inhibition of protein synthesis in isolated hepatocytes incubated under different pO2 levels. Arch Biochem Biophys. 1993;304:322–31. doi: 10.1006/abbi.1993.1357. [DOI] [PubMed] [Google Scholar]
- 3.Lluis JM, Morales A, Blasco C, Colell A, Mari M, Garcia-Ruiz C, et al. Critical role of mitochondrial glutathione in the survival of hepatocytes during hypoxia. J Biol Chem. 2005;280:3224–32. doi: 10.1074/jbc.M408244200. [DOI] [PubMed] [Google Scholar]
- 4.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Simon LM, Phillips JR, Robin ED. Superoxide dismutase (SOD) activity in hypoxic mammalian systems. J Appl Physiol. 1977;42:107–10. doi: 10.1152/jappl.1977.42.1.107. [DOI] [PubMed] [Google Scholar]
- 6.de Groot H, Littauer A. Hypoxia, reactive oxygen, and cell injury. Free Radic Biol Med. 1989;6:541–51. doi: 10.1016/0891-5849(89)90047-6. [DOI] [PubMed] [Google Scholar]
- 7.Geetha S, Sai Ram M, Singh V, Ilavazhagan G, Sawhney RC. Anti-oxidant and immunomodulatory properties of seabuckthorn (Hippophae rhamnoides) – An in vitro study. J Ethnopharmacol. 2002;79:373–8. doi: 10.1016/s0378-8741(01)00406-8. [DOI] [PubMed] [Google Scholar]
- 8.Saggu S, Divekar HM, Gupta V, Sawhney RC, Banerjee PK, Kumar R. Adaptogenic and safety evaluation of seabuckthorn (Hippophae rhamnoides) leaf extract: A dose dependent study. Food Chem Toxicol. 2007;45:609–17. doi: 10.1016/j.fct.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Tulsawani RK, Sharma P, Divekar HM, Meena RN, Singh M, Kumar R. Supplementation of fruit extract of Hippophae rhamnoides speeds adaptation to simulated high altitude stressors in rats. J Complement Integr Med. 2010:7. [Google Scholar]
- 10.Rousi A. The genus Hippophae L., a taxonomic study. Annals Bot. 1970;8:177–227. [Google Scholar]
- 11.Dhyani D, Maikhuri RK, Misra S, Rao KS. Endorsing the declining indigenous ethnobotanical knowledge system of seabuckthorn in Central Himalaya, India. J Ethnopharmacol. 2010;127:329–34. doi: 10.1016/j.jep.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 12.Li TS, Beveridge HJ. Introduction. In: Cavers PB, editor. Seabuckthorn (Hippophae rhamnoides): Production and utilization. Chap. 1. Ottawa, Ontariopp: NRC Research Press; 2003. pp. 1–6. [Google Scholar]
- 13.Beveridge T, Li TS, Oomah BD, Smith A. Sea buckthorn products: Manufacture and composition. J Agric Food Chem. 1999;47:3480–8. doi: 10.1021/jf981331m. [DOI] [PubMed] [Google Scholar]
- 14.Dhyani D, Maikhuri RK, Rao KS, Kumar L, Purohit VK, Sundriyal M, et al. Basic nutritional attributes of Hippophae rhamnoides (seabuckthorn) populations from Uttarakhand Himalaya, India. Current Science. 2007;92:1148–52. [Google Scholar]
- 15.Eccleston C, Baoru Y, Tahvonen R, Kallio H, Rimbach GH, Minihane AM. Effects of an antioxidant-rich juice (sea buckthorn) on risk factors for coronary heart disease in humans. J Nutr Biochem. 2002;13:346–54. doi: 10.1016/s0955-2863(02)00179-1. [DOI] [PubMed] [Google Scholar]
- 16.Kallio H, Yang B, Peippo P. Effects of different origins and harvesting time on vitamin C, tocopherols, and tocotrienols in sea buckthorn (Hippophaë rhamnoides L.) berries. J Agric Food Chem. 2002;50:6136–42. doi: 10.1021/jf020421v. [DOI] [PubMed] [Google Scholar]
- 17.Zu Y, Li C, Fu Y, Zhao C. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J Pharm Biomed Anal. 2006;41:714–9. doi: 10.1016/j.jpba.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 18.Purushothaman J, Suryakumar G, Shukla D, Malhotra AS, Kasiganesan H, Kumar R, et al. Modulatory effects of seabuckthorn (Hippophae rhamnoides L.) in hypobaric hypoxia induced cerebral vascular injury. Brain Res Bull. 2008;77:246–52. doi: 10.1016/j.brainresbull.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Narayanan S, Ruma D, Gitika B, Sharma SK, Pauline T, Ram MS, et al. Antioxidant activities of seabuckthorn (Hippophae rhamnoides) during hypoxia induced oxidative stress in glial cells. Mol Cell Biochem. 2005;278:9–14. doi: 10.1007/s11010-005-7636-2. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A, Kumar R, Pal K, Banerjee PK, Sawhney RC. A preclinical study of the effects of seabuckthorn (Hippophae rhamnoides L.) leaf extract on cutaneous wound healing in albino rats. Int J Low Extrem Wounds. 2005;4:88–92. doi: 10.1177/1534734605277401. [DOI] [PubMed] [Google Scholar]
- 21.Jain M, Ganju L, Katiyal A, Padwad Y, Mishra KP, Chanda S, et al. Effect of Hippophae rhamnoides leaf extract against Dengue virus infection in human blood-derived macrophages. Phytomedicine. 2008;15:793–9. doi: 10.1016/j.phymed.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Kumar MS, Dutta R, Prasad D, Misra K. Subcritical water extraction of antioxidant compounds from Seabuckthorn (Hippophae rhamnoides) leaves for the comparative evaluation of antioxidant activity. Food Chem. 2001;127:1309–16. doi: 10.1016/j.foodchem.2011.01.088. [DOI] [PubMed] [Google Scholar]
- 23.Singleton VL, Rossi JA. Colorimetric determination of total phenolic with phosho-molybidic-phospho-tungstic acid reagents. Am J Enol Viticult. 1965;16(3):144–58. [Google Scholar]
- 24.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid content in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–9. [Google Scholar]
- 25.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 26.Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43:506–20. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 29.Latour I, Pregaldien JL, Buc-Calderon P. Cell death and lipid peroxidation in isolated hepatocytes incubated in the presence of hydrogen peroxide and iron salts. Arch Toxicol. 1992;66:743–9. doi: 10.1007/BF01972625. [DOI] [PubMed] [Google Scholar]
- 30.Tulsawani RK, Hariharakrishnan J, Jatav PC, Bhattacharya R. Effect of alpha ketoglutarate on some hepatic variables altered by cyanide in vivo and in vitro. J Cell Tissue Res. 2006;6:719–25. [Google Scholar]
- 31.Nakanishi K, Tajima F, Nakamura A, Yagura S, Ookawara T, Yamashita H, et al. Effects of hypobaric hypoxia on antioxidant enzymes in rats. J Physiol. 1995;489:869–76. doi: 10.1113/jphysiol.1995.sp021099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tribble DL, Jones DP, Edmondson DE. Effect of hypoxia on tert-butylhydroperoxide-induced oxidative injury in hepatocytes. Mol Pharmacol. 1988;34:413–20. [PubMed] [Google Scholar]


