Abstract
OBJECTIVES
To determine whether increasing kyphosis angle was independently associated with poorer mobility as measured according to the Timed Up and Go Test (TUG), after controlling for other established risk factors.
DESIGN
Prospective cohort study.
SETTING
Eleven clinical centers in the United States.
PARTICIPANTS
Two thousand seven hundred seventy-seven women aged 55 to 80 randomized to the placebo arms of the Fracture Intervention Trial, a randomized controlled trial of the effect of alendronate on risk for osteoporotic fractures.
MEASUREMENTS
The primary predictor was change in kyphosis angle, measured using the Debrunner Kyphometer; the outcome was change in mobility, measured as performance time on the TUG. Covariates were baseline age, kyphosis angle, body mass index (BMI), self-reported health status, grip strength, change in total hip bond mineral density (BMD), and number of vertebral fractures over a mean of 4.4 years.
RESULTS
Greater kyphosis angle predicted longer mobility performance times (P<.001), independent of other significant predictors of worsening mobility including age, baseline kyphosis, health status, grip strength, BMI, change in hip BMD, and new vertebral fractures. TUG performance times increased by 0.02 seconds (95% confidence interval (CI) =0.01–0.03) for every 5° increase in kyphosis angle, more than the increase in mobility time of 0.01 seconds (95% CI =0.005–0.03) over 1 year observed in this cohort.
CONCLUSION
Increasing kyphosis angle is independently associated with worsening mobility. Interventions are needed to prevent or reduce increasing kyphosis and mobility decline.
Keywords: hyperkyphosis, kyphosis, Timed Up and Go, mobility, aging
Spinal deformities, particularly those of the sagittal plane, have an important and measurable effect on physical mobility. Excessive kyphosis, an exaggerated curvature in the thoracic spine, is the leading cause of sagittal plane deformity in older adults, and affected individuals may suffer from impaired mobility, including slower gait speed, greater difficulty climbing stairs, poorer ability to turn 360°, and poorer balance,1–5 which places them at risk of falls and fractures.1,6 A small amount of anterior thoracic curvature is normal because of the shape of the underlying vertebrae and discs, and the level of kyphosis remains relatively stable during early adulthood, but around the fifth decade of life, kyphosis tends to increase with age according to cross-sectional data,7,8 from a mean of 43° in women aged 55 to 60 to a mean of 51.8° in women aged 76 to 80.8 Longitudinal studies that have followed kyphosis progression in the same individuals are scant but indicate changes that range from 2° per decade to 5.6° over 3 years.9,10 Although an increasing number of studies suggest that kyphosis itself is a potential cause of worsening physical function, it can be debated that the underlying causes of worsening kyphosis are the reason for worse mobility. Putative causes of excessive kyphosis include vertebral fractures,8 degenerative disc disease,11 decreased height since the age of 25,8 and spinal extensor muscle weakness.12 To the knowledge of the authors of the current study, no one has investigated the association between concurrent kyphosis change and declines in physical function.
Furthermore, although it is important to target treatment of vertebral fractures, for example, preventing age-related progression of kyphosis may be prove to be just as important. Even though the scope and scale of intervention studies have remained small, data suggest that the level of kyphosis can be reduced in persons with excessive kyphosis through exercise13–16 and bracing,17 and increasing kyphosis may be attenuated using targeted spinal extensor strengthening exercises.7
It was previously shown that women with greater kyphosis had slower Timed Up and Go Test (TUG) performance time,18 a test of functional mobility that has been used clinically to predict falls. Taking more than 12 seconds to perform this mobility test is regarded as impaired mobility in women aged 65 to 85 and discriminates between those who are community dwelling and those who are institutionalized.19 However, these study results, and many others are cross-sectional in design, and it was hypothesized that increasing kyphosis is coincident with an active geriatric syndrome of decline in physical status that negatively affects physical mobility. Therefore, longitudinal data from the Fracture Intervention Trial (FIT) were used to determine the relationship between increasing kyphosis angle and worsening mobility, as measured according to the TUG, after controlling for other established risk factors. The effect of baseline kyphosis on worsening mobility over time was also investigated.
