Abstract
Lamivudine (3TC) is a nucleoside reverse transcriptase inhibitor (NRTI) licensed for as a first line drug in Human immunodeficiency virus (HIV) infection and also in the treatment of hepatitis B. It is relatively nontoxic in nature and potentiates the antiviral effects of other NRTIs like zidovudine. Although lamivudine is well-tolerated, certain dermatological side effects like severe skin rash may appear. We report a case series of severe skin rashes in four HIV-infected patients, probably due to lamivudine.
KEY WORDS: Lamivudine, skin rash, Stevens-Johnson syndrome
Introduction
With an alarming increase in the incidence of Human immunodeficiency virus (HIV) infections, use of antiretroviral drugs has been delegated a vital role in improving quality of life and disease-related morbidity of these patients. However, the flipside of its benefits are a number of adverse effects including dermatological reactions. Although non-nucleoside reverse transcriptase inhibitors (NNRTIs), like nevirapine, are most commonly responsible for cutaneous reactions, lamivudine (a nucleoside reverse transcriptase inhibitor, NRTI) can also cause severe skin reactions including toxic epidermal necrolysis (TEN), which necessitates its discontinuation and prevent its reintroduction. We report a total of four cases where skin rashes developed after lamivudine therapy.
Case Reports
Case 1
Antiretroviral therapy (ART) was initiated with zidovudine, lamivudine, and nevirapine in a 30-year-old HIV positive, asymptomatic female with CD4 count of 174 cells/μL. She was already on co-trimoxazole prophylaxis for more than 2 weeks and was tolerating it well. She was hospitalized with diffuse erythematous, maculopapular, pruritic rash, which started from the 8th day after initiation of ART, appearing first on extremities, gradually spreading with systemic symptoms like fever and myalgia. Physical examination revealed increased body temperature, rash on trunk and extremities without any mucosal involvement. Routine laboratory investigations including complete hemogram, liver, and kidney functions were normal. All medications including co-trimoxazole were stopped, and within a few hours, the patient improved and the rash disappeared completely by 10th day. Assuming nevirapine to be the offending agent, a combination of zidovudine, lamivudine, and efavirenz (co-trimoxazole was not started) were initiated 2 weeks following complete resolution of rash but similar type of rash with more extensive distribution re-appeared after second dose of ART. All the antiretrovirals (ARV) were withdrawn and the reaction resolved. The next regimen did not include any NNRTIs; instead, lopinavir/ritonavir was started along with zidovudine and lamivudine. The rash once again reappeared after the first dose and all the drugs were withdrawn. The rash disappeared within 7 days. As zidovudine and lamivudine were the common denominator in all three ARV regimens, lamivudine was suspected and withdrawn, and substituted with didanosine in the next regimen. The patient did not develop any rash with zidovudine, didanosine, and lopinavir/ritonavir. After 2 months, efavirenz was substituted instead of lopinavir/ritonavir.
Case 2
Severe maculopapular, pruritic rash, with conjunctival redness and mucosal involvement of the oral cavity and genitalia were observed on the 10th day of ARV initiation with zidovudine, lamivudine, and nevirapine in a 32-year-old female with CD4 count of 25 cells/μL. She was found to be allergic to co-trimoxazole earlier and the attempts at desensitization had failed. She was diagnosed with Steven-Johnson syndrome (SJS). Her laboratory parameters were normal. She improved following discontinuation of all the drugs and symptomatic management. ART was re-initiated with lopinavir/ritonavir, zidovudine, and lamivudine. However, she was admitted again with desquamation of skin of more than 30% of total body area, with exfoliative erythroderma, mucosal ulcers, conjunctival erosion, lacrimation and photophobia, and a diagnosis of toxic epidermal necrolysis (TEN) was made. All medicines were stopped and she recovered gradually in the next 4 weeks. Two months later, ART was re-started with tenofovir, zidovudine, and lopinavir/ritonavir, presuming lamivudine to be offending drug. She continued the drugs without any further adverse effects.
Case 3
In a 38-year-old male patient diagnosed to be HIV positive for last 6 years, ART was started with zidovudine, lamivudine, and nevirapine when his CD4 count dropped to 223/μL. He was admitted with pruritic, erythematous, maculopapular rash with blisters over genitalia, and multiple ulcers in buccal mucosa [Figure 1] along with constitutional symptoms like fever and myalgia on the 7th day of starting ART. All medications including co-trimoxazole were stopped. On examination, he was febrile with maculopapular rash, patchy areas of desquamation, [Figure 2] and multiple ulcers over oral and genital mucous membrane. SJS was diagnosed. His kidney and liver functions were normal. He was managed symptomatically in the hospital with disappearance of the rash by 7th day. Following complete resolution of rash, ART was re-started with lopinavir/ritonavir along with zidovudine and lamivudine. However, similar but more severe rash re-appeared after the first dose. All medicines were withdrawn. ART was subsequently restarted with tenofovir, zidovudine, and lopinavir/ritonavir after complete recovery of rash. He tolerated the drugs well and rash did not re-appear. Two months later lopinavir/ritonavir was substituted with efavirenz which was well-tolerated by the patient.
