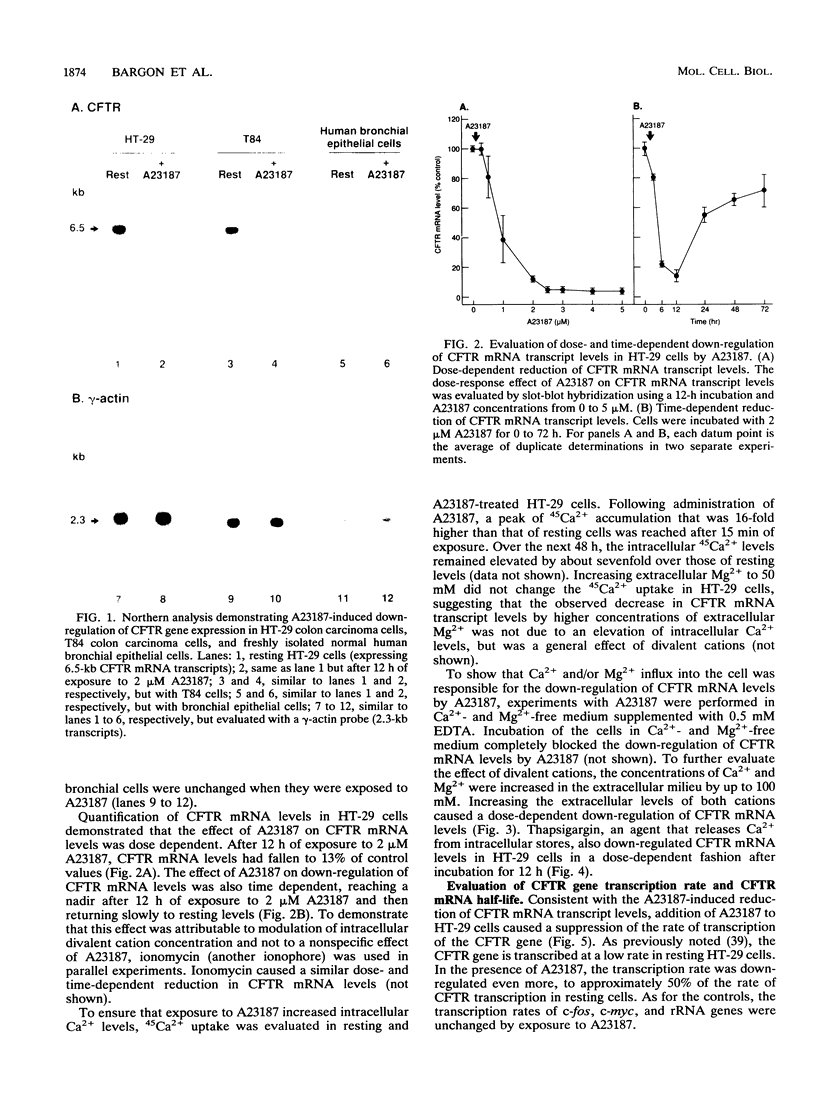
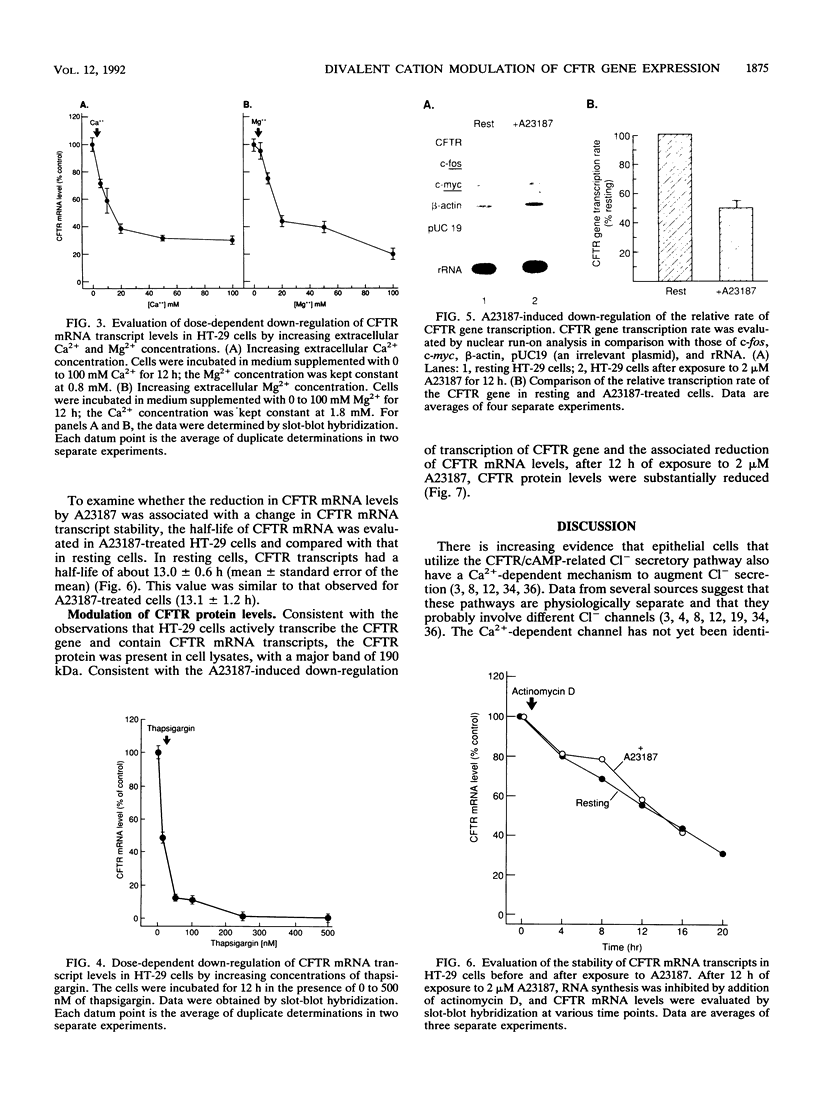
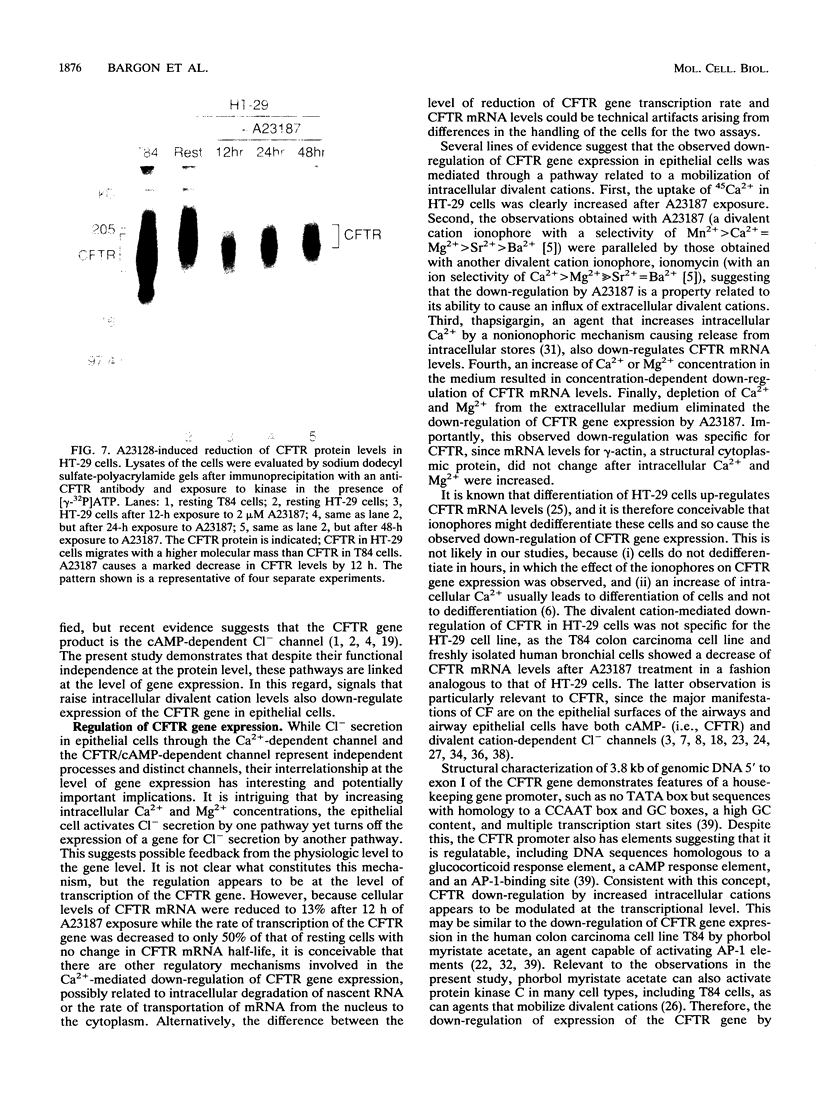
Abstract
In cystic fibrosis (CF), epithelial cells are unable to normally up-regulate apical membrane Cl- secretion in response to agents which increase cyclic AMP, but they do increase Cl- secretion in response to increases in intracellular Ca2+. Since intracellular divalent cations regulate the expression of many genes, we hypothesized that mobilization of intracellular Ca2+ and/or other divalent cations might modulate not only Ca(2+)-dependent Cl- channels but also cystic fibrosis transmembrane conductance regulator (CFTR) gene expression. To evaluate this concept, HT-29 human colon carcinoma cells were cultured under various conditions designed to manipulate intracellular divalent cation concentrations and CFTR gene expression was quantified at the levels of transcription, mRNA accumulation, mRNA half-life, and protein. Exposure to the divalent cation ionophores A23187 and ionomycin (agents which increase intracellular divalent cation concentrations) caused dose- and time-dependent reductions of CFTR mRNA levels, which could be blocked by the use of Ca(2+)- and Mg(2+)-free media. Ionophore-induced CFTR gene modulation was also observed with T84 human colon carcinoma cells and freshly isolated normal human bronchial epithelial cells. Incubation of HT-29 cells with thapsigargin, an agent that releases Ca2+ from intracellular stores, or in medium containing increased extracellular concentrations of Ca2+ or Mg2+ also caused down-regulation of CFTR mRNA levels. Transcription run-on analysis showed that, parallel with the decrease in CFTR mRNA levels, A23187 reduced the rate of transcription of the CFTR gene, while CFTR mRNA transcript half-life was unaffected. Consistent with the down-regulation of CFTR gene expression, CFTR protein levels also decreased after exposure to A23187. Thus, despite the independence of Ca(2+)-dependent Cl- channels and cyclic AMP-dependent CFTR-related Cl- channels in epithelial cells, increases in intracellular divalent cation concentrations down-regulate the expression of the CFTR gene at the transcriptional level, with consequent decreases in CFTR mRNA and protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. Generation of cAMP-activated chloride currents by expression of CFTR. Science. 1991 Feb 8;251(4994):679–682. doi: 10.1126/science.1704151. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Welsh M. J. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear C. E., Duguay F., Naismith A. L., Kartner N., Hanrahan J. W., Riordan J. R. Cl- channel activity in Xenopus oocytes expressing the cystic fibrosis gene. J Biol Chem. 1991 Oct 15;266(29):19142–19145. [PubMed] [Google Scholar]