METHODS
The FIT was a randomized, controlled trial in 6,439 women with osteopenia or osteoporosis recruited from 11 clinical centers in the United States to test the efficacy of alendronate for prevention of osteoporotic fractures.20 Women in FIT were aged 55 to 80, were postmenopausal for at least 2 years, lived independently in the community, and had a bone mineral density (BMD) of the femoral neck 1.6 or more standard deviations (SDs) below peak premenopausal femoral neck BMD (<0.68 g/cm2).20 Women were randomized into two study arms, those with and without prevalent vertebral fractures; those in both placebo arms of FIT (n =3,223) were eligible for inclusion in the analyses. Women in the intervention arm were excluded to prevent potential confounding effects of alendronate on progression of kyphosis. One-third of the women randomized to the placebo arms of the study had prevalent fractures at baseline.
Measurements
Kyphosis angle was measured using a Debrunner Kyphometer (Proteck AG, Bern, Switzerland), a protractor-like instrument that measures Cobb’s angle of kyphosis (Figure 1). The arms of the device are placed over the spinous process of C7 superiorly and T12 inferiorly after identifying the bony landmarks by palpation of the vertebrae and lower ribs.8 Recent studies have demonstrated that interrater reliability of the kyphometer measurement of kyphosis angle using the Debrunner Kyphometer, assessed using intraclass correlation, ranged from 0.89 to 0.96.13
Figure 1.
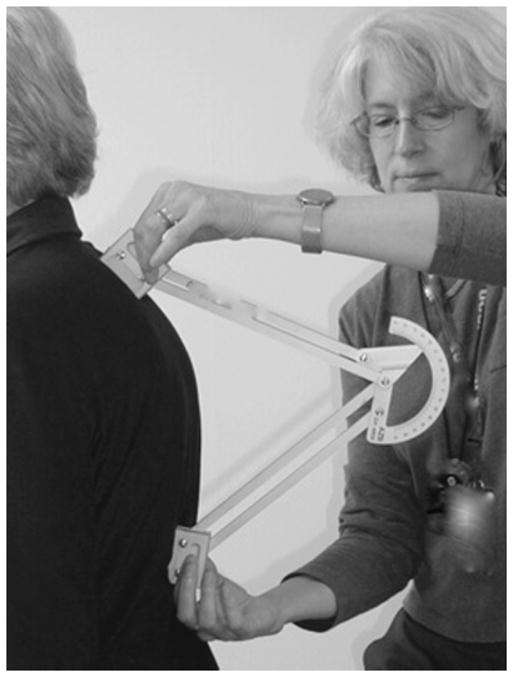
Kyphometer measurement of Cobb’s angle of kyphosis.
The TUG is a widely used clinical tool that detects mobility impairments in older adults. This test measures the time to rise from a 48-cm-high armchair, walk 3 m, turn, and return to a fully seated position in the chair.21 This test has excellent reliability (intraclass correlation coefficient 0.91–0.96)19 and is a sensitive (87%) and specific (87%) measure for identifying elderly individuals prone to falls.22
Grip strength was measured using a handheld dynamometer according to standardized protocol. Body mass index (BMI) was calculated from height and weight measurements using a standard formula (weight (kg)/height (m)2). BMD was measured using the QDR 2000 (Hologic, Inc., Waltham, MA). Quality control measures have been detailed elsewhere.20 Vertebral fractures were assessed using a standardized digitization and semiquantitative classification method from lateral radiographs of the thoracic and lumbar spine that has been described previously.23 A prevalent vertebral fracture was defined as any deformity in vertebral height ratio more than 3 SDs below the mean of normal.23 Incident vertebral fractures were defined as a 20% or greater decrease in any of three heights of a vertebral body (anterior, middle, or posterior height) between baseline and follow-up radiograph at Year 4.23
All participants had measurements of kyphosis angle, mobility on the TUG, grip strength, height, weight, BMD of the hip, and vertebral fractures at baseline visits in 1993, and all measurements except grip strength were repeated a mean of 4.4 years later. Other baseline characteristics included age and self-reported health status.
