Abstract
Objective:
In this study, we investigated the role of peroxisome proliferator-activated receptors (PPAR)-β/δ receptors in carrageenan-induced inflammation and in the anti-inflammatory effects of all-trans retinoic acid (ATRA).
Materials and Methods:
The λ-carrageenan (0.1 ml of 1% w/v) was injected into intra-plantar (i.pl.) region of the hind paw to produce acute inflammation. Paw volume was measured by using the mercury plethysmography. Further, mechanical and thermal hyperalgesia (TH) were assessed by using the dynamic plantar aesthesiometer and plantar test apparatus, respectively. In addition, markers of oxido-nitrosative stress were assessed spectrophotometrically in the hind paw tissue 5 h post-carrageenan.
Results:
An i.pl injection of carrageenan has produced a marked mechanical hyperalgesia (MH) and TH in ipsilateral paw, which was associated with significant elevated oxido-nitrosative stress. Treatment with ATRA (5 mg/kg/p.o/4 days) and GW0742, a selective PPAR-β/δ receptor agonist (0.1 mg/kg/i.p/4 days), significantly decreased the paw volume, mechanical and TH as compared to vehicle control. Administration of GSK0660, selective PPAR-β/δ receptor antagonist, at a dose of (0.3 mg/kg/i.p/4 days), did not produce a significant effect on carrageenan-induced paw edema, MH and TH. However, co-administration of GSK0660 (0.3 mg/kg/i.p/4 days) along with both ATRA (5 mg/kg/p.o/4 days) and GW0742 (0.1 mg/kg/i.p/4 days), significantly reverse the decreased paw edema, MH, and TH. These observed ameliorative effects on inflammatory pain symptoms are correlated with the extent of reduction of oxido-nitrosative stress.
Conclusion:
From above findings, it can be concluded that ATRA exerts anti-inflammatory and anti-hyperalgesic effect, possibly through activation of PPAR-β/δ and subsequent reduction of oxido-nitrosative stress.
Keywords: GSK0660, GW0742, inflammatory pain, peroxisome proliferator-activated receptor-β/δ-receptors, retinoic acid
Introduction
Peroxisome proliferator-activated receptors (PPARs) are transcription factors belonging to the nuclear receptor superfamily. The three known PPAR subtypes, α, γ, and δ, show different tissue distributions and are associated with selective ligands and primarily involved in regulation of lipid metabolism, cellular proliferation, and inflammatory response.[1] Accumulating evidences clearly suggest that PPAR-β/δ receptor play an important role in the regulation of inflammation, in a wide array of experimental models, including LDL-/-knock-out mice,[2] experimental autoimmune encephalitis,[3] and diabetic nephropathy in the rat.[4] Although, PPAR-β/δ has been suggested as a novel therapeutic anti-inflammatory target site for the management of chronic inflammatory diseases, but its role in carrageenan-induced paw edema in rats, a well-established and commonly used model of acute inflammation and inflammatory pain, has not yet been studied. GW0742 and GSK0660 have been reported to be a selective PPAR-β/δ receptor agonist and antagonist, respectively.[5,6] The all-trans retinoic acid (ATRA) is an acid form of vitamin A, which is also known as tretinoin. It is commonly used to treat acne vulgaris and promyelocytic leukemia (APL).[7] ATRA has been shown to protect neurons from inflammation-associated injury via suppression of pro-inflammatory cytokines and inducible nitric oxide (NO) synthase.[8] In vitro studies using human chondrocytes have demonstrated that ATRA suppresses pro-inflammatory cytokine-induced matrix metalloproteinases (MMPs) production andIL-1-induced TNF -α production.[9] We have recently reported that 2-week administration of ATRA significantly alleviated the allodynia and hyperalgesia in chronic constriction injury of sciatic nerve-induced neuropathy, possibly via decreased levels of oxido-nitrosative stress, along with improved anti-oxidant enzymes.[10] However, molecular mechanisms involved in the observed beneficial effects are not delineated.
An in vitro transcription/translation assay using COS-2 cell line demonstrated that ATRA acts as a high affinity ligand for PPAR-β/δ.[11] Therefore, it may be probable to speculate that ATRA-induced anti-inflammatory and anti-hyperalgesic effects may be mediated through activation of PPAR-β/δ receptors. Thus, the present study was designed to investigate the role of PPAR-β/δ receptors in carrageenan-induced inflammation and in the anti-inflammatory effects of ATRA.
