Abstract
Objective:
Present investigation was undertaken to study the effectiveness of hydroalcoholic extract of roots of Boerhaavia diffusa in experimental benign prostatic hyperplasia (BPH) in rats using various animal models.
Materials and Methods:
BPH in rats was induced by subcutaneous injection of testosterone (5 mg/kg) daily for 28 days. Rats were divided in to five groups (six rats each). A negative control group received arachis oil (1 ml/kg s.c.) and four groups were injected testosterone. These four groups were further divided into reference group (finasteride 1 mg/kg), model group (testosterone), study group A (B. diffusa 100 mg/kg), and study group B (B. diffusa 250 mg/kg). On the 29th day, rats were sacrificed and body weight, prostate weight, bladder weight, and serum testosterone level were measured and histological studies were carried out. Further in vitro analysis of B. diffusa extract on contractility of isolated rat vas deferens and prostate gland, produced by exogenously administered agonists were carried out. All results were expressed as mean ± SEM. 0 Data were analyzed by one-way analysis of variance followed by Tukey's test.
Results:
B. diffusa (100 mg/kg) treatment for 28 days resulted in significant inhibition of prostate growth (P < 0.05). Drug extract did not have significant change on serum testosterone level. Histopathological analysis of prostate gland supported above results. Results of in vitro experiment suggest that extracts had attenuated the contractile responses of isolated vas deferens and prostate gland to exogenously applied agonists.
Conclusion:
The results suggested that treatment with B. diffusa may improve symptoms of disease and inhibit the increased prostate size. In vitro study implies that herbal extracts has the machinery to produce beneficial effect on prostatic smooth muscle, which would relieve the urinary symptoms of disease. B. diffusa could be a potential source of new treatment of prostatic hyperplasia.
KEY WORDS: Benign prostatic hyperplasia, Boerhaavia diffusa, dihydrotestosterone, isolated vas deferens, prostate gland, testosterone
Introduction
Benign prostatic hyperplasia (BPH) is a non-malignant, uncontrolled proliferation of the epithelial cells and stroma that occurs in the peri urethral transition zone of the prostate gland that surrounds the urethra.[1] The functions of prostate are to secret fluids that make up a portion of the ejaculate volume; and to provide secretions with possible antibacterial effect. BPH is a common condition in elderly men, with an estimated prevalence of up to 85%. According to autopsy studies, approximately 80% of elderly men develop microscopic evidence of disease. About half of the patient with microscopic changes develop an enlarged prostate gland and as a result have a difficulty emptying the content of the urinary bladder.[2]
Although, the etiology of BPH is not completely elucidated, it involves hormonal changes in the aging men.[3] The development and growth of prostate depends on androgen stimulation, mainly by dihydrotestosterone (DHT), an active metabolite formed due to enzymatic conversation of testosterone by steroid 5α-reductase enzyme. Production and accumulation of DHT in the prostate increase with the aging which results in the induction of disease. Furthermore, as men age, prostatic estrogen level rise, resulting in increased activity of substances stimulating cell growth, possibly causing disease. BPH also involves augmented adrenergic tone in prostate smooth muscle, regulated through α1-adrenoreceptor.[4]
It results from static and dynamic factors. Static factors relate to anatomical enlargement of prostate gland, dynamic factors relates to excessive α-adrenergic tone of the stromal component of the prostate gland, bladder neck, and posterior urethra, which results in contraction of prostate gland around the urethra and narrowing of the urethral lumen.
Management option of prostatic hyperplasia includes watchful waiting, drug treatment, and surgical interventions. Conventional drug treatments include 5α-reductase inhibitors and α-adrenergic antagonists. Although these drugs have great efficacy in treating patients, their adverse effect should not be overlooked. For example, 5α-reductase inhibitors can cause adverse effect such as impotence, gynecomestia, impairment of muscle growth, and decreased libido; α-adrenergic antagonists can cause orthostatic hypotension, fatigue, dizziness, abnormal ejaculation. Furthermore, long-term treatment is required and surgical treatments are costly and a risk for aged men excludes their use as routine treatment.[1]
India has vast ethnobotanical knowledge since ancient time. The modern medicine has contributed significantly in combating against many disease conditions. But there are various problems with modern drugs, which can indirectly increase the interest for search of safer plant drugs. Boerhaavia diffusa has been of keen interest in phytochemical and pharmacological research due to their excellent medicinal values. It possesses hepatoprotective,[5] diuretic,[6] anti-inflammatory,[7] anti-stress, and immunomodulation,[8] antifertility,[9] activities. In vitro anti-proliferative and anti-estrogenic properties were also proved.[10]
Therefore, the present study was carried out to study effectiveness of B. diffusa in experimental BPH in rats using various animal models.