Statistical Analyses
To assess the effects of increases in kyphosis on change in mobility, analysis of covariance (ANCOVA) models were used for the change in TUG from baseline to Year 4 as the outcome, adjusting for the baseline values of the kyphosis angle and TUG. The primary predictor in this model was change in kyphosis from baseline to Year 4. Potential confounders of the association between change in kyphosis and change in TUG were considered in separate analyses. Factors that had strong biological relevance or were significantly related to change in kyphosis and change in TUG (P<.10) were included in the multivariate analysis. Age only was first adjusted for and then age and self-reported health status, grip strength, baseline BMI, change in total hip BMD, and number of new vertebral fractures. For some covariates, change rather than baseline values were adjusted for if the changes were more-powerful predictors of change in TUG. Two thousand seven hundred seventy-seven women with complete data at baseline and Year 4 were included. On the basis of visual inspection of scatter plots of the data, 30 (1.1 %) observations with change in kyphosis angle of more than 3 SDs greater than the mean change in kyphosis angle were removed, as well as 14 (0.4%) with change in TUG of more than 3 SDs greater than the mean change in TUG; these were considered measurement or gross data errors. Of the 2,777 women with complete data, 2,735 were included in the analysis after trimming. Change in kyphosis was normally distributed after trimming, whereas the changes in TUG were slightly right skewed; because this was a large sample, the skewing had essentially no effect on inferences, as shown by a sensitivity analysis using the bootstrap to obtain standard errors. The magnitude of change in kyphosis and TUG was calculated using a distribution-based method of quantifying the difference between the means on a unitless standard scale with effect size δ =(μ1 − μ2)σ1, where μ1 and μ2 are the means at baseline and Year 4 follow-up respectively, and σ1 is the SD at baseline.24 All analyses were performed using STATA software, version 11 (Statacorp LP, College Station, TX).
RESULTS
The mean change in kyphosis angle was 3.9 ± 8.6° and the mean change in TUG performance time was 0.70 ± 2.0 seconds, corresponding to small magnitude effects (0.34 and 0.24, respectively). Average kyphosis at baseline was 47.5 ± 11.7°. There was a declining trend in baseline kyphosis with increasing change in kyphosis (P<.001) (Table 1). Of the 2,735 participants, 65% (n =1,816) rated their health as very good or excellent. Those rating their health as very good or excellent had lower levels of baseline kyphosis (46.5 ± 12.0° vs 49.2 ± 11.6°) and faster TUG times (9.9 ± 2.7 seconds) than those rating their health fair to poor (11.1 ± 3.3 seconds). Average BMI was 25.0 kg/m2; 2% had BMI less than 18.5 kg/m2, and 12% had BMI greater than 30.0 kg/m2.
Table 1.
Baseline Characteristics of 2,735 Subjects in the Placebo Arm of the Fracture Intervention Trial According to Quartile of Change in Kyphosis
| Characteristic | Q1–Q4 | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|---|
| Change in kyphosis, °, mean (range) | 3.9 (− 24–31.5) | − 6.2 (− 24 to − 1.5) | 1.1 (− 1.0–3.0) | 6.0 (3.5–9.0) | 15.5 (9.5–31.5) |
| Age, mean ± SD | 68.0 ± 6.0 | 68.2 ± 5.9 | 67.8 ± 6.1 | 67.9 ± 6.0 | 68.2 ± 6.1 |
| Kyphosis, °, mean ± SD* | 47.5 ± 11.7 | 53.4 ± 10.5 | 48.3 ± 11.5 | 46.2 ± 11.3 | 41.7 ± 10.4 |
| Timed Up and Go, seconds, mean ± SD | 9.6 ± 2.0 | 9.5 ± 2.1 | 9.5 ± 1.8 | 9.6 ± 1.9 | 9.6 ± 2.0 |
| Self-reported health very good to excellent, % | 65 | 65 | 67 | 63 | 68 |
| Grip strength, kg, mean ± SD | 23.8 ± 5.1 | 23.7 ± 4.9 | 23.8 ± 5.3 | 23.8 ± 5.2 | 23.8 ± 4.9 |
| Body mass index, kg/m2, mean ± SD | 25.2 ± 4.0 | 25.1 ± 3.9 | 25.0 ± 4.0 | 25.1 ± 3.9 | 25.5 ± 4.3 |
| Total hip bone mineral density, g/cm2, mean ± SD | 0.69 ± 0.09 | 0.69 ± 0.08 | 0.69 ± 0.09 | 0.69 ± 0.09 | 0.69 ± 0.09 |
| Number of vertebral fracture at baseline, % | |||||
| 0 | 70 | 69 | 72 | 70 | 68 |
| 1 | 21 | 21 | 20 | 21 | 21 |
| ≥2 | 9 | 10 | 8 | 9 | 11 |
There was a significant (P<.001) declining trend in baseline kyphosis with higher quartiles of change in kyphosis.
