Abstract
Background and Purpose
Pulmonary arterial hypertension (PAH) is characterized by enhanced pulmonary vascular resistance, right ventricular hypertrophy and increased right ventricular systolic pressure. Here, we investigated the effects of a N-acylhydrazone derivative, 3,4-dimethoxyphenyl-N-methyl-benzoylhydrazide (LASSBio-1359), on monocrotaline (MCT)-induced pulmonary hypertension in rats.
Experimental Approach
PAH was induced in male Wistar rats by a single i.p. injection of MCT (60 mg·kg−1) and 2 weeks later, oral LASSBio-1359 (50 mg·kg−1) or vehicle was given once daily for 14 days. Echocardiography was used to measure cardiac function and pulmonary artery dimensions, with histological assay of vascular collagen. Studies of binding to human recombinant adenosine receptors (A1, A2A, A3) and of docking with A2A receptors were also performed.
Key Results
MCT administration induced changes in vascular and ventricular structure and function, characteristic of PAH. These changes were reversed by treatment with LASSBio-1359. MCT also induced endothelial dysfunction in pulmonary artery, as measured by diminished relaxation of pre-contracted arterial rings, and this dysfunction was reversed by LASSBio-1359. In pulmonary artery rings from normal Wistar rats, LASSBio-1359 induced relaxation, which was decreased by the adenosine A2A receptor antagonist, ZM 241385. In adenosine receptor binding studies, LASSBio-1359 showed most affinity for the A2A receptor and in the docking analyses, binding modes of LASSBio-1359 and the A2A receptor agonist, CGS21680, were very similar.
Conclusion and Implications
In rats with MCT-induced PAH, structural and functional changes in heart and pulmonary artery were reversed by treatment with oral LASSBio-1359, most probably through the activation of adenosine A2A receptors.
Keywords: pulmonary hypertension, monocrotaline, ventricular dysfunction, pulmonary vascular remodelling, A2A adenosine agonist
Introduction
Pulmonary arterial hypertension (PAH), a progressive vasculopathy associated with a poor prognosis, results in right ventricular dysfunction (Soon et al., 2010; Schermuly et al., 2011). PAH is related to increased vasoconstrictor tone and thrombosis which cause sustained elevation of the pulmonary arterial pressure, leading to increased pressure in the right ventricle (RV) and, consequently, ventricular hypertrophy and failure. The disease is characterized by increased vascular cell growth and inflammation, in which the recruitment and infiltration of circulating cells play important roles (Schermuly et al., 2011). Extensive research supports the involvement of inflammatory cytokines in the development of PAH. Increased circulating levels of TNF-α, IL-1 and IL-6 have been observed in the lungs of patients with PAH (Soon et al., 2010).
Adenosine is an important intermediate of purine synthesis and energy metabolism that is distributed throughout the body. Several lines of experimental evidence have suggested that adenosine plays a crucial role in controlling vascular tone and vascular remodelling by activation of adenosine A2A receptors, acting as an important regulator of inflammation and a powerful vasodilator affecting the systemic arterial pressure, with possible similar effects on the pulmonary arteries (Impellizzeri et al., 2011; Xu et al., 2011). A study using A2A receptor knockout mice revealed that the absence of this receptor led to PAH and increased pulmonary vascular remodelling (Xu et al., 2011).
Recently, new N-acylhydrazone derivatives were synthesized from safrole, a component of sassafras oil, with structures based on the molecular simplification of pyridazinone phosphodiesterase inhibitors (Barreiro, 2002; Silva et al., 2005). These novel compounds were potent vasodilators (Silva et al., 2005; Zapata-Sudo et al., 2010) and to increase their vasodilator and anti-inflammatory properties, a new N-acylhydrazone derivative, 3,4-dimethoxyphenyl-N-methyl-benzoylhydrazide (named LASSBio-1359; Figure 1), was synthesized and showed anti–TNF-α properties in vitro and in vivo (Kummerle et al., 2012). Here we have investigated the effects of oral treatment with LASSBio-1359 on the vascular reactivity and ventricular function in rats with monocrotaline (MCT)-induced PAH, and the possible mechanism(s) involved in the relaxation of pulmonary artery rings by LASSBio-1359 in vitro.
Figure 1.
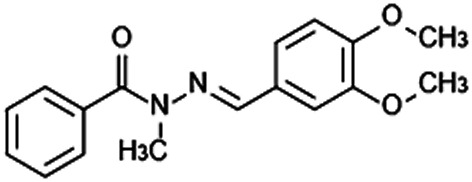
Chemical structure of 3,4-dimethoxyphenyl-N-methyl-benzoilidrazide (LASSBio-1359).
Methods
Animals and experimental design
All animal care and experimental protocols used in the present study were approved by the Animal Care and Use Committee at Universidade Federal do Rio de Janeiro. Male Wistar rats (220–300 g) were housed at 20 ± 3°C under a 12-h light/12-h dark cycle with free access to food and water. Animals received a single i.p. injection of saline or MCT (60 mg·kg−1) and were randomly divided into the following groups (n = 6 per group): (i) control (injected with saline), (ii) MCT, (iii) MCT + vehicle and (iv) MCT + LASSBio-1359 (50 mg·kg−1 p.o.). Two weeks after the MCT or saline injection, rats were dosed via oral gavage once a day for 2 weeks with vehicle (DMSO) or a solution of LASSBio-1359 in DMSO (1.6 mol L−1; approximately 100 μL per rat). Rats were weighed daily, and the dosages of LASSBio-1359 were adjusted appropriately. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 24 animals were used in the experiments described here.