Materials and Methods
Animals
Male Wistar rats weighing 160-290 g were used in present study and were housed in polypropylene cage. Animals were maintained at 21-25°C and 45-65% humidity with 12-h light/dark cycle and had free access to food and water. All experimental procedures were carried out in accordance with Committee for the Purpose of Control and Supervision of Experiments in Animals (CPCSEA) guidelines. The study was reviewed and approved by the Institutional Animal Ethics Committee (Protocol Number: MPC/12/2012). Animals were randomly divided in different groups for different treatments.
Plant extract
The authenticated dried hydro-alcoholic extract of root of B. diffusa was provided by Asian Drugs and Pharma, 50/3, G.I.D.C., Kabilpore - 396 424, Navsari, Gujarat, India. Extract was stored in airtight container in dry place and used throughout the experiment.
Quality assessment of extract
The extract was subjected to high performance thin layer chromatography (HPTLC) examinations[11] to detect the presence of various phyto constituents. One gram of powdered extract was dissolved in 10 ml of methanol and filtered. HPTLC plate (3 cm × 5 cm) silica gel 60 F254 (E. Merck, Germany) of 200 ìm layer thickness was used. 10 μl of test solution was applied on HPTLC plates coated with silica gel by use of Automatic device “CAMAG LINOMAT-5” sample applicator equipped with a 100 ìl micro-syringe and an automatic sampler. The linear development was carried out in a chamber (20 cm × 10 cm) previously saturated with 20 ml mobile phase with solvent system toluene:ethyl acetate (5:1.5 v/v) at room temperature. The plate was dried in the air and after air drying the plate was placed in HPTLC scanner for analysis.
In vivo study
Induction of disease and experimental design
Male Wistar rats weighing 160-290 g were randomly divided in five groups (n = 6). Experimentally developed BPH model was created by subcutaneous administration of testosterone (5 mg/kg) dissolved in arachis oil for 28 days.[12,13,14] Both extracts were dissolved in to distilled water. Drugs were administered orally once daily for 28 consecutive days. Experimental design and treatments are described in Table 1. Body weight was measured weekly during the study. On the 29th day, blood was collected from retro orbital plexus and animals were sacrificed. Immediately prostate gland and bladder were dissected and weighed and various parameters were measured.
Table 1.
Effectiveness of Boerhavia diffusa in experimental prostatic hyperplasia in rats: Study groups
Prostate weight (P) to body weight (BW) ratio
Prostate weight (P) to body weight (BW) ratio were calculated by dividing prostate weight with that of animal body weight for the individual study group animal.
Determination of serum testosterone level
Blood samples were collected and centrifuged at 2000×g for 20 min to obtain serum for determination of testosterone using enzyme-linked immunosorbent assay kit.
Determination of total protein in prostate
Prostate glands were dissected and homogenates were made in phosphate buffer solution (0.01 M sodium phosphate buffer, pH 7.4, containing 0.14 M NaCl) at a ml volume/g gland wet weight ratio of 4:1. Homogenates were centrifuged at 13,000×g for 20 min and supernant collected.[15] Supernant was used as source of proteins and concentration was determined by modified biuret end point assay method.
Histopathological investigation
All prostatic specimen and bladder in each group were fixed with 10% formalin and sent at Jenny Laboratory, Surat, for histology. Microscope images were then captured and slides were analyzed by pathologist of Nakoda Diagnostics, Surat.
Percentage of recovery
On the basis of mean prostatic weight and P/BW ratios, we also calculated the percentage recovery in P/BW ratio by test group compared with model group. The increase induced by testosterone alone was considered to be 100% and all other test groups were compared with this reading. The formula used was as follows:
Percent recovery by the test sample = [A – B]
where A is the percentage increase in prostatic weight induced by testosterone and B is the percentage increase in prostatic weight induced by test sample.