Figure 1.
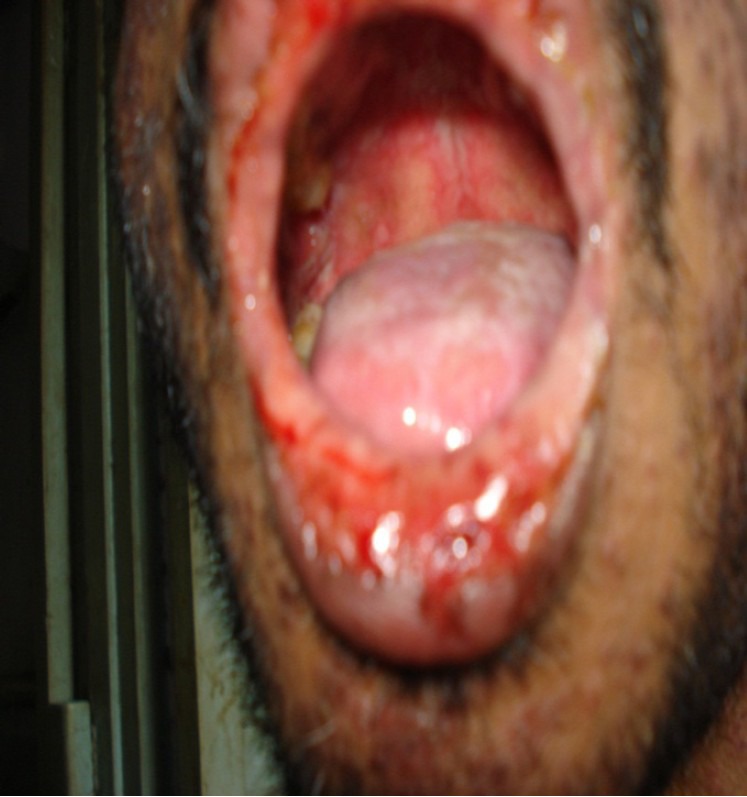
Mucosal involvement of oral cavity
Figure 2.
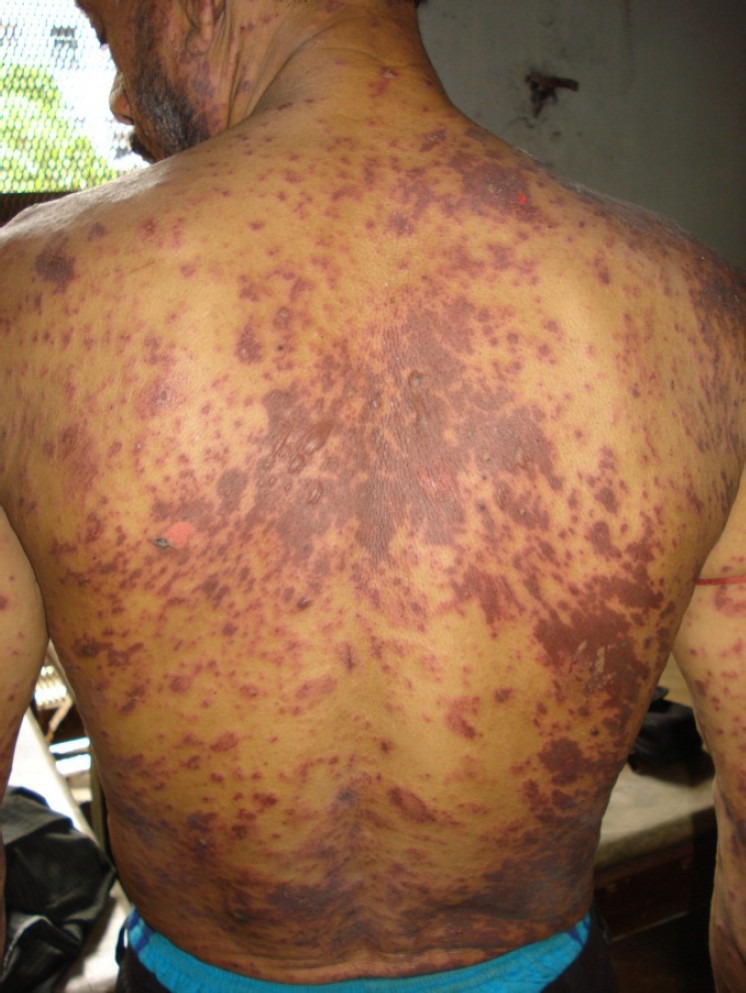
Extensive skin rash with desquamation
Case 4
A 26-year-old HIV positive female patient was admitted with rapidly progressive pruritic, maculopapular, erythematous rash all over the body, without mucosal involvement that developed on 10th day after initiation of ART with zidovudine, lamivudine, and nevirapine. She was already on co-trimoxazole for 2 months. On physical examination, she was febrile and generalized rash was present all over the body without mucosal lesion. Her laboratory and biochemical parameters were normal; CD4 count was 34/μL. All medicines, including co-trimoxazole, were discontinued and the rash disappeared in 1 week. Similar type of rash with fever was observed after first dose of ART re-initiation with efavirenz, zidovudine, and lamivudine. Antiretroviral therapy was stopped. Six weeks after disappearance of cutaneous rash, ART was re-initiated with lopinavir/ritonavir, zidovudine, and lamivudine. Rash reappeared after the 1st dose of ART. Tenofovir was substituted for lamivudine following disappearance of rash. Subjective improvement started within 4 hours of lamivudine discontinuation with complete recovery being noted by two weeks. Rash was not associated with hepatosplenomegaly or lymphadenopathy in all the cases. Histopathology from the rash revealed mild acanthosis, focal spongiosis, and strong sub-epidermal edema.