- Black B. L., Smith J. E. Regulation of goblet cell differentiation by calcium in embryonic chick intestine. FASEB J. 1989 Dec;3(14):2653–2659. doi: 10.1096/fasebj.3.14.2512193. [DOI] [PubMed] [Google Scholar]
- Boucher R. C., Cheng E. H., Paradiso A. M., Stutts M. J., Knowles M. R., Earp H. S. Chloride secretory response of cystic fibrosis human airway epithelia. Preservation of calcium but not protein kinase C- and A-dependent mechanisms. J Clin Invest. 1989 Nov;84(5):1424–1431. doi: 10.1172/JCI114316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990 Nov 16;63(4):827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Rich D. P., Marshall J., Gregory R. J., Welsh M. J., Smith A. E. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 1991 Sep 6;66(5):1027–1036. doi: 10.1016/0092-8674(91)90446-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppens H., Marynen P., De Boeck C., De Baets F., Eggermont E., Van den Berghe H., Cassiman J. J. A child, homozygous for a stop codon in exon 11, shows milder cystic fibrosis symptoms than her heterozygous nephew. J Med Genet. 1990 Nov;27(11):717–719. doi: 10.1136/jmg.27.11.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting G. R., Kasch L. M., Rosenstein B. J., Tsui L. C., Kazazian H. H., Jr, Antonarakis S. E. Two patients with cystic fibrosis, nonsense mutations in each cystic fibrosis gene, and mild pulmonary disease. N Engl J Med. 1990 Dec 13;323(24):1685–1689. doi: 10.1056/NEJM199012133232407. [DOI] [PubMed] [Google Scholar]
- Gregory R. J., Cheng S. H., Rich D. P., Marshall J., Paul S., Hehir K., Ostedgaard L., Klinger K. W., Welsh M. J., Smith A. E. Expression and characterization of the cystic fibrosis transmembrane conductance regulator. Nature. 1990 Sep 27;347(6291):382–386. doi: 10.1038/347382a0. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamosh A., Trapnell B. C., Zeitlin P. L., Montrose-Rafizadeh C., Rosenstein B. J., Crystal R. G., Cutting G. R. Severe deficiency of cystic fibrosis transmembrane conductance regulator messenger RNA carrying nonsense mutations R553X and W1316X in respiratory epithelial cells of patients with cystic fibrosis. J Clin Invest. 1991 Dec;88(6):1880–1885. doi: 10.1172/JCI115510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T. C., Lu L., Zeitlin P. L., Gruenert D. C., Huganir R., Guggino W. B. Cl- channels in CF: lack of activation by protein kinase C and cAMP-dependent protein kinase. Science. 1989 Jun 16;244(4910):1351–1353. doi: 10.1126/science.2472005. [DOI] [PubMed] [Google Scholar]