Echocardiography
Male Wistar rats were anaesthetized by i.p. injection of sodium pentobarbital (40 mg·kg−1). The room temperature was maintained around 25°C to avoid hypothermia. Cardiac function was assessed by an echocardiography system equipped with a 10 MHz mechanical transducer (Esaote model, CarisPlus, Firenze, Italy). Short- and long axis B-dimensional parasternal views of both ventricles at the level of the papillary muscles were acquired to obtain the areas of the left ventricle (LV) and RV. Cardiac output and stroke volume were obtained from the B-mode long axis according to Simpson's method (Lang et al., 2006). The pulmonary artery diameter and RV wall thickness were obtained in M-mode. Doppler from the pulmonary artery was applied to obtain the pulmonary artery acceleration time. All measurements were obtained according to the American Society of Echocardiography Guidelines.
Haemodynamic measurements
Rats were anaesthetized with sodium pentobarbital (40 mg·kg−1, i.p.; Rhobifarma Indústria Farmacêutica Ltda, Hortolândia, São Paulo, Brazil). The depth of anaesthesia was evaluated by pinching the animal's paw with forceps and no supplemental analgesia was needed.
The thoracic cavity was opened, and a heparinized 19-G scalp weplast (Embramac) was inserted into the RV. The RV systolic pressure (RVSP) was measured with PowerLab (ADInstruments, Sydney, Australia) monitoring equipment. The animals were killed at the end of the experiment while still anaesthetized. Haemodynamic values were automatically calculated with the physiological data acquisition system LabChart 7.0 (ADInstruments).
RV hypertrophy measurements
At the end of the study, the rats were killed and their hearts were isolated, flushed with saline, and dissected to separate the RV from the LV + septum (S). As indices of RV hypertrophy, the ratios of the RV weight to LV + S weight [RV/LV + S] and of the RV weight to body weight [RV/BW] were determined.
Non-invasive BP measurements
Non-invasive BP measurements were performed in Wistar rats by tail-cuff plethysmography (Letica model LE 5001, Cornella, Barcelona, Spain). Animals were treated p.o. daily for 14 days with either vehicle (DMSO) or LASSBio-1359 (50 mg·kg−1). The BP of rats was measured before and at 1, 3, 5, 7, 11 and 14 days of treatment.
Morphometric analysis
Lungs were collected from rats of the experimental groups and immersed in 10% neutral buffered formalin. The left and right lung lobes were longitudinally cut and processed as described below. Tissues were dehydrated in three ethyl alcohol solutions (70, 100 and 100%) for 30 min and then were immersed in xylol solution for 30 min, twice. Tissues were then infiltrated with paraffin solution (60°C) and, after this procedure, all lungs were embedded in paraffin blocks. Sections (4 μm thick) were stained with hematoxylin and eosin and examined by light microscopy.
All histological material was randomly numbered and analysis was carried out without knowledge of the treatment groups. Afterwards, another observer collected the data and carried out the statistical analysis. For pulmonary vascular morphometry, images of the terminal arterioles were captured (magnification ×40) and the arterial area was measured with an image analysis programme (ImageJ). The diameter of the vessels analysed ranged between <50 μm and 50–150 μm. Ten vessels of comparable size per rat were measured from six rats per group. The percent wall thickness was calculated with the following formula: wall thickness (%) = [(areaext − areaint)/areaext] × 100, where areaext and areaint are the areas bounded by the external and internal elastic lamina respectively.
Determination of collagen volume fraction in lung and arterial tissue
In sections of lung, the volume fraction of collagen (%) was determined by measuring the area of stained tissue within a given field after Picrosirius Red staining, under light microscopy (magnification 40×). The area stained was calculated as a percentage of the total area within a field (Image-Pro Plus, Silver Spring, MD, USA). For each lung, 20 fields were analysed and averaged. The diameter of pulmonary arterioles, which had collagen volume fraction quantified, ranged between 50 and 150 μm.
Determination of endothelial dysfunction of the pulmonary artery
After the rats were treated with LASSBio-1359 or vehicle for 2 weeks, the first 3 mm of pulmonary artery closest to the heart of each rat was excised, connective tissue removed and rings (3 mm wide) prepared for isometric tension recording. The rings were placed in chambers filled with saline solution (120 × 10−3 mol·L−1 NaCl, 5.9 × 10−3 mol·L−1 KCl, 1.2 × 10−3 mol·L−1 MgCl2, 1.2 × 10−3 mol·L−1 NaH2PO4, 18 × 10−3 mol·L−1 NaHCO3, 1.2 × 10−3 mol·L−1 CaCl2 and 11 × 10−3 mol·L−1 glucose; pH 7.4) and oxygenated with carbogen gas at 37.0 ± 0.5°C. After an equilibrium period of 2 h at 1.5 g resting tension, the preparations were exposed to increasing concentrations of phenylephrine (10−9 mol·L−1–10−5 mol·L−1). On reaching a maximum response to phenylephrine, the pulmonary artery rings were exposed to increasing concentrations of ACh (10−9 mol·L−1–10−5 mol·L−1) to induce relaxation and hence to determine endothelial dysfunction.