Materials and Methods
Animals
Adult male Wistar rats, weight about (180-250 g), were fed on standard chow diet (Ashirwad Industries, Ropar, India) and water ad libitum. The experimental protocol used in the present study was approved by the Institutional Animal Ethical Committee (approval no. ISF/IAEC/M1/Committee for the Purpose of Supervision and Control of Experiments [CPCSEA]/P9/2011; dated on 8.10.2011) and carried out in accordance with the guidelines of the CPCSEA on animals for the use and care of experimental animals.
Drugs and chemicals
λ-Carrageenan, ATRA, PPAR-β/δ agonist (GW0742), PPAR-β/δ antagonist (GSK0660) were purchased from Sigma-Aldrich Corporation, India. ATRA for oral (p.o) administration was freshly prepared by suspending in Carboxymethylcellulose (CMC) (0.5% w/v in saline). GW0742 and GSK0660 for (i.p) administration were freshly prepared by dissolving in DMSO (10% w/v in saline).
Study design and protocol
Rats were randomly allocated to the following groups: Group I: Vehicle treated carrageenan control; Group II: ATRA (5 mg/kg/p.o, 4 days) treated; Group III: GW0742 (PPAR-β/δ agonist) (0.1 mg/kg/i.p, 4 days) treated; Group V: GSK0660 (0.3 mg/kg/i.p, 4 days) treated; Group VI: GSK0660 (0.3 mg/kg/i.p, 4 days) + ATRA (5 mg/kg/p.o,4 days) treated; Group VII: GSK0660 (0.3 mg/kg/i.p, 4 days) + GW0742 (0.1 mg/kg/i.p, 4 days) treated.
Induction and assessment of paw edema
The λ-carrageenan (0.1 ml of 1% w/v) was injected into intra-plantar (i.pl.) region of the hind paw was to produce acute paw inflammation. The paw volume, up to the ankle joint, was recorded using mercury plethysmography (INCO, Ambala), before (-96 and 0 h) and at 1, 2, 3 and 4 h post-carrageenan injection.[12]
Assessment of mechanical hyperalgesia (MH)
The threshold for touch sensitivity was measured in both hind paws, using an automated apparatus for applying reproducible light touch (Dynamic plantar Aesthesiometer 37400-002; UgoBasile, Comerio, Italy). The maximum value of force in grams (50 g) was previously fixed.[13]
Assessment of thermal hyperalgesia (TH)
The paw withdrawal latencies (PWLs) to thermal stimuli were determined using a Plantar Test Apparatus that records automatically using the photodiode motor sensors (37370-002 UgoBasile, Comerio, Italy). Rats were placed individually in Plexiglas cubicles mounted on a glass surface maintained at 25 ± 2°C. A cut-off latency of 20 s was imposed to avoid tissue damage.[13]
Estimation of Biochemical Parameters
Ipsilateral rat paw homogenate preparation
Animals were sacrificed 5 h after carrageenan injection, by survical dislocation, the ipsilateral paw was cut and skin removed. Tissue from the pads of the rat hind paw was removed with a scalpel and 5-mm pieces were then obtained with a tissue punch and each piece was homogenized in a phosphate buffer solution. The homogenate was centrifuged at 10,000 ×g for 15 min, aliquots of supernatant separated and used for biochemical estimation.
Measurement of malondialdehyde (MDA)
The thiobarbituric acid reactive substances assay, based on MDA measurement by spectrophotometrically at 532 nm as described previously was used. Results were expressed as nmol of MDA per mg protein.13
Measurement of Nitrite Content
The nitrite content, an indicator of the production of NO, was determined spectrophotometrically at 540 nm, as described previously.[14]
Measurement of myeloperoxidase (MPO) activity
MPO activity, an indicator of polymorphonuclear leukocyte (PMN) accumulation, was determined spectrophotometrically at 650 as described previously.[15] MPO activity was defined as the quantity of enzyme degrading 1 μmol peroxide/min at 37°C and was expressed in milliunits per gram wet tissue.