Causality Assessment
The reaction seen in our cases was observed between 7 to 11 days of starting ART and re-exposure to lamivudine triggered the reaction within 1–2 days. The temporal relation between appearance of rash and lamivudine initiation, rapid regression after its discontinuation, relapse following its re-introduction, and tolerance to lamivudine sparing regimens, confirms the causal relationship. As per the World Health Organization-The Uppsala Monitoring Centre (WHO-UMC) standardized case causality assessment criteria,[1] these events can be considered as a “certain” reaction due to lamivudine. The Naranjo's Adverse Drug Reaction (ADR) probability score[2]9, also confirmed this causality as “definite”.
Discussion
HIV-infected patients are at an increased risk of developing mucocutaneous drug reaction.[3] Nearly 80% patients experience adverse drug reaction at some time during treatment either from immune dysregulation or altered drug metabolism or polypharmacy.[4] It is routine to consider the NNRTI component as the culprit agent when the patients are initiated simultaneously with zidovudine/stavudine, lamivudine, and nevirapine/efavirenz and cutaneous reaction appears. Following recovery, in cases with mild-moderate rash following nevirapine, efavirenz is often used under close medical supervision. In severe cases like SJS/TEN, NNRTIs are never re-introduced and alternate agents like protease inhibitors (PIs) are started (Ritonavir-boosted atazanavir or lopinavir). However, the next common drug group suspected are the NRTIs. Zidovudine and lamivudine have been reported to be associated with skin rash very rarely. An extensive literature search revealed only a few case reports of drug allergy to lamivudine alone. The first is a case of an anaphylactoid reaction, 30 minutes after the first dose of lamivudine (150 mg) in a 49-year-old man.[5] A full recovery was observed within the next 24 hours. A case of contact dermatitis was reported in a healthcare worker taking lamivudine for post exposure prophylaxis.[6] There is another reported incident of severe skin eruption caused by lamivudine that needed discontinuation in a patient with chronic hepatitis B.[7] Lamivudine is available as fixed dose co-formulation with other NRTIs (zidovudine/stavudine/tenofovir) in most of the public health program, including India; it's difficult to manage such patients with lamivudine intolerance without availability of single drug formulations. Since Lamivudine has been an effective, safe, and widely used antiretroviral drug, clinicians must be aware of such severe adverse reactions to lamivudine, which may require treatment discontinuation so that swift treatment can be given in such patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Uppsala: The Uppsala Monitoring Centre; 2005. [Last accessed on 2011 May 6]. The use of the WHO-UMC system for standardized case causality assessment. Available from: http://whoumc.org/Graphics/24734.pdf . [Google Scholar]
- 2.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 3.Singh H, Kachhap VK, Kumar BN, Nayak K. Navirapine induced Stevens-Johnson syndrome in an HIV infected patient. Indian J Pharmacol. 2011;43:84–6. doi: 10.4103/0253-7613.75680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borras-Blasco J, Navarro-Ruiz A, Borras C, Castera E. Adverse cutaneous reactions associated with the newest antiretroviral drugs in patients with human immunodeficiency virus infection. J Antimicrob Chemother. 2008;62:879–88. doi: 10.1093/jac/dkn292. [DOI] [PubMed] [Google Scholar]
- 5.Kainer MA, Mijch A. Anaphylactoid reaction, angioedema and urticaria associated with Lamivudine. Lancet. 1996;348:1519. doi: 10.1016/s0140-6736(05)65939-9. [DOI] [PubMed] [Google Scholar]
- 6.Smith KJ, Buckley R, Skelton H. Lamivudine (3TC)-induced contact dermatitis. Cutis. 2000;65:227–9. [PubMed] [Google Scholar]
- 7.Kim SB, Seo PJ, Baik du S, Yun SY, Kim BH, Shin JE, et al. A case of severe skin eruption caused by lamivudine in a patient with chronic hepatitis B. Korean J Gastroenterol. 2006;48:281–5. [PubMed] [Google Scholar]