Effects of LASSBio-1359 on pulmonary arterial rings
The pulmonary artery was excised from normal male Wistar rats (220–300 g), connective tissue removed and rings prepared for isometric tension recording, as described above. After an equilibrium period of 2 h at 1.5 g resting tension, the preparations were contracted with phenylephrine (10−5 mol·L−1) and exposed to ACh (10−5 mol·L−1) to test the integrity of the endothelium. Vascular endothelium was considered intact when the ACh-induced relaxation was >60% of the phenylephrine-induced contraction. In some experiments the vascular endothelium was mechanically disrupted by gently rubbing the luminal surface with plastic tubing and, in these preparations, the ACh-induced relaxation was <10%. Increasing concentrations of LASSBio-1359 (5 × 10−6 − 5 × 10−4 mol·L−1) were added at the plateau of the phenylephrine-induced contraction. ZM 241385 (10−7 mol·L−1), a selective antagonist of the adenosine A2A receptor (Gołembiowska and Dziubina, 2012; receptor nomenclature follows Alexander et al., 2011), was used to evaluate the possible mechanism mediating the effects of the derivative. Experiments with vehicle alone were performed on endothelium-intact rings to eliminate a possible interference with the contractile response. Treatment with the maximum concentration of DMSO (0.2% v/v) did not significantly alter the vascular contractility.
Binding assays
Receptor binding assays were carried out as Study no. 100003173 by Cerep (Celle l'Evescault, France). Binding of LASSBio-1359 (10−5 mol·L−1) to the human recombinant adenosine A1, A2A or A3 receptors transfected into CHO cells (A1) or HEK-293 cells (A2A and A3) was determined. [3H]CCPA (10−9 mol·L−1), [3H]CGS21680 (6 × 10−9 mol·L−1) and [125I]AB-MECA (1.5 × 10−10 mol·L−1) were used as the A1, A2A and A3 receptor agonist radioligands respectively. The data were expressed as the percent inhibition of control specific binding obtained in the presence of LASSBio-1359, by using the equation: % inhibition = 100 − [(measured specific binding/control specific binding) × 100].
Docking of LASSBio-1359 and CGS21680 in the adenosine A2A receptor
The docking experiment of LASSBio-1359 was performed with the programme GOLD 5.1 (CCDC) and its fitness function GoldscoreX. The crystal structure of the adenosine A2A receptor co-crystallized with an agonist (N-ethylcarboxamido-adenosine; NECA) was retrieved from the RCSB Protein Data Bank (PDB 2YDV). The redocking of NECA was performed in the A2A crystal to demonstrate that the programme could predict an experimental binding mode. Three consecutive dockings were made with LASSBio-1359 and CGS21680 (the agonist used in the binding assay). The set of amino acid residues selected as the binding site for docking studies was determined at a distance of 10 Å from the amino acid residue Asn253. The conformation with the highest score was analysed.
Data analysis
All data are reported as the mean ± SEM. For the comparison of multiple groups, one-way anova was used, followed by the Newman–Keuls test. Differences with P < 0.05 were considered statistically significant.
Materials
LASSBio-1359 was synthesized by Laboratório de Avaliação e Síntese de Substâncias Bioativas of the Universidade Federal do Rio de Janeiro, as described earlier (Kummerle et al., 2012). DMSO was purchased from Merck (Darmstadt, Germany). MCT, phenylephrine, ACh and ZM 241385 were purchased from Sigma-Aldrich (St. Louis, MO, USA). LASSBio-1359 was dissolved in DMSO to give a solution of 1.6 mol L−1 for each day's use and not stored. Phenylephrine, ACh and ZM 241385 were dissolved in distilled water. MCT was dissolved in 1 N HCl, neutralized with 0.5 N NaOH and diluted with PBS.
Results
Evaluation of cardiac function by echocardiography
At the end of the treatment, cardiac function in rats was assessed by echocardiography. The single injection of MCT significantly increased the pulmonary artery diameter and reduced the pulmonary artery acceleration time, compared with the control group. Treatment of MCT-injected rats with LASSBio-1359 restored the pulmonary artery acceleration time to control values (Table 1).
Table 1.
Echocardiographic data from saline-injected controls and MCT-injected rats receiving oral vehicle (DMSO) or LASSBio-1359 (50 mg·kg−1) for 14 days
| Control | MCT | MCT + vehicle | MCT + LASSBio-1359 | |
|---|---|---|---|---|
| Pulmonary artery acceleration time, ms | 44.2 ± 0.7 | 24.8 ± 1.6* | 25.5 ± 1.3* | 39.8 ± 2.0†‡ |
| LV cardiac output, mL·min−1 | 127.4 ± 1.9 | 80.0 ± 7.0* | 77.4 ± 2.1* | 126.6 ± 5.7†‡ |
| RV wall thickness, cm | 0.10 ± 0.02 | 0.16 ± 0.01* | 0.15 ± 0.09* | 0.11 ± 0.03†‡ |
| RV area, mm2 | 9.6 ± 0.4 | 21.9 ± 1.2* | 23.4 ± 1.4* | 13.8 ± 1.0*†‡ |
| LV area, mm2 | 21.3 ± 2.1 | 16.4 ± 0.9 | 20.5 ± 1.5 | 19.7 ± 1.9 |
| Pulmonary artery diameter, cm | 0.29 ± 0.01 | 0.38 ± 0.01* | 0.41 ± 0.01* | 0.31 ± 0.01†‡ |
| Heart rate, bpm | 332.0 ± 1.4 | 321. ± 0.9 | 334.2 ± 1.8 | 329.4 ± 0.9 |
| LV stroke volume, mL | 0.23 ± 0.30 | 0.11 ± 0.50* | 0.13 ± 0.80* | 0.24 ± 1.20†‡ |
All values are mean ± SEM, n = 6 per group.
LV, left ventricle; RV, right ventricle.
P < 0.05 versus control;
P < 0.05 versus MCT;
P < 0.05 versus MCT + vehicle (DMSO).
