Abstract
Objective:
To investigate the anti-thrombotic activity of DT-13 in experimental animal models.
Materials and Methods:
The anti-thrombotic activity of DT-13 was evaluated by measuring the thrombus induced by inferior vena cava (IVC) ligation in mice and rats. The anti-thrombotic mechanism of DT-13 was investigated by assessing the mRNA expression levels of interleukin-6 (IL-6) and tissue factor (TF) in rat IVC tissue around thrombus.
Results:
DT-13 markedly inhibited thrombosis induced by IVC ligation for 6 h in mice (2.0 and 4.0 mg/kg, p.o.) and for 18 h in rats (1.4 mg/kg, p.o.). Furthermore, DT-13 down-regulated the increased mRNA expression levels of IL-6 and TF in rats.
Conclusions:
DT-13 has an anti-thrombotic activity due to down-regulation of the increased mRNA expression levels of IL-6 and TF.
KEY WORDS: Anti-thrombotic activity, DT-13, interleukin-6, radix Liriope muscari, tissue factor
Introduction
Thrombosis is a widespread pathological condition and one of the leading causes of mortality in old people.[1] Deep vein thrombosis (DVT) is a common clinical problem and often regarded as a serious complication of diseases such as cancer and renal failure. It has been reported that 1 per 1000 people in the world are affected by venous thrombosis and 1:100 patients die due to the disease.[2] Several prospective studies have shown a strong relationship between thrombosis and the incidence of ischemic cardio/cerebrovascular diseases, such as cerebral infarction and myocardial ischemia.[3]
The medicinal plant Liriope muscari has been used in Chinese traditional medicine to treat various diseases such as pharyngitis, bronchitis, pneumonia, cough, and cardiovascular diseases.[4] Steroidal saponin DT-13 (25(R,S)-ruscogenin-1-O-[β-d-glucopyranosyl-(1→2)] [β-d-xylopyranosyl-(1→3)]-β-d-fucopyranoside), one of the major active compounds of L. muscari (Decne.) Baily, possesses strong anti-inflammatory, immunopharmacological, and cardioprotective activities.[5,6,7] DT-13 has shown to prolong the survival time of mice under normobaric hypoxia condition, improve the immunological liver injury and inhibit the growth of S180 and ascites tumors.[8,9] Previous studies have revealed that DT-13 inhibits human breast cancer cell metastasis during hypoxia via regulation of tissue factor (TF) and by early growth response gene-1.[10]
The present study was undertaken to investigate the anti-thrombotic activity of DT-13 and its potential mechanisms using animal models to provide pharmacological evidence in the treatment of thrombotic diseases.
Materials and Methods
Preparation of DT-13
DT-13 was prepared according to the previous method (Ma et al., 2011) and determined as 25(R,S)-ruscogenin 1-O-[β-d-glucopyranosyl(1→2)] [β-d-xylopyranosyl (1→3)]-β-d-frucopyranoside by comparison of its physical data (1H NMR, 13C NMR, MS) with published values.
Animals
The experiments were performed on male Institute of Cancer Research (ICR) mice (weighting 25-30 g) and male Sprague-Dawley rats (weighing 280-340 g), supplied by the experimental animal center of China Pharmaceutical University. All the animal experiments were performed in accordance with the Regulations of the Experimental Animal Administration issued by the State Committee of Science and Technology of the People's Republic of China (November 14, 1988).
Inferior vena cava (IVC) thrombosis model in mice and rats
DT-13 at doses of 1.0, 2.0 and 4.0 mg/kg, warfarin sodium (Shanghai Jiufu Pharmaceutical Co., Ltd, Shanghai, China.) 2.0 mg/kg, or vehicle was administered to mice as a single oral dose. After 1 h, mice were anesthetized by intraperitoneal injection of chloral hydrate at a dose of 400 mg/kg. Abdomen of each animal was opened surgically, and after careful dissection, the IVC was exposed and dissected free from the surrounding tissue. Venous thrombosis was induced by tight ligation of the IVC just below the left renal venous branch using a cotton thread. The abdominal cavity was closed provisionally and the stasis was maintained for 6 h. The cavity was then reopened, the ligated segment was opened longitudinally and the clot was harvested. To quantify the weight of the formed thrombus, the clot was dried at 60°C for 24 h and then weighed using TB-215D electronic balance (Sartorius, Germany).
Similarly, rats were anesthetized by intraperitoneal injection of chloral hydrate at a dose of 350 mg/kg. Abdomen of each animal was opened surgically, and after careful dissection, the IVC was exposed and dissected free from the surrounding tissue. Venous thrombosis was induced by tight ligation of the IVC just below the left renal venous branch using a cotton thread. The abdominal cavity was closed provisionally and the stasis was maintained for 18 h. The cavity was then reopened, the ligated segment was opened longitudinally and the clot was harvested. To quantify the weight of the formed thrombus, the clot was dried at 60°C for 24 h and then weighed using TB-215D electronic balance (Sartorius, Germany). DT-13 at a dose of 1.4 mg/kg was administered orally to rats 1 h before ligation and 17 h after ligation.