Percentage of Inhibition of increase in prostate weight
Percentage of inhibition[13] was calculated as follows:

In vitro study
Isolated prostate gland contractility study
Animals were sacrificed by cervical dislocation and a lower abdominal incision was made and prostate gland was identified and dissected carefully. The connective fascia of the rat prostate was bisected to provide paired lobe preparations. Immediately following excision, prostates were placed in to a petridish containing physiological salt solution (Krebs-Henseleit solution [mM: NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, glucose 11.7]) and the surrounding prostatic capsule along with excess fatty tissue was dissected away to facilitate drug distribution throughout the tissue. Tissue was mounted in 30 ml organ bath containing Krebs-Henseleit solution warmed at 37°C with continuous aeration. One end of the prostate was attached to MLTF500/ST force-displacement transducer and other to a tissue holder. Force was recorded via PowerLab data acquisition system (Lab Chart 7.3). Preparations were equilibrated for 60 min under a resting tension of ~0.7 g. The organ bath medium was replaced after every 10 min during equilibration period.
The effect of extract on direct muscle stimulation by exogenously administered agonists was assessed by constructing concentration-response curve of acetylcholine and noradrenaline using a dose progression ratio of approximately half a log unit. Once the contractile response to each concentration of agonists had reached a maximum, tissue was washed with bath solution and allowed to recover for 15 min. Following the initial concentration response curve, tissue was exposed to an extract solution (10 μg/ml, 0.3 ml) for 3 min every time and second concentration response curve was recorded in presence of an extract. The organ bath medium was replaced after each response with fresh medium. Mean log molar concentration of agonist versus percentage response curves were constructed by pooling data from individual curves constructed from tissue taken from three rats.[16,17,18]
Isolated vas deference contractility study
Animals were sacrificed by cervical dislocation and a lower abdominal incision was made followed by location of testicles. Vas deferens was carefully dissected and epididymal end of vas deferens was made free from connective tissue and was cut. The tissue was kept moist during dissection and tugging and stretching of the preparation was kept to a minimum. Once removed from the animal, vas deference was further cleaned of its connective tissue sheath and blood vessels with the tissue immersed in physiological salt solution (Ringer-Locke solution [mM: NaCl 110, KCl 1.9, CaCl2 1.1, NaHCO3 2.4, NaH2PO4 0.06, glucose 11.1]).[19] Tissue was mounted in 50 ml organ bath containing Ringer-Locke solution warmed at 37°C with continuous aeration. One end of the vas deferens was attached to MLTF500/ST force-displacement transducer and other to a tissue holder. Force was recorded via PowerLab data acquisition system (Chart 7.3). Preparations were equilibrated for 60 min under a resting tension of <“0.5 g. The organ bath medium was replaced after every 10 min during equilibration period.
The effect of extract on α-receptor by exogenously administered agonists was assessed by constructing concentration-response curve of noradrenaline using a dose progression ration of approximately half a log unit. Once the contractile response to each concentration of agonists had reached a maximum, tissue was washed with bath solution and allowed to recover for 15 min. Following the initial concentration response curve, tissue was exposed to extract solutions (10μg/ml, 0.3 ml) for 3 min every time and second concentration response curve was recorded in presence of an individual extract. The organ bath medium was replaced after each response with fresh medium. Mean log molar concentration of agonist versus percentage response curves were constructed by pooling data from individual curves constructed from tissue taken from three rats.[20,21]
Statistical analysis
All results were expressed as mean ± SEM. 0 Data were analyzed by one-way analysis of variance followed by Tukey's test.
Results
Quality assessment of extract
The result of HPTLC profile of B. diffusa has Rf values 0.13, 0.23, 0.30, 0.45, 0.57, 0.71, and 0.98 at 254 nm. HPTLC profile of B. diffusa showed presence of seven substances with maximum area 59%.
Evaluation of prostate enlargement
Effect of B. diffusa on prostatic parameters
When the animals were treated with testosterone injection (5 mg/kg) it showed significant increase in prostate weight and prostate weight index compared to negative control group. There was significant change (P < 0.05) in prostate weight after treatment with B. diffusa extract 100 mg/kg when compared with model group. There was 80.76% prostate growth was increased at dose of 250 mg/kg while at dose of 100 mg/kg it was 68.26% [Table 2].
Table 2.
Effect of Boerhaavia diffusa extract on prostatic parameters

Effect of B. diffusa extract on other parameters
There was no significant difference between bladder weights of the animals but protein level in prostate gland was significantly changed in groups compared with model group. Serum testosterone level was significantly increased in model group compared to negative control group. Administration of B. diffusa extract did not significantly affect serum testosterone level [Table 3].
Table 3.
Effect of Boerhaavia diffusa extract on other parameters in rats

Effect on contractile response of prostate gland
Contractile responses to exogenous application of noradrenaline and acetylcholine, elicited concentration dependent contraction of isolated rat prostate. Contractile response to all two exogenously administered agonists was attenuated following incubation with B. diffusa extract compared to vehicle controls [Figures 1 and 2].