MCT also increased the RV area and wall thickness but not the LV area and heart rate, compared with saline-injected rats (Table 1). The rats injected with MCT and treated orally with vehicle for 2 weeks also had increased pulmonary artery diameter, RV area and wall thickness results compared to control rats. Stroke volume was reduced in both MCT and MCT + vehicle groups and was ameliorated in the group treated with LASSBio-1359. Cardiac output was significantly reduced in the MCT and MCT + vehicle groups, compared with controls. In MCT-injected rats treated orally with LASSBio-1359, cardiac output was increased to values not different from control, due to enhanced stroke volume but without altering heart rate. In addition, daily oral treatment of MCT-injected rats with LASSBio-1359 (50 mg·kg−1) decreased the pulmonary artery diameter and reversed the RV hypertrophy, as assessed by the RV area and wall thickness (Table 1).
LASSBio-1359 administration normalizes haemodynamic values and RV hypertrophy in MCT-induced PAH
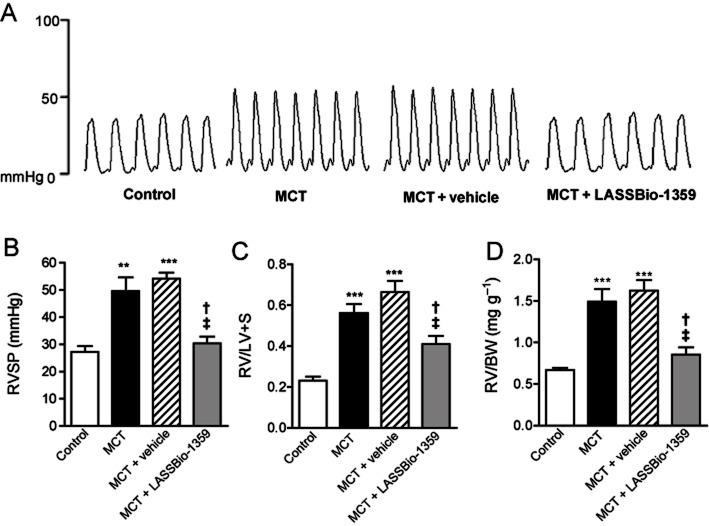
Starting at 14 days after exposure to MCT (60 mg·kg−1 i.p.) or saline, rats were treated orally with vehicle (DMSO) or LASSBio-1359 (50 mg·kg−1) for 2 weeks. After this period, the rats were subjected to haemodynamic and RV hypertrophy evaluations. Figure 2A shows representative tracings of RVSP, which increased significantly in MCT-treated rats, compared with saline-injected controls (Figure 2B). Treatment of MCT-injected rats with LASSBio-1359 restored RVSP to control levels; Figure 2B). Similarly, MCT administration increased the RV/LV + S and RV/BW ratios, compared with ratios in the control group (Figure 2C and D). Treatment with LASSBio-1359 decreased the RV/LV + S and RV/BW ratios, indicating reduction of cardiac hypertrophy. Daily clinical evaluation showed no evidence of physical or behavioural drug-related toxicity.
Figure 2.
Effects of oral treatment with LASSBio-1359 (50 mg·kg−1, p.o.) for 2 weeks on RVSP and on right ventricular (RV) hypertrophy in MCT-injected rats. (A) Representative tracings of RVSP of rats injected with saline (control), MCT, MCT + vehicle (DMSO), and MCT + LASSBio-1359, respectively. (B) MCT-injected rats developed PAH, as shown by the increased RVSP. Oral treatment with LASSBio-1359 (50 mg·kg−1·day−1) reversed the development of PAH. (C) RV weight to left ventricle + septum weight ratio [RV/LV + S]. (D) RV weight to body weight ratio [RV/BW]. Treatment with LASSBio-1359 decreased the RV hypertrophy. Each column represents the mean ± SEM (n = 6). **P < 0.01, ***P < 0.001 versus control; †P < 0.05 versus MCT; ‡P < 0.05 versus MCT + vehicle.
In a separate experiment, LASSBio-1359 was given p.o. (50 mg·kg−1) to normal Wistar rats during 14 days. During oral treatment of rats, neither vehicle nor LASSBio-1359 had a significant effect on BP (Figure 3), indicating the absence of systemic hypotension.
Figure 3.
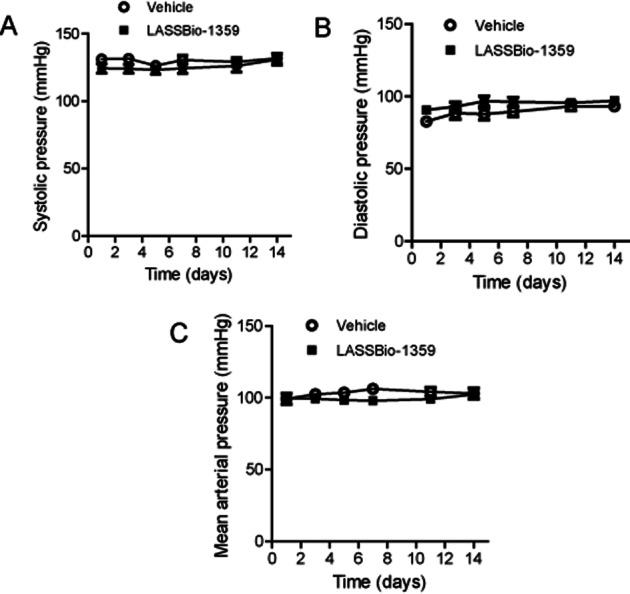
Effects of oral treatment of Wistar rats with LASSBio-1359 (50 mg·kg−1) or vehicle (DMSO) for 14 days on (A) systolic pressure, (B) diastolic pressure and (C) mean arterial pressure. Data are mean ± SEM (n = 6).