RNA extraction and Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) assay for TF and interleukin-6 (IL-6) in rat IVC tissue around thrombus.
Rat IVC tissue around thrombus was isolated after the clot was harvested as previously mentioned. Total RNA from vessel wall tissue was isolated using Trizol reagent (Invitrogen Life Technologies, USA) following manufacturer's instructions. The purity and the amount of RNA were determined by measuring the Optical density (OD) at a ratio of 260-280 nm using Tecan Safire2 microplate reader (Tecan, Zurich, Switzerland). One microgram of total RNA was transcribed into DNA using a MiniCycler PCR (MJ Research, USA) by RT-PCR that was performed for reduced glyceraldehyde-phosphate dehydrogenase (GAPDH), IL-6, and TF. The primers used for rat GAPDH, TF, and IL-6 were as follows: GAPDH, 5'-ACATCTGCTGGAAGGTGGAC (forward) and (3'-GGTACCACCATGTACCCAGG (reverse); TF, 5'-GGAGTGGCAACCGAAACC (forward) and 3'-TGTAAGGAGTGAGACGCCG (reverse); IL-6, 5'-CCTTCTTGGGACTGATGT (forward) and 3'-TCTTTCTGTTTCGGTCTC (forward) (Beijng Sanbo Biotechnology Company, Beijing, China). The thermal cycling conditions of the PCR were 94 °C for 5 min, followed by 30 cycles for 30 s at 94 °C, 60 s at 55 °C, 60 s at 72 °C. The amplified products (10 μL) were separated by electrophoresis in 1 × Tris-acetate ethylene diamine tetraacetic acid (EDTA) buffer with 1.5% agarose gels containing 1 mg/mL of ethidium bromide. The DNA bands were visualized and analyzed by JD-801 Gel Electrophoresis Imagine Analytic System (Jiangsu, China). The ratios of TF mRNA, IL-6 mRNA and GAPDH mRNA were calculated.
Statistical analysis
Data were presented as means ± SD. Statistical differences in data were evaluated by unpaired Student's t-test and a probability value (P) < 0.05 was considered significant.
Results
The inhibitory effect of DT-13 on venous thrombosis by IVC ligation in mice and rats
The IVC ligation procedure led to a dramatic thrombus formation after 6 or 18 h of stasis in all mice or rats administered with vehicle and a red thrombus was present below the ligature. As shown in Figure 1a, DT-13 (2.0-4.0 mg/kg, p.o.) significantly (P < 0.005) inhibited venous thrombosis in mice compared to the control and displayed a dose-dependent manner. Warfarin sodium also markedly inhibited venous thrombosis. Similarly, DT-13 at a single dose of 1.4 mg/kg by oral administration 2 times in 18 h also significantly (P < 0.005) inhibited venous thrombosis in rats compared to the model group [Figure 1b].
Figure 1.
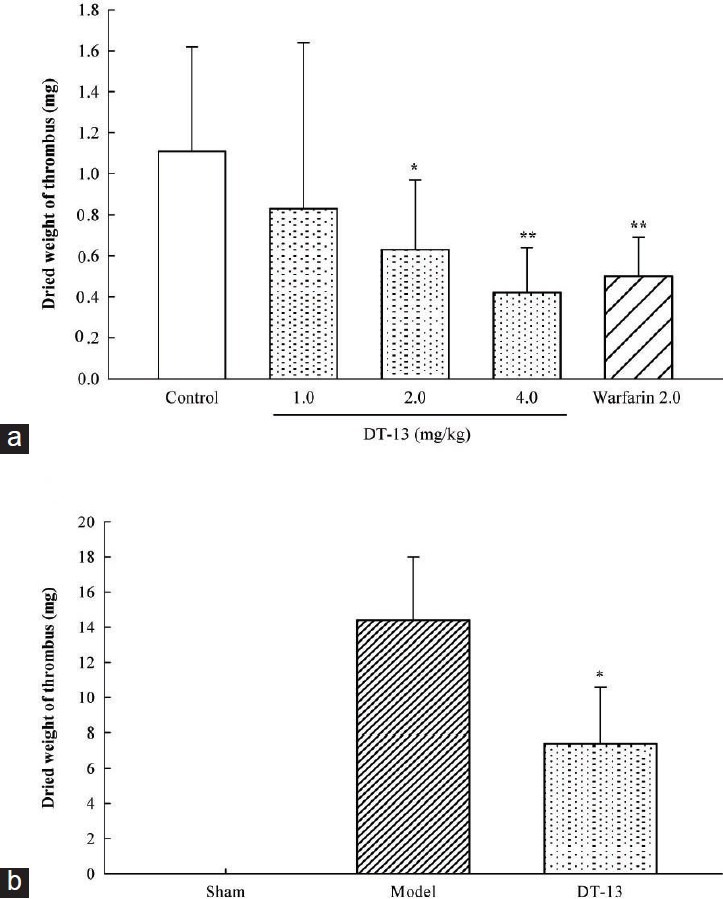