Statistical analysis
The data were expressed as Mean ± SEM. The obtained data for the biochemical parameters was analysed by means one-way ANOVA followed by Tukeys multiple test. Whereas, data obtained for behavior testing was analysed by two-way ANOVA followed by Bonferroni post-hoc test. P < 0.05 was adopted as a criterion of significance. Statistical analysis was performed by means of graph PAD prism Software programs (Version 5.0).
Results
Effect of various PPAR-β/δ-receptor modulators on paw volume in carrageenan-induced paw edema in Wistar rats
The i.pl injection of carrageenan (0.1 ml, 1%w/v) significantly increased the paw volume after 1, 2, 3and 4 h post-injection, as compared to basal paw volume. Treatment with ATRA (5 mg/kg/p.o/4 days) and GW0742 (0.1 mg/kg/i.p/4 days), significantly decreased the paw volume, as compared to vehicle treated control. Administration of GSK0660, a selective PPAR-β/δ antagonist (0.3 mg/kg/i.p/4 days), did not produce any significant effect on carrageenan-induced increase in paw volume. However, co-administration of GSK0660 (0.3 mg/kg/i.p/4 days) along with both ATRA (5 mg/kg/p.o/4 days) and GW0742 (0.1 mg/kg/i.p/4 days), significantly attenuated the decrease in paw volume, as compared to ATRA and GW0742, alone treated groups [Figure 1].
Figure 1.
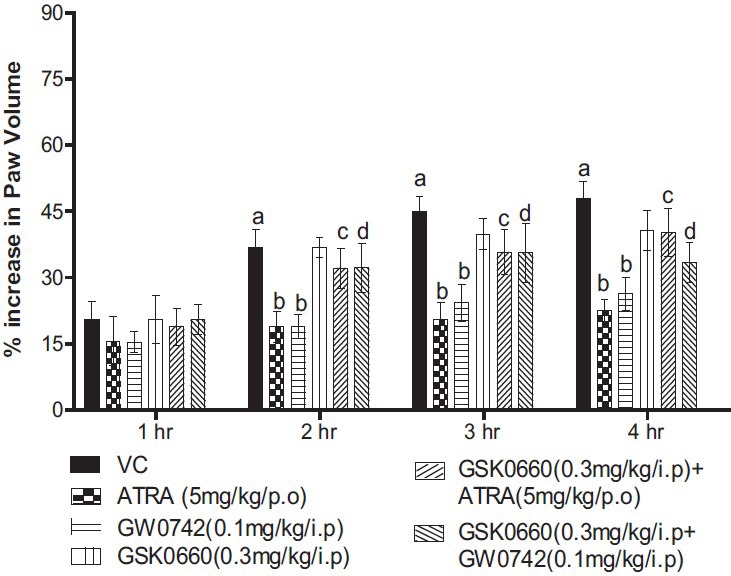
Effect of peroxisome proliferator-activated receptors-β/δ modulators on paw volume in carrageenan-induced paw edema in Wistar rats. All values are expressed as a mean ± SEM (n = 6). a = P < 0.05 versus Basal; b = P < 0.05 versus VC; c = P < 0.05 versus all-trans retinoic acid (5 mg/kg/p.o/4 days); d = P < 0.05 versus GW0742 (0.1 mg/kg/i.p/4 days) versus GW0742
Effect of various PPAR-β/δ-receptor modulators on MH and TH in carrageenan-induced paw edema in Wistar rats
The i.pl injection of carrageenan significantly decreased the mechanical threshold and PWL to thermal stimuli, at both observed intervals. It indicates the development of mechanical and TH. Treatment with ATRA (5 mg/kg/p.o/4 days), GW0742 (0.1 mg/kg/i.p/4 days), significantly attenuated both mechanical and TH as compared to vehicle treated control. Administration of GSK0660, a selective PPAR-β/δ antagonist, at a dose of (0.3 mg/kg/i.p/4 days), did not produce any significant effect on carrageenan-induced mechanical and TH. However, co-administration of GSK0660 (0.3 mg/kg/i.p/4 days) along with ATRA (5 mg/kg/p.o/4 days) and GW0742 (0.1 mg/kg/i.p/4 days) significantly reversed the decreased mechanical and TH as compared to ATRA and GW0742, alone treated groups [Figure 2].
Figure 2.