Reduction of pulmonary vascular remodelling in MCT-injected rats treated with LASSBio-1359
Representative images of the pulmonary terminal arterioles are shown in Figure 4A. The wall thickness of the pulmonary arterioles with external diameter of <50 μm was significantly increased in the MCT and MCT + vehicle groups, compared with controls. Oral treatment with LASSBio-1359 (50 mg·kg−1) reduced the wall thickness of these vessels to control values (Figure 4B). In larger blood vessels, with diameter ranging between 50 and 150 μm, the wall thickness was similarly increased by MCT treatment and normalized after LASSBio-1359 (Figure 4C).
Figure 4.
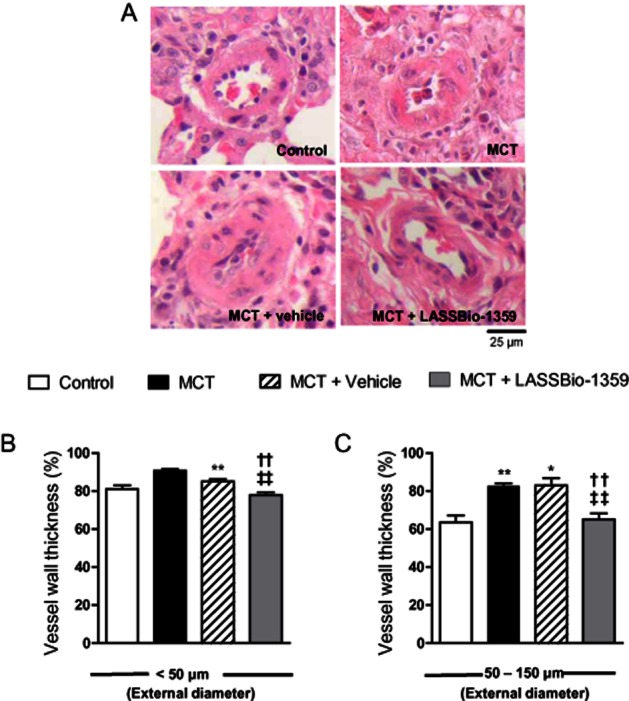
LASSBio-1359 attenuates pulmonary vascular remodelling in MCT-induced PAH. (A) Representative images of H&E-stained lung sections of rats exposed to saline (control), MCT, MCT + vehicle (DMSO), or MCT + LASSBio-1359 (50 mg·kg−1, p.o.). Images show vessels at 40 × magnification. (B) Wall thickness expressed as a percentage of the total area of the vessel (<50 μm). (C) Wall thickness of vessels ranging between 50 and 150 μm in external diameter. Each column represents the mean ± SEM (n = 6). *P < 0.05, **P < 0.01 versus. control; ††P < 0.01 versus MCT; ‡‡P < 0.01 versus MCT + vehicle.
After oral treatment with LASSBio-1359 or vehicle, collagen deposition was determined in the left lung of control and MCT-injected rats (Figure 5A). Deposition was determined as the collagen volume fraction (%) in relation to the tissue area and this fraction was significantly increased in MCT and MCT + vehicle groups, compared with controls, and was reduced in MCT-injected rats treated with LASSBio-1359 (Figure 5B).
Figure 5.
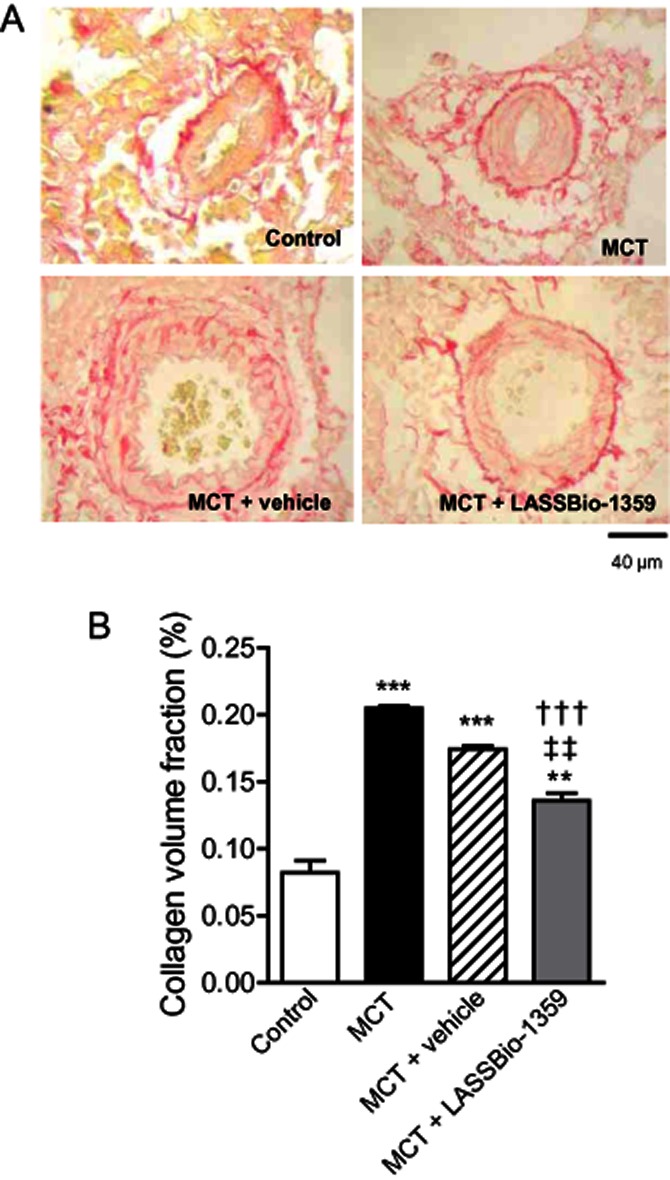
Collagen volume analysis of the pulmonary terminal arterioles from MCT-injected rats treated orally with vehicle or LASSBio-1359 (50 mg·kg−1) for 14 days. (A) Picrosirius Red staining under light microscopy (magnification 40×), showing collagen fibers in red. (B) Collagen volume fraction of pulmonary arterioles in relation to the tissue area. Data are mean ± SEM (n = 6). **P < 0.01, ***P < 0.001 versus control; †††P < 0.001 versus MCT; ‡‡P < 0.01 versus MCT + vehicle.