Effects of DT-13 on venous thrombosis by ligation of the inferior vena cava in mice and rats. (a) DT-13 (1.0, 2.0 or 4.0 mg/kg), warfarin sodium (2.0 mg/kg) or vechicle (control) were administered orally 1 h before ligation respectively. Thrombus was obtained 6 h after ligation in mice. (b) DT-13 (1.4 mg/kg) was administered orally 1 h before ligation and 17 h after ligation. Thrombus was obtained 18 h after ligation in rats. Data are expressed as mean±SD (n=18). *P<0.05 and **P<0.01, compared with control or model
The inhibitory effects of DT-13 on mRNA expressions of IL-6 and TF in blood vessel tissue in rats by IVC ligation
Based on the inhibition of DT-13 on IVC thrombosis in rats, the IL-6 and TF responses to DT-13 were assessed by mRNA levels. In saline-administered rats (model group), IL-6 and TF mRNAs were detectable in blood vessel tissue surrounding IVC thrombosis and their expression were 3.5 and 5.1 times of shame group, respectively [Figures 2a and 2b]. In contrast, in the tissue of DT-13-administered rats, IL-6 and TF mRNAs levels decreased significantly (P < 0.005) [Figure 2b], and their inhibitory rates were 60% and 57.58%, respectively.
Figure 2.
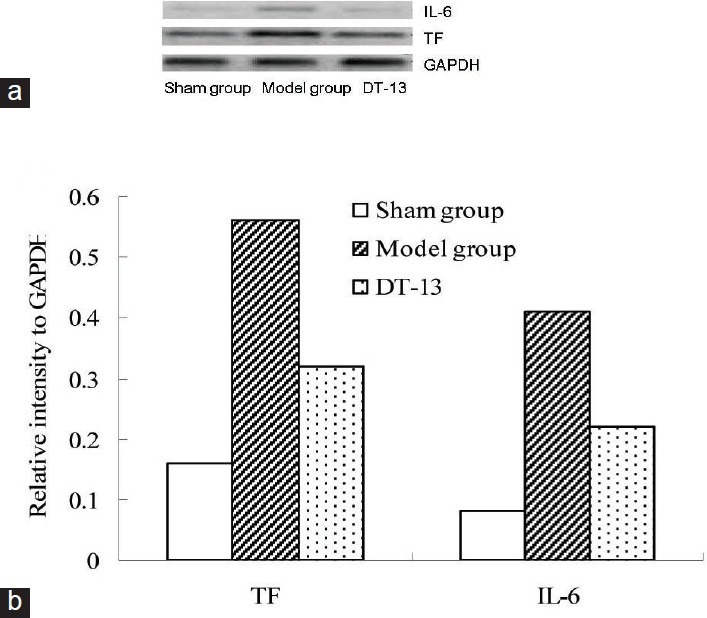
Effects of DT-13 on mRNA expressions of interleukin-6 (IL-6) and tissue factor (TF) in inferior vena cava (IVC) tissue of rat venous thrombosis model. DT-13 (1.4 mg/kg) was administered orally 1 h before ligation and 17 h after ligation. Rat IVC tissue around thrombus was isolated and total RNA was prepared for gene expression by reverse transcriptase polymerase chain reaction (RT-PCR) analysis. IL-6 (384 bp) and TF sequences (329 bp) were detected by agarose gel electrophoresis. PCR of GAPDH was performed to correct for uneven loading of cDNA (a). The result was semi-quantitatively analyzed by JD-801 gel electrophoresis assay system (b)
Discussion
This study showed the antithrombotic properties and the possible mechanisms of DT-13, isolated from L. muscari, in the classical thrombosis model of IVC ligation in mice and rats.
The results demonstrated that DT-13 produced a dose-dependent inhibition of venous thrombus formation in mice at doses of 1.0-4.0 mg/kg. DT-13 at 1.4 mg/kg (comparable to 2 mg/kg in mice) also inhibited venous thrombus formation in rats.
Extensive literatures have shown that inflammatory factors and venous thrombosis are closely related. Serum IL-6 has been shown to increase in an experimental baboon with DVT and patients with post-thrombotic syndrome, suggesting that IL-6 is a risk factor of thrombosis.[11] On the other hand, TF, the surface receptor of coagulation factor VIIa, is a promoter of extrinsic coagulation pathway, and is usually not expressed on monocytes and endothelial cells.