SD = standard deviation.
In the unadjusted ANCOVA model, within-person increases in kyphosis angle independently predicted increases in TUG times (Table 2). These effects were attenuated but remained significant in the age-adjusted model and the fully adjusted model (P<.001). Baseline kyphosis was a significant predictor of increase in TUG in the age-adjusted (P<.001) and fully adjusted (P =.04) models. Other independent predictors of longer TUG time were older age, poorer self-reported health status, weaker grip strength, greater BMI, lower hip BMD, and new vertebral fractures during the trial (P<.001).
Table 2.
Association Between Increasing Kyphosis Angle and Timed Up and Go (TUG) Performance Time
| Kyphosis Angle | Increase in TUG, Seconds (95% Confidence Interval) P-Value
|
||
|---|---|---|---|
| Unadjusted | Age Adjusted | Fully Adjusted* | |
| Baseline (per 5°) | 0.02 (0.01–0.02) < .001 | 0.01 (0.005–0.02) <.001 | 0.007 (0.002–0.01) .04 |
| Change (per 5°) | 0.025 (0.02–0.03) < .001 | 0.02 (0.01–0.03) <.001 | 0.02 (0.01–0.03) <.001 |
Adjusted for age, poor-self reported health, baseline body mass index, grip strength, change in bone mineral density, and new vertebral fractures.
DISCUSSION
This study found that longitudinal increases in kyphosis angle were independently associated with increases in TUG times. To the knowledge of the authors, this is the first study to suggest the presence of an independent association between kyphosis and mobility in longitudinal analysis. It showed that changes in kyphosis are correlated with concurrent changes in mobility, strengthening the evidence for a causal link. Accounting for the increase in mobility in the fully adjusted model, TUG performance times were 0.02 seconds (95% confidence interval (CI) =0.01–0.03 seconds) longer for every 5° increase in kyphosis angle, more than the increase in mobility time of 0.01 seconds (95% CI =0.005–0.03) over 1 year observed in this cohort. These effects were small in magnitude but clearly independent of other known correlates of impaired mobility, including age, self-reported poor health, grip strength, BMD, and history of vertebral fractures. Furthermore, these effects would likely accrue given more time or after an intervention.
Although baseline kyphosis and change in kyphosis were significantly associated with worsening mobility in age-adjusted models, the association with change remained significant and was twice as strong in the fully adjusted model. Thus, worsening kyphosis may be a more-sensitive marker of increasing impairment than any single kyphosis measurement. Kyphosis increased by 3.9 ± 8.6° in this cohort over 4 years, more than the 1.7° increase over 10 years observed in men and women aged 50 years old at baseline without vertebral fractures10 but less than the 5.6 ± 0.7° increase over 3 years reported in postmenopausal women with a recent vertebral fracture.9 The current cohort had TUG performance times of 9.6 ± 2.7 seconds at baseline, comparable with those of other age-matched cohorts,25 and the 0.70 ± 2.0-second increase in TUG observed over 4 years in the current study was comparable with the increase in TUG reported by able-bodied adults who transitioned to disabled over 2 years.26 Furthermore, for every new vertebral fracture