Oral treatment with LASSBio-1359 reverses the endothelial dysfunction of pulmonary artery rings in MCT-induced PAH
At the end of the treatment with LASSBio-1359, the pulmonary artery was removed from the rats and rings prepared for isometric tension recording, to evaluate the endothelial dysfunction. The relaxation of pulmonary artery rings, pre-contracted with phenylephrine, to increasing concentrations of ACh was reduced in MCT rats and MCT + vehicle rats, compared with control values (Figure 6A), indicating that MCT treatment induced endothelial dysfunction of the pulmonary artery rings. Oral treatment of MCT-injected rats with LASSBio-1359 (50 mg·kg−1) restored the maximal relaxation induced by ACh to control values, showing beneficial effects of LASSBio-1359 in reducing the endothelial dysfunction of the pulmonary artery rings of PAH rats. In addition, the ACh-induced relaxation of pulmonary artery rings prepared from normal rats was also increased after oral treatment with LASSBio-1359 (Table 2).
Figure 6.
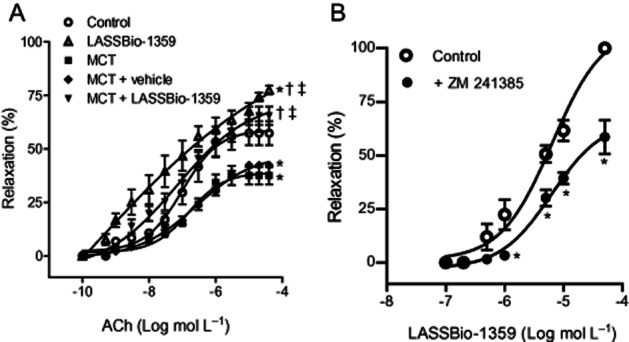
(A) ACh-induced relaxation of pulmonary artery rings from control, MCT, MCT + vehicle (DMSO), and MCT + LASSBio-1359 (50 mg·kg−1, p.o.). (B) Concentration-response curves for LASSBio-1359 in pulmonary artery rings from normal Wistar rats, contracted with phenylephrine (10−5 mol·L−1), in the presence or absence of ZM 241385 (10−7 mol·L−1). Data are mean ± SEM (n = 6–7). *P < 0.05 versus control; †P < 0.05 versus MCT; ‡P < 0.05 versus MCT + vehicle (DMSO).
Table 2.
ACh-induced relaxation of pulmonary artery rings of control rats and MCT-injected rats receiving oral vehicle (DMSO) or LASSBio-1359 (50 mg·kg−1) for 14 days
| IC50 (10−6 mol·L−1) | Relaxation to ACh 10−5 mol·L−1 (%) | |
|---|---|---|
| Control | 0.80 ± 0.18 | 57.3 ± 5.5 |
| LASSBio-1359 | 0.66 ± 0.54 | 77.5 ± 2.1*†‡ |
| MCT | ND | 37.5 ± 4.0* |
| MCT + vehicle | ND | 43.6 ± 1.3* |
| MCT + LASSBio-1359 | 0.72 ± 1.05 | 61.4 ± 8.4†‡ |
All values are mean ± SEM, n = 6 per group.
IC50, concentration of ACh for 50% of inhibition.
P < 0.05 versus control;
P < 0.05 versus MCT;
P < 0.05 versus MCT + vehicle (DMSO).
LASSBio-1359–induced relaxation of the rat pulmonary artery is mediated by activation of the adenosine A2A receptor
The vasorelaxant activity of LASSBio-1359 was investigated in vitro using pulmonary artery rings from normal Wistar rats. Added to phenylephrine-contracted rings, LASSBio-1359 induced relaxation in a concentration-dependent manner (Figure 6B), yielding a IC50 value (as the concentration reducing the phenylephrine-induced contraction to 50%) of 6.6 ± 1.7 × 10−6 mol·L−1. This relaxant effect of LASSBio-1359 was then measured in the presence of ZM 241385 (10−7 mol·L−1), a selective antagonist of the adenosine A2A receptor. Pretreatment of pulmonary arteries with ZM 241385 induced a rightward shift of the concentration-response curve to LASSBio-1359 and reduced the maximal relaxation (to 10−4 mol·L−1) by about 50% (Figure 6B).
Binding assays
The ability of LASSBio-1359 to inhibit the binding of agonists to the adenosine A1, A2A and A3 receptors was tested. LASSBio-1359 (10−5 mol·L−1) inhibited binding of [3H]CCPA (10−9 mol·L−1) to the A1 receptor (n = 2) by an average of 4.9% (range: 4.7–5.0%), inhibited binding of [3H]CGS21680 (6 × 10−9 mol·L−1) to the A2A receptor (n = 2) by an average of 78.6% (range: 75.2–81.9%) and inhibited binding of [125I]AB-MECA (1.5 × 10−10 mol·L−1) to the A3 receptor (n = 2) by an average of 20.6% (range: 17.5–23.7%).