Figure 1.

Effect of Boerhaavia diffusa extract on acetylcholine induced contraction of prostate gland. Each point represents mean±SEM
Figure 2.

Effect of Boerhaavia diffusa extract on noradrenaline induced contraction of prostate gland. Each point represents mean±SEM
Effect on contractile response of vas deferens
Exogenously administered noradrenaline elicited concentration dependent contraction of isolated rat vas deferens. Incubation of isolated vas deferens with drug extract attenuated the contractile response to exogenously applied noradrenaline [Figure 3].
Figure 3.
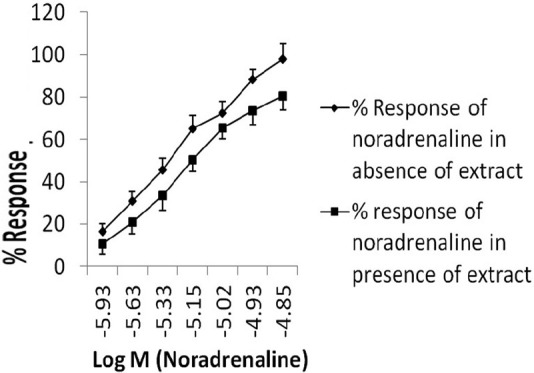
Effect of Boerhaavia diffusa extract on noradrenaline induced contraction of vas deferens. Each point represents mean±SEM
Histopathological investigation
Group I: Negative control group (arachis oil 1 mg/kg)
Histology of prostate gland [Figure 4] showed normal histological features of prostate gland, acini of variable diameter were visible. The tubules of variable diameter and irregular lumen with single layer of epithelium were there. The lumens were filled with prostatic secretions in connective tissue the matrix was normal. Stroma and gland revealed histology suggestive of normal prostate gland.
Figure 4.

Effect of Boerhaavia diffusa on histology of prostate gland
Group II: Model group (testosterone 5 mg/kg)
Sections from prostate glands showed mild to moderate disruption of histo-architecture structure of gland. In the group treated with testosterone alone, tubules appear to have become wider compared with the control group. The walls of tubules are thickened and almost every tubule developed large involutions projecting into the lumen, reducing the volume of the lumen compared with the control group. The connective tissue was compressed compared with the control. The distinct nucleus and normal sarcoplasmic texture were not visible. The shape of the tubules was obliterated. Lumen was narrow, but at most places, the transitional nature of the epithelium persisted. Overall histology revealed prostatic hyperplasia.
Group III: Reference group (testosterone + finasteride l mg/kg)
With normal distribution of stroma, this group had a texture similar to that observed in control group. The projections were not as prominent as observed in the group treated with testosterone alone. Although, finasteride appeared to antagonize the effects of testosterone to a certain degree, several cells with increased volume were observed throughout the transitional epithelium. Cells with swollen nuclei were prominent in many places. Reduced involutions in lesser number were observed. Overall histology revealed normal prostate gland with a few foci showing changes of prostatic hyperplasia.
Group IV: Study group A (testosterone + B. diffusa extract l00 mg/kg)
The lumens of the tubules were normal, and in some places the epithelium was thicker than that of the control group. Connective tissue between the tubules was reduced. Tubules appear to have large lumen. There was improvement in histo-architecture of prostate gland compared to model group.
Group V: Study group B (testosterone + B. diffusa extract 250 mg/kg)
Groups treated with B. diffusa extract (250 mg/kg) appeared to be similar to the group treated with testosterone alone. No improvement in volume of the lumen, sarcoplasmic texture, or stromal distribution was visible in this group, except in the shape and size of the tubules compared with the testosterone-treated group. The epithelium was little more maintained compared with the group treated with testosterone alone.
Discussion
BPH involves microscopic benign proliferation of prostatic stroma and epithelium and enlargement of prostate gland. Although the incidence of hypertrophy of the canine prostate increase with age and closely resembles that in humans, BPH have been experimentally induced in rats following prolonged treatment with testosterone.[13,14,15] As androgen exhibited stimulating action on the growth of prostate cell, Testosterone and related hormones are demonstrated to play an important role in disease.[3] Data from large scale clinical study have demonstrated that treatment with 5α-reductase inhibitors not only significantly ameliorates symptoms, but also reduces the long-term risk of progression in patients treated surgically.
The model rats, treated with subcutaneous injection of testosterone exhibited enlargement of prostate as a consequence of progressive hyperplasia of glandular and stromal tissue of glands clearly confirmed influence of androgen on prostate growth. Treatment with B. diffusa at dose of 100 mg/kg significantly decreases the prostate weight. Prostatic enlargement is used as one of important marker of disease, the prostatic index used in this study was the prostate weight to body weight ratio. In the present study, oral administration of B. diffusa extract at 100 mg/kg resulted in significant reduction in prostatic index and inhibited the prostatic growth more significantly than at dose of 250 mg/kg confirms that B. diffusa have significant activity at low dose (100 mg/kg) compared to high dose (250 mg/kg). These results were consistent with histopathological examination of prostatic tissue. Animals with BPH show stomatal proliferation and glandular hyperplasia in the prostate, whereas animals treated with test drug showed mild glandular hyperplasia.
DHT, a potent androgen converted from testosterone by 5α-reductase enzyme, plays more critical role in pathogenesis.[2,3] Inhibition of enzyme results in decrease in conversion of DHT and might increase serum testosterone. Measurement of serum testosterone suggested that treatment with B. diffusa did not significantly affect serum testosterone level. Measurement of serum DHT and tissue estradiol is warranted to evaluate effect of drug on 5α-reductase enzyme. The data suggest that B. diffusa significantly decreased total protein content suggesting enzymatic changes in the prostate gland. The development of disease is also associated with enhanced proliferation and suppressed apoptosis of prostatic cell. Polypeptide growth factors play an important role in disease development. Effect of B. diffusa is supposed to be due to its reported anti-inflammatory and anti-proliferative action.
Previous studies suggest that drug which relieve urinary symptoms caused by prostatic hyperplasia by relaxing prostatic smooth muscle are able to inhibit contractility in isolated rat prostate gland.[19] The mammalian prostate is densely innervated by hypogastric and pelvic nerves that play an important role in regulating the growth and function of the gland. Cholinergic autonomic nerves, as well as noradrenergic nerves, influence prostate development. Moreover, acetylcholine may have a general role in cellular growth and function apart from its role as neurotransmitter. In antibody studies on rat prostate, Ruggieri et al., found muscarinic M2 receptors and presence of M3 receptors. Noradrenaline acts on α1-adrenoreceptors in the neck and sphincter of the urinary bladder to promote contraction and urinary retention, and controls the smooth muscle in the prostate capsule and prostate urethra. Dynamic factors relate to excessive α-adrenergic tone resulting in contraction of prostate gland around urethra and narrowing of the urethral lumen; causing lower urinary tract symptoms. Contraction of rat epididymal vas deference is regulated via release of neurotransmitters from autonomic nerves and is mediated by α1-receptors. Relaxation of prostatic smooth muscle is the most effective mechanism of action to relieve urinary symptoms caused by urethral obstruction due to BPH.[14]
Results of this study suggest that B. diffusa extract contains bioactive components, which are able to relax prostatic smooth muscle and also attenuated α-receptor response at some extent. B. diffusa extract inhibit isolated vas deference contractility induced by noradrenaline suggest that it might have action on α-receptor and further study is required to confirm mechanism action of B. diffusa.
In conclusion, extracts of B. diffusa (100 mg/kg) significantly inhibited the prostate growth in experimentally induced BPH. In vitro study implies that herbal extracts has the machinery to produce beneficial effect on prostatic smooth muscle, which would relieve the urinary symptoms of disease. The possible mechanism of B. diffusa extract might be its reported anti-inflammatory and anti-proliferative activity. Further study on inhibition of extracts on the isolated preparations and measurement of DHT and estradiol is warranted.