- Kartner N., Hanrahan J. W., Jensen T. J., Naismith A. L., Sun S. Z., Ackerley C. A., Reyes E. F., Tsui L. C., Rommens J. M., Bear C. E. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell. 1991 Feb 22;64(4):681–691. doi: 10.1016/0092-8674(91)90498-n. [DOI] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Anderson M. P., Clancy J. P., Liedtke C. M., Nairn A. C., Greengard P., Welsch M. J. Regulation of chloride channels by protein kinase C in normal and cystic fibrosis airway epithelia. Science. 1989 Jun 16;244(4910):1353–1356. doi: 10.1126/science.2472006. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- Montrose-Rafizadeh C., Guggino W. B., Montrose M. H. Cellular differentiation regulates expression of Cl- transport and cystic fibrosis transmembrane conductance regulator mRNA in human intestinal cells. J Biol Chem. 1991 Mar 5;266(7):4495–4499. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Quinton P. M. Cystic fibrosis: a disease in electrolyte transport. FASEB J. 1990 Jul;4(10):2709–2717. doi: 10.1096/fasebj.4.10.2197151. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rommens J. M., Iannuzzi M. C., Kerem B., Drumm M. L., Melmer G., Dean M., Rozmahel R., Cole J. L., Kennedy D., Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989 Sep 8;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Tabcharani J. A., Chang X. B., Riordan J. R., Hanrahan J. W. Phosphorylation-regulated Cl- channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991 Aug 15;352(6336):628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Dawson A. P., Scharff O., Foder B., Cullen P. J., Drøbak B. K., Bjerrum P. J., Christensen S. B., Hanley M. R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989 Apr;27(1-2):17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- Trapnell B. C., Chu C. S., Paakko P. K., Banks T. C., Yoshimura K., Ferrans V. J., Chernick M. S., Crystal R. G. Expression of the cystic fibrosis transmembrane conductance regulator gene in the respiratory tract of normal individuals and individuals with cystic fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6565–6569. doi: 10.1073/pnas.88.15.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell B. C., Zeitlin P. L., Chu C. S., Yoshimura K., Nakamura H., Guggino W. B., Bargon J., Banks T. C., Dalemans W., Pavirani A. Down-regulation of cystic fibrosis gene mRNA transcript levels and induction of the cystic fibrosis chloride secretory phenotype in epithelial cells by phorbol ester. J Biol Chem. 1991 Jun 5;266(16):10319–10323. [PubMed] [Google Scholar]
- Wagner J. A., Cozens A. L., Schulman H., Gruenert D. C., Stryer L., Gardner P. Activation of chloride channels in normal and cystic fibrosis airway epithelial cells by multifunctional calcium/calmodulin-dependent protein kinase. Nature. 1991 Feb 28;349(6312):793–796. doi: 10.1038/349793a0. [DOI] [PubMed] [Google Scholar]
- Weber B., Horiguchi J., Luebbers R., Sherman M., Kufe D. Posttranscriptional stabilization of c-fms mRNA by a labile protein during human monocytic differentiation. Mol Cell Biol. 1989 Feb;9(2):769–775. doi: 10.1128/mcb.9.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J. Abnormal regulation of ion channels in cystic fibrosis epithelia. FASEB J. 1990 Jul;4(10):2718–2725. doi: 10.1096/fasebj.4.10.1695593. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H. Cystic fibrosis and beta-adrenergic response of airway epithelial cell cultures. Am J Physiol. 1986 Oct;251(4 Pt 2):R818–R822. doi: 10.1152/ajpregu.1986.251.4.R818. [DOI] [PubMed] [Google Scholar]
- Willumsen N. J., Boucher R. C. Activation of an apical Cl- conductance by Ca2+ ionophores in cystic fibrosis airway epithelia. Am J Physiol. 1989 Feb;256(2 Pt 1):C226–C233. doi: 10.1152/ajpcell.1989.256.2.C226. [DOI] [PubMed] [Google Scholar]
- Yoshimura K., Nakamura H., Trapnell B. C., Chu C. S., Dalemans W., Pavirani A., Lecocq J. P., Crystal R. G. Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res. 1991 Oct 11;19(19):5417–5423. doi: 10.1093/nar/19.19.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K., Nakamura H., Trapnell B. C., Dalemans W., Pavirani A., Lecocq J. P., Crystal R. G. The cystic fibrosis gene has a "housekeeping"-type promoter and is expressed at low levels in cells of epithelial origin. J Biol Chem. 1991 May 15;266(14):9140–9144. [PubMed] [Google Scholar]
- Zielenski J., Rozmahel R., Bozon D., Kerem B., Grzelczak Z., Riordan J. R., Rommens J., Tsui L. C. Genomic DNA sequence of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Genomics. 1991 May;10(1):214–228. doi: 10.1016/0888-7543(91)90503-7. [DOI] [PubMed] [Google Scholar]