Proposed binding mode of LASSBio-1359 in the adenosine A2A receptor
Before the predicted binding mode of LASSBio-1359 and CSG21680 in the adenosine A2A receptor was analysed, the ability of GOLD 5.1 (CCDC) to predict an experimental binding mode was evaluated. The conformations of redocked NECA and of NECA co-crystallized with the A2A receptor were very close in the active site, with a root mean square deviation of 0.6 Å for all atoms (Figure 7A). The highest scoring conformation of LASSBio-1359 and CSG21680 in the A2A receptor was analysed. LASSBio-1359 seemed to interact with the amino acid residue Asn253 by hydrogen bonds and other amino acid residues by hydrophobic interactions, for example Phe168 (Figure 7B). A comparison of the binding modes of both agonists, CGS21680 and LASSBio-1359, suggested common interactions with the amino acid residues Asn253and Phe168and interactions with different amino acid residues, which might explain the binding results obtained (Figure 7C).
Figure 7.
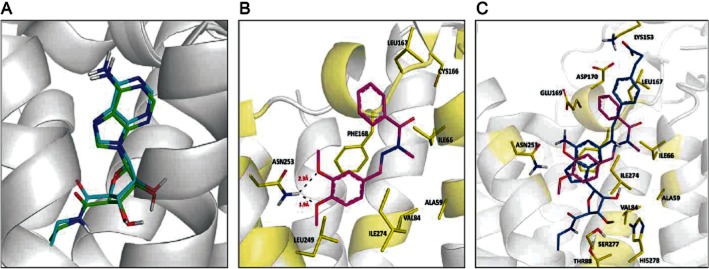
(A) Superposition of the NECA conformation in the crystal structure of the A2A receptor (green) and that obtained after redocking (blue) using the program GOLD. RMSD = 0.60 Å. (B) Predicted binding mode of the compound LASSBio-1359 (magenta) in the adenosine A2A receptor (PDB ID: 2YDV). (C) Superimposition of the adenosine A2A receptor agonist CSG21680 (blue) with the agonist LASSBio-1359 (magenta).
Discussion and conclusions
The present study demonstrated that oral treatment with LASSBio-1359 reversed the development of PAH in rats. A single injection of MCT led to fibromuscular hypertrophy and hyperplasia in the walls of pulmonary arterioles from PAH rats. This condition increased RVSP and led to RV hypertrophy, as indicated by the RV/LV + S and RV/BW ratios, RV area and wall thickness. Pulmonary arteries from MCT-treated rats showed endothelial dysfunction due to reduced ACh-induced relaxation. Daily oral treatment with LASSBio-1359 for 14 days after disease establishment abolished the increase in RVSP and reduced RV hypertrophy. These beneficial effects were probably caused by the decreased resistance and pulmonary vascular remodelling, and consequent reduction in RV systolic work.
Echocardiography is widely used in the evaluation of PAH in the rat MCT model (Koskenvuo et al., 2010). In the present study, echocardiographic analysis of normal and MCT-treated rats revealed an increased diameter of the pulmonary artery and reduction of the pulmonary artery acceleration time, due to the hypertrophy and stiffness of the pulmonary artery. Treatment with LASSBio-1359 reduced these changes in the pulmonary artery and normalized the pulmonary artery diameter and the pulmonary artery flow. Additionally, LASSBio-1359 ameliorated the LV stroke volume and cardiac output, due to the reduction of cardiac dysfunction and hypertrophy.
The structural changes observed in MCT-induced PAH resemble characteristics of human pulmonary hypertension, in terms of the marked medial wall thickening of the terminal pulmonary arterioles that subsequently increased the pulmonary arterial resistance (Jeffery and Wanstall, 2001). The current study corroborates previous reports showing increased medial thickening and neomuscularization of pulmonary arterioles of MCT-treated rats (Schermuly et al., 2005). MCT induced endothelial damage and dysfunction, resulting in the decreased production of NO by the vascular endothelium of pulmonary arterioles (Zhang et al., 2005). The decreased bioavailability of NO reduces the antiproliferative effects of NO and thus contributes to the increased pulmonary vascular resistance. These events lead to increased pulmonary arterial pressure and pulmonary vascular remodelling.
Treatment with LASSBio-1359 reduced the proliferative changes in the pulmonary arterioles and the pulmonary vascular remodelling and corrected the endothelial dysfunction of pulmonary artery rings, as assessed by the normalized ACh-induced relaxation. This result could be due to activation of adenosine A2A receptors because they represent an important regulatory mechanism to control the development of PAH and pulmonary vascular remodelling (Xu et al., 2011). Pretreatment of the pulmonary artery rings with the the A2A receptor antagonist ZM 241385 significantly decreased the vasorelaxant effect of LASSBio-1359, suggesting the involvement of A2A receptors. The binding assay results showed that LASSBio-1359 exhibited adenosine agonist activity, most potently at the A2A receptor subtype, because an inhibition (or stimulation, for assays run in basal conditions) of >50% is considered significant.