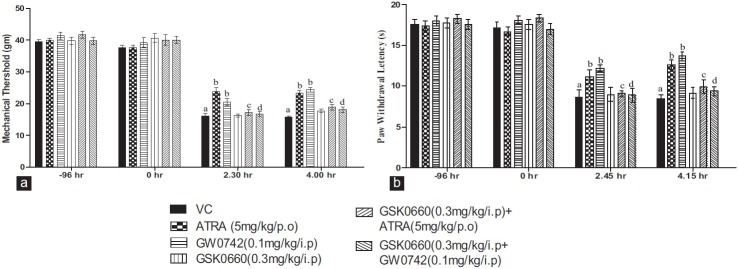
Effect of peroxisome proliferator-activated receptors-β/δ modulators on mechanical hyperalgesia (a) and thermal hyperalgesia (b) in carrageenan-induced paw edema in Wistar rats. All values are expressed as a mean ± SEM (n = 6). a = P < 0.05 versus Basal; b = P < 0.05 versus VC; c = P < 0.05 versus all-trans retinoic acid (5 mg/kg/p.o/4 days); d = P < 0.05 versus GW0742 (0.1 mg/kg/i.p/4 days) versus GW074
Effect of PPAR-β/δ-receptor modulators on increased levels of MDA, nitrate and MPO in carrageenan injected ipsilateral rat paw
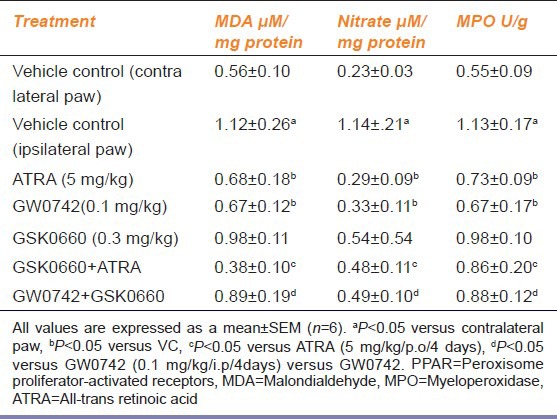
The i.pl injection of carrageenan significantly increased MDA, nitrate and MPO levels in ipisilateral paw, as compared to contralateral paw (the vehicle treated control). Treatment with ATRA (5 mg/kg/p.o/4 days), and GW0742 (0.1 mg/kg/i.p/4 days), significantly decreased levels of MDA, nitrate and MPO as compared to vehicle control. Administration of GSK0660, a selective PPAR-β/δ antagonist, at a dose of (0.3 mg/kg/i.p/4 days), did not produce any significant effect on carrageenan-induced increase in levels of MDA, nitrate and MPO as compared vehicle treated control. However, co-administration of GSK0660 (0.3 mg/kg/i.p/4 days) along with ATRA (5 mg/kg/p.o/4 days) and GW0742 (0.1 mg/kg/i.p/4 days), significantly reversed the decrease in the levels of MDA, nitrate and MPO as compared to ATRA and GW0742, alone treated groups [Table 1].
Table 1.
Effect of PPAR-β/δ modulators on MDA, nitrate and MPO levels in carrageenan-induced paw edema in Wistar rats
Discussion
The results of the present study demonstrate that 4 days repeated administration of both ATRA (5 mg/kg/p.o.) and PPAR-β/δ agonist, GW0742 (0.1 mg/kg/i.p.), produced significant anti-inflammatory and anti-hyperalgesic activity. Administration of PPAR-β/δ antagonist, GSK0660 at a dose (0.3 mg/kg/i.p, for 4 days), per se, did not produce any significant effect, however, concurrent administration of GSK0660 (0.3 mg/kg/i.p, for 4 days) along with ATRA (5 mg/kg/p.o, 4 for days) significantly reversed the anti edematogenic and anti-hyperalgesic effect of both GW0742 and ATRA. The observed beneficial effects of GW0742 and ATRA are correlated with decreased levels of oxido-nitrosative stress markers and MPO.