The stimulation by IL-1, IL-6 and tumor necrosis, TF is expressed and activated to trigger coagulation. TF is the key factor in thrombosis and plays an important role in diseases such as venous thrombosis, atherosclerosis, hypertension, angina and disseminated intravascular coagulation.[12,13] Recently immunohistochemical results showed that TF increases in vessel tissue in the early period of thrombosis in the IVC ligation model.[14]
Furthermore, the result of RT-PCR showed that gene expressions of IL-6 and TF in the thrombotic part of the IVC tissue significantly increased. On the other hand, DT-13 reduced gene expressions of IL-6 and TF, which might be one part of its mechanisms for inhibiting venous thrombosis.
In summary, DT-13 a major component of L. muscari inhibits venous thrombosis partly by down-regulating TF and IL-6 expressions. For the in-depth mechanism further research needs to be undertaken.
Acknowledgments
This work was supported by the Major National Science and Technology Project of China for Significant New Drugs Development (No. 2009ZX09103-308 and 2012ZX09102201-015), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
Source of Support: This work was supported by the Major National Science and Technology Project of China for Significant New Drugs Development (No. 2009ZX09103-308 and 2012ZX09102201-015), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. (We have described these foundations in acknowledgements)
Conflict of Interest: No
References
- 1.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 2.Kyrle PA, Eichinger S. Deep vein thrombosis. Lancet. 2005;365:1163–74. doi: 10.1016/S0140-6736(05)71880-8. [DOI] [PubMed] [Google Scholar]
- 3.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–58. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 4.Xiao PG. Modern Chinese Materia Medica. Beijing, China: Chemical Industry Press; 2002. RADIX LIRIOPES; pp. 77–81. [Google Scholar]
- 5.Liu J, Chen T, Yu B, Xu Q. Ruscogenin glycoside (Lm-3) isolated from Liriope muscari inhibits lymphocyte adhesion to extracellular matrix. J Pharm Pharmacol. 2002;54:959–65. doi: 10.1211/002235702760089081. [DOI] [PubMed] [Google Scholar]
- 6.Tao J, Wang H, Zhou H, Li S. The saponin monomer of dwarf lilyturf tuber, DT-13, reduces L-type calcium currents during hypoxia in adult rat ventricular myocytes. Life Sci. 2005;77:3021–30. doi: 10.1016/j.lfs.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Tian YQ, Kou JP, Li LZ, Yu BY. Anti-inflammatory effects of aqueous extract from Radix Liriope muscari and its major active fraction and component. Chin J Nat Med. 2011;9:222–6. [Google Scholar]
- 8.Yu BY, Yin X, Rong ZY, Yang T, Zhang CH, Xu GJ. Biological activities of ruscogenin 1-O-[β-D-glucopyranosyl (1→2)] [β-D-xylopyranosyl (1→3)]-β-D-frucopy-ranoside from tuberous roots of Liriope muscari (Decene.) Baily. J Chin Pharm Univ. 1994;25:286–8. [Google Scholar]
- 9.Wu F, Cao J, Jiang J, Yu B, Xu Q. Ruscogenin glycoside (Lm-3) isolated from Liriope muscari improves liver injury by dysfunctioning liver-infiltrating lymphocytes. J Pharm Pharmacol. 2001;53:681–8. doi: 10.1211/0022357011775802. [DOI] [PubMed] [Google Scholar]
- 10.Sun L, Lin S, Zhao R, Yu B, Yuan S, Zhang L. The saponin monomer of dwarf lilyturf tuber, DT-13, reduces human breast cancer cell adhesion and migration during hypoxia via regulation of tissue factor. Biol Pharm Bull. 2010;33:1192–8. doi: 10.1248/bpb.33.1192. [DOI] [PubMed] [Google Scholar]
- 11.Shbaklo H, Holcroft CA, Kahn SR. Levels of inflammatory markers and the development of the post-thrombotic syndrome. Thromb Haemost. 2009;101:505–12. [PubMed] [Google Scholar]
- 12.Chu AJ. Role of tissue factor in thrombosis. Coagulation-inflammation-thrombosis circuit. Front Biosci. 2006;11:256–71. doi: 10.2741/1796. [DOI] [PubMed] [Google Scholar]
- 13.Steffel J, Lüscher TF, Tanner FC. Tissue factor in cardiovascular diseases: Molecular mechanisms and clinical implications. Circulation. 2006;113:722–31. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, May L, Liao P, Gross PL, Weitz JI. Inferior vena cava ligation rapidly induces tissue factor expression and venous thrombosis in rats. Arterioscler Thromb Vasc Biol. 2009;29:863–9. doi: 10.1161/ATVBAHA.109.185678. [DOI] [PubMed] [Google Scholar]