The docking study in the A2A receptor crystal structure showed how LASSBio-1359 might interact with the amino acid residues of the A2A receptor binding site, compared with the agonist CGS21680 used in the binding assay, supporting the A2A agonist action proposed in this work. The adenosine A2A receptor is located in the vascular endothelium and is coupled with a stimulatory G-protein, which induces NO release by activating the adenylate cyclase-PKA pathway and promotes vasodilatation. Adenosine is a potent pulmonary vasodilator that has been used to treat clinical and experimental pulmonary hypertension (McLaughlin and McGoon, 2006). Adenosine also produces endothelium-independent relaxation, which is partly induced by the activation of smooth muscle adenosine A2A receptors, which regulate the opening of a mixed population of potassium channels (Miroslav et al., 2005). LASSBio-1359 may thus activate A2A receptors in the pulmonary arteries, leading to normal levels of endothelial NO, improvement of endothelial dysfunction and reduction of pulmonary vascular resistance.
Genetic deletion of the A2A receptor in mice induced pulmonary vascular modifications that resembled idiopathic PAH, indicating an important role of this receptor in PAH development (Xu et al., 2011). Agonists of the adenosine A2A receptor may emerge as new substances to treat PAH. Furthermore, A2A receptor agonists may be important regulators of inflammation (Impellizzeri et al., 2011). Thus, the activation of the adenosine A2A receptor by LASSBio-1359 may be an important therapeutic strategy for the treatment of PAH.
In summary, oral treatment with LASSBio-1359 reversed the development of PAH in a rat MCT model. Activation of the adenosine A2A receptor may contribute to the beneficial effects of LASSBio-1359. This compound reversed endothelial dysfunction of the pulmonary artery and pulmonary vascular remodelling, which in turn reduced RV hypertrophy. Further studies are required to determine whether LASSBio-1359 reduces the oxidative stress and apoptosis induced by PAH and whether these findings could be applied to a clinical setting.
Acknowledgments
This work was supported by grants of Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Universitária Jose Bonifácio (FUJB), Programa de Apoio a Núcleos de Experiência (PRONEX), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Instituto Nacional de Ciência e Tecnologia (INCT).
Glossary
- LV
left ventricle
- NECA
N-ethylcarboxamido adenosine
- PAH
pulmonary arterial hypertension
- RV
right ventricle
- RVSP
right ventricular systolic pressure
- S
septum
Conflict of interest
None.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro EJ. Estratégia de simplificação molecular no planejamento racional de fármacos: a descoberta de novo agente cardioativo. Quim Nova. 2002;25:1172–1180. [Google Scholar]
- Gołembiowska K, Dziubina A. The effect of adenosine A2A receptor antagonists on hydroxyl radical, dopamine, and glutamate in the striatum of rats with altered function of VMAT2. Neurotox Res. 2012;2:150–157. doi: 10.1007/s12640-012-9316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impellizzeri D, Di Paola R, Esposito E, Mazzon E, Paterniti I, Melani A, et al. CGS 21680, an agonist of the adenosine (A2A) receptor, decreases acute lung inflammation. Eur J Pharmacol. 2011;668:305–316. doi: 10.1016/j.ejphar.2011.06.049. [DOI] [PubMed] [Google Scholar]
- Jeffery TK, Wanstall JC. Pulmonary vascular remodeling: a target for therapeutic intervention in pulmonary hypertension. Pharmacol Ther. 2001;92:1–20. doi: 10.1016/s0163-7258(01)00157-7. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskenvuo JW, Mirsky R, Zhang Y, Angeli FS, Jahn S, Alastalo TP, et al. A comparison of echocardiography to invasive measurement in the evaluation of pulmonary arterial hypertension in a rat model. Int J Cardiovasc Imaging. 2010;26:509–518. doi: 10.1007/s10554-010-9596-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummerle AE, Schmitt M, Cardoso SVS, Lugnier C, Villa P, Lopes AB, et al. Design, synthesis, and pharmacological evaluation of N-Acylhydrazones and novel conformationally constrained compounds as selective and potent orally active phosphodiesterase-4 inhibitors. J Med Chem. 2012;55:7525–7545. doi: 10.1021/jm300514y. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- Miroslav R, Leposava G, Srðan P, Dragica S. Isolated rat inferior mesenteric artery response to adenosine: possible participation of Na+/K+-ATPase and potassium channels. Pharmacol Rep. 2005;57:824–832. [PubMed] [Google Scholar]
- Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermuly RT, Ghofrani MRW, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8:443–455. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AG, Zapata-Sudo G, Kummerle AE, Fraga CAM, Barreiro EJ, Sudo RT. Synthesis and vasodilatory activity of new N-acylhydrazone derivatives, designed as LASSBio-294 analogues. Bioorg Med Chem. 2005;13:3431–3437. doi: 10.1016/j.bmc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–927. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- Xu MH, Gong YS, Su MS, Dai ZY, Dai SS, Bao SZ, et al. Absence of the adenosine A2A receptor confers pulmonary arterial hypertension and increased pulmonary vascular remodeling in mice. J Vasc Res. 2011;48:171–183. doi: 10.1159/000316935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata-Sudo G, Pereira SL, Beiral HJ, Kummerle AE, Raimundo JM, Antunes F, et al. Pharmacological characterization of (3-thienylidene)-3,4-methylenedioxybenzoylhydrazide: a novel muscarinic agonist with antihypertensive profile. Am J Hypertens. 2010;23:135–141. doi: 10.1038/ajh.2009.238. [DOI] [PubMed] [Google Scholar]
- Zhang T, Cui B, Dai D, Su W. CPU 86017, p-chlorobenzyltetrahydroberberine chloride, attenuates monocrotaline-induced pulmonary hypertension by suppressing endothelin pathway. Acta Pharmacol Sin. 2005;26:1309–1316. doi: 10.1111/j.1745-7254.2005.00214.x. [DOI] [PubMed] [Google Scholar]