Acknowledgments
The authors are thankful to Mr. Bipin Jameria, Asian drugs and Pharma, Kabilpore, Navsari for providing drug extract to carry out the present research work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Dhingra N, Bhagwat D. Benign prostatic hyperplasia: An overview of existing treatment. Indian J Pharmacol. 2011;43:6–12. doi: 10.4103/0253-7613.75657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee M. Management of benign prostatic hyperplasia. In: DiPiro JT, editor. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. South Carolina: McGraw Hill Companies; 2008. pp. 1387–98. [Google Scholar]
- 3.Mirone V, Fusco F, Verze P, Schulman C, Debruyne F, Imbimbo C. Androgens and benign prostatic hyperplasia. Eur Urol Suppl. 2006;5:410–7. [Google Scholar]
- 4.Muramatsu I, Oshita M, Ohmura T, Kigoshi S, Akino H, Gobara M, et al. Pharmacological characterization of alpha 1-adrenoceptor subtypes in the human prostate: Functional and binding studies. Br J Urol. 1994;74:572–8. doi: 10.1111/j.1464-410x.1994.tb09186.x. [DOI] [PubMed] [Google Scholar]
- 5.Desai SK, Gawali VS, Naik AB, D’souza LL. Potentiating effect of piperine on hepatoprotective activity of Boerhaavia diffusa to combat oxidative stress. Int J Pharmcol. 2008;4:393–7. [Google Scholar]
- 6.Chowdhury A, Sen PB. Studies on Boerhaavia diffusa Linn.– Effect on diuresis and some renal enzymes. Ann Biochem Exp Med. 1995;15:119–26. [Google Scholar]
- 7.Oladele GM, Ode OJ, Ogunbodede MA. Evaluation of anti-inflammatory and membrane stabilizing effects of aqueous root extract of Boerhavia diffusa Linn. in rats. Int J Appl Biol Pharm Technol. 2011;2:84–8. [Google Scholar]
- 8.Sumanth M, Mustafa SS. Antistress, adoptogenic and immunopotentiating activity roots of Boerhaavia diffusa in mice. Int J Pharmacol. 2007;3:416–20. [Google Scholar]
- 9.Agrawal B, Das S, Pandey A. Boerhaavia diffusa Linn.: A review on its phytochemical and pharmacological profile. Asian J Appl Sci. 2011;4:663–84. [Google Scholar]
- 10.Sreeja S, Sreeja S. An in vitro study on antiproliferative and antiestrogenic effects of Boerhaavia diffusa L. extracts. J Ethnopharmacol. 2009;126:221–5. doi: 10.1016/j.jep.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 11.Meena AK, Niranjan US, Yadav AK, Ajit K, Singh B, Kiran, et al. A quality assessment of Boerhaavia diffusa Linn. commonly known as ‘Punarnava’ plant. Int J Pharmacog Phytochem Res. 2010;2:25–8. [Google Scholar]
- 12.Nandecha C, Nahata A, Dixit VK. Effect of benincasa hispida fruits on testosterone-induced prostatic hypertrophy in albino rats. Curr Ther Res. 2010;71:331–43. doi: 10.1016/j.curtheres.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veeresh Babu SV, Veeresh B, Patil AA, Warke YB. Lauric acid and myristic acid prevent testosterone induced prostatic hyperplasia in rats. Eur J Pharmacol. 2010;626:262–5. doi: 10.1016/j.ejphar.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 14.Sun H, Li TJ, Sun LN, Qiu Y, Huang BB, Yi B, et al. Inhibitory effect of traditional Chinese medicine Zi-Shen Pill on benign prostatic hyperplasia in rats. J Ethnopharmacol. 2008;115:203–8. doi: 10.1016/j.jep.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Schøyen H, Wassdal I, Toft K, Almendingen M, Berg T. Purification of enzymes of the kallikrein gene family (rK8 and rK9) from the rat prostate. Biochem J. 1994;302:229–35. doi: 10.1042/bj3020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahapokai W, Van Sluijs FJ, Schalken JA. Models for studying benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2000;3:28–33. doi: 10.1038/sj.pcan.4500391. [DOI] [PubMed] [Google Scholar]
- 17.Brandli A, Simpson JS, Ventura S. Isoflavones isolated from red clover (Trifolium pratense) inhibit smooth muscle contraction of the isolated rat prostate gland. Phytomedicine. 2010;17:895–901. doi: 10.1016/j.phymed.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Ventura S. Extracts of bark from the traditional Chinese herb Phellodendron amurense inhibit contractility of the isolated rat prostate gland. J Ethnopharmacol. 2010;127:196–9. doi: 10.1016/j.jep.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh MN. Some standard isolated muscle preaprations. In: Ghosh MN, editor. Fundamentals of Experimental Pharmacology. 3rd ed. Kolkata: Hilton and Company; 2005. pp. 110–20. [Google Scholar]
- 20.Burnstock G, Verkhratsky A. Vas deferens – A model used to establish sympathetic cotransmission. Trends Pharmacol Sci. 2010;31:131–9. doi: 10.1016/j.tips.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Westfall TD, Westfall DP. Pharmacological techniques for the in vitro study of the vas deferens. J Pharmacol Toxicol Methods. 2001;45:109–22. doi: 10.1016/s1056-8719(01)00144-7. [DOI] [PubMed] [Google Scholar]