Although, carrageenan-induced paw edema is a most commonly used the standard technique to screen anti-inflammatory activity, it can also be used as simple routine animal model for the evaluation of inflammatory pain, without any injury to the inflamed tissue.[12] Carrageenan-induced paw edema is a biphasic response. The first phase (0-2 h) is associated with the release of several mediators such as histamine, serotonin, and kinins. The second phase (3 h onward) inflammation is sensitive to most clinically effective anti-inflammatory drugs, which is primarily due to the enhancement of inducible cyclooxygenase isoenzyme, (COX-2) and subsequent formation of prostaglandins.[16,17] It is now well-accepted that the sensitization of primary sensory neurons is essential to inflammatory pain. In humans, this nociceptor sensitization usually leads to clinical conditions known as hyperalgesia (an increased response to a painful stimulus) or allodynia (pain evoked by non-noxious stimuli).[18] Similarly, carrageenan-induced inflammatory hypernociception is characterized by mechanical allodynia and TH in rats, which is due to release of a cascade of the hypernociceptive cytokines such as TNF-α and interleukin-1 β. These cytokines induce the expression of COX-2 and iNOS,[19,20] which ultimately result in elevated oxido-nitrosative stress in the inflamed paw.[21,22,23] Consistent with this, in the present study, the i.pl administration of carrageenan significantly increased paw volume, thermal and MH, along with increased oxido-nitrosative markers.
Pre-treatment with PPAR-β/δ agonist, GW0742 (0.03 mg/kg, i.v), has been shown to attenuate lipopolysaccharide-induced organs dysfunction and decreased mortality in mice. These beneficial effects are mediated through activation of PPAR-β/δ and subsequent inhibition of NF-ĸB activation and iNOS expression.[6] GW0742 (0.3 mg/kg, i.p.) also shown to cause a reduction in inflammatory responses such as PMN accumulation and oxido-nitrosative stress in carrageenan-induced pleurisy test.[23] GW0742 also significantly ameliorated the recovery of spinal cord injury-induced limb dysfunction due to activation of PPAR-β/δ receptors.[24] Further, GW0742 reduced Lipopolysaccharide (LPS) -induced iNOS protein and activity in the rat microglia and astrocytes in vitro.[3] In addition, PPAR-β/δ ligand has also been shown to release anti-inflammatory co-repressor Bcl-6, from PPAR-β/δ and becomes free to repress pro-inflammatory pathway.[25,26,27] Thus, in the present study the observed anti-inflammatory and anti-hyperalgesic effect of GW0742 may be due to activation of PPAR-β/δ.
ATRA, the most active metabolite of vitamin A, has been shown to regulate a wide range of biological processes; such as cell proliferation, differentiation, morphogenesis, and immunomodulation etc. ATRA modulates the cellular activity by binding with specific nuclear receptors, which can be grouped into two families, the Retinoid Acid Receptors (RAR) and the Retinoid X Receptors (RXR).[11] Vitamin A deficiency is associated with enhanced inflammatory reactions, hyperplasia, and cancer.[28] In vitro studies, using human chondrocytes demonstrated that ATRA suppressed pro-inflammatory cytokine-induced MMPs production, and reduced IL-1-induced TNF-α production in chondrocytes.[9] The in vitro transcription/translation assay using COS-2 cell line demonstrated that all trans-retinoic acid act as a high affinity ligand for PPAR-β/δ.11 In addition, in vivo studies using the high fat diet in the mouse model of obesity, demonstrated that the beneficial effect such as decreased food intake, body temperature, triglyceride and insulin levels, induced by ATRA are largely mediated by PPAR-β/δ.[29]
Recently, we have demonstrated that ATRA significantly alleviated the mechanical and TH and cold allodynia in CCl-induced peripheral neuropathy, which was associated with decreased levels of oxido-nitrosative stress, along with improved anti-oxidant enzymes levels.[10] In the present study, concurrent administrations of GSK0660 at dose (0.3 mg/kg/i.p, 4 days) significantly reversed the ATRA-induced anti-inflammatory and anti-hyperalgesic effect. This indicates that ATRA induced beneficial effects may be due to activation of PPAR-β/δ receptor, since, exogenous administration of SOD, Superoxide Dismutase (SOD) mimetic and NO synthase inhibitors are shown to produce anti-inflammatory and anti-nociceptive effect in carrageenan induced inflammatory pain in rats;[21,30] Therefore, these data, along with the results of the present study, reveal that pharmacological administration of GW0742, PPAR-β/δ receptor agonist and ATRA produced beneficial effects may due to an inhibitory effect on release of pro-inflammatory cytokines and subsequent generation of oxido-nitrosative stress, which are secondary to activation of PPAR-β/δ receptors; since, the observed beneficial effects were almost completely abolished by co-administration of GSK0660, a selective PPAR-β/δ receptor antagonist.
Conclusion
From the above findings, it can be concluded that ATRA exerts anti-inflammatory and anti-hyperalgesic effect, possibly through activation of PPAR-β/δ receptors and subsequent reduction of oxido-nitrosative stress.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Bishop-Bailey D, Bystrom J. Emerging roles of peroxisome proliferator-activated receptor-beta/delta in inflammation. Pharmacol Ther. 2009;124:141–50. doi: 10.1016/j.pharmthera.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Graham TL, Mookherjee C, Suckling KE, Palmer CN, Patel L. The PPARdelta agonist GW0742X reduces atherosclerosis in LDLR(-/-) mice. Atherosclerosis. 2005;181:29–37. doi: 10.1016/j.atherosclerosis.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 3.Polak PE, Kalinin S, Dello Russo C, Gavrilyuk V, Sharp A, Peters JM, et al. Protective effects of a peroxisome proliferator-activated receptor-beta/delta agonist in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;168:65–75. doi: 10.1016/j.jneuroim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Collino M, Benetti E, Miglio G, Castiglia S, Rosa AC, Aragno M, et al. Peroxisome proliferator-activated receptor β/δ agonism protects the kidney against ischemia/reperfusion injury in diabetic rats. Free Radic Biol Med. 2011;50:345–53. doi: 10.1016/j.freeradbiomed.2010.10.710. [DOI] [PubMed] [Google Scholar]
- 5.Shearer BG, Steger DJ, Way JM, Stanley TB, Lobe DC, Grillot DA, et al. Identification and characterization of a selective peroxisome proliferator-activated receptor beta/delta (NR1C2) antagonist. Mol Endocrinol. 2008;22:523–9. doi: 10.1210/me.2007-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapoor A, Shintani Y, Collino M, Osuchowski MF, Busch D, Patel NS, et al. Protective role of peroxisome proliferator-activated receptor-β/δ in septic shock. Am J Respir Crit Care Med. 2010;182:1506–15. doi: 10.1164/rccm.201002-0240OC. [DOI] [PubMed] [Google Scholar]
- 7.Romero-Sandoval EA, Molina C, Alique M, Moreno-Manzano V, Lucio FJ, Herrero JF. Vitamin A active metabolite, all-trans retinoic acid, induces spinal cord sensitization. I. Effects after oral administration. Br J Pharmacol. 2006;149:56–64. doi: 10.1038/sj.bjp.0706829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dheen ST, Jun Y, Yan Z, Tay SS, Ling EA. Retinoic acid inhibits expression of TNF-alpha and iNOS in activated rat microglia. Glia. 2005;50:21–31. doi: 10.1002/glia.20153. [DOI] [PubMed] [Google Scholar]
- 9.Ho LJ, Lin LC, Hung LF, Wang SJ, Lee CH, Chang DM, et al. Retinoic acid blocks pro-inflammatory cytokine-induced matrix metalloproteinase production by down-regulating JNK-AP-1 signaling in human chondrocytes. Biochem Pharmacol. 2005;70:200–8. doi: 10.1016/j.bcp.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 10.Bijjem KR, Padi SS, Sharma PL. Ameliorative effects of all-trans retinoic acid on behavioural symptoms of mononeuropathic pain in wistar rat. Int J Recent Adv Pharm Res. 2012;2:56–64. [Google Scholar]
- 11.Shaw N, Elholm M, Noy N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor beta/delta. J Biol Chem. 2003;278:41589–92. doi: 10.1074/jbc.C300368200. [DOI] [PubMed] [Google Scholar]
- 12.Dirig DM, Isakson PC, Yaksh TL. Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. J Pharmacol Exp Ther. 1998;285:1031–8. [PubMed] [Google Scholar]
- 13.Kaur S, Bijjem KR, Sharma PL. Anti-inflammatory and antihyperalgesic effects of the combination of ibuprofen and hemin in adjuvant-induced arthritis in the Wistar rat. Inflammopharmacology. 2011;19:265–72. doi: 10.1007/s10787-011-0090-8. [DOI] [PubMed] [Google Scholar]
- 14.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and 15N nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 15.Cuzzocrea S, Di Paola R, Mazzon E, Genovese T, Muià C, Centorrino T, et al. Role of endogenous and exogenous ligands for the peroxisome proliferators activated receptors alpha (PPAR-alpha) in the development of inflammatory bowel disease in mice. Lab Invest. 2004;84:1643–54. doi: 10.1038/labinvest.3700185. [DOI] [PubMed] [Google Scholar]
- 16.Panthong A, Kanjanapothi D, Taesotikul T, Phankummoon A, Panthong K, Reutrakul V. Anti-inflammatory activity of methanolic extracts from Ventilago harmandiana Pierre. J Ethnopharmacol. 2004;91:237–42. doi: 10.1016/j.jep.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Calixto JB, Medeiros R, Fernandes ES, Ferreira J, Cabrini DA, Campos MM. Kinin B1 receptors: Key G-protein-coupled receptors and their role in inflammatory and painful processes. Br J Pharmacol. 2004;143:803–18. doi: 10.1038/sj.bjp.0706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napimoga MH, Souza GR, Cunha TM, Ferrari LF, Clemente-Napimoga JT, Parada CA, et al. 15d-prostaglandin J2 inhibits inflammatory hypernociception: Involvement of peripheral opioid receptor. J Pharmacol Exp Ther. 2008;324:313–21. doi: 10.1124/jpet.107.126045. [DOI] [PubMed] [Google Scholar]
- 19.Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 2005;102:1755–60. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verri WA, Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol Ther. 2006;112:116–38. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Salvemini D, Wang ZQ, Wyatt PS, Bourdon DM, Marino MH, Manning PT, et al. Nitric oxide: A key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol. 1996;118:829–38. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beloeil H, Asehnoune K, Moine P, Benhamou D, Mazoit JX. Bupivacaine's action on the carrageenan-induced inflammatory response in mice: Cytokine production by leukocytes after ex-vivo stimulation. Anesth Analg. 2005;100:1081–6. doi: 10.1213/01.ANE.0000146964.05212.65. [DOI] [PubMed] [Google Scholar]
- 23.Di Paola R, Esposito E, Mazzon E, Paterniti I, Galuppo M, Cuzzocrea S. GW0742, a selective PPAR-beta/delta agonist, contributes to the resolution of inflammation after gut ischemia/reperfusion injury. J Leukoc Biol. 2010;88:291–301. doi: 10.1189/jlb.0110053. [DOI] [PubMed] [Google Scholar]
- 24.Paterniti I, Esposito E, Mazzon E, Galuppo M, Di Paola R, Bramanti P, et al. Evidence for the role of peroxisome proliferator-activated receptor-beta/delta in the development of spinal cord injury. J Pharmacol Exp Ther. 2010;333:465–77. doi: 10.1124/jpet.110.165605. [DOI] [PubMed] [Google Scholar]
- 25.Lee CH, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM, et al. Transcriptional repression of atherogenic inflammation: Modulation by PPARdelta. Science. 2003;302:453–7. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 26.Barish GD, Atkins AR, Downes M, Olson P, Chong LW, Nelson M, et al. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc Natl Acad Sci U S A. 2008;105:4271–6. doi: 10.1073/pnas.0711875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takata Y, Liu J, Yin F, Collins AR, Lyon CJ, Lee CH, et al. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc Natl Acad Sci U S A. 2008;105:4277–82. doi: 10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu PT, Krutzik SR, Kim J, Modlin RL. Cutting edge: All-trans retinoic acid down-regulates TLR2 expression and function. J Immunol. 2005;174:2467–70. doi: 10.4049/jimmunol.174.5.2467. [DOI] [PubMed] [Google Scholar]
- 29.Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol. 2009;29:3286–96. doi: 10.1128/MCB.01742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handy RL, Moore PK. Effects of selective inhibitors of neuronal nitric oxide synthase on carrageenan-induced mechanical and thermal hyperalgesia. Neuropharmacology. 1998;37:37–43. doi: 10.1016/s0028-3908(97)00201-3. [DOI] [PubMed] [Google Scholar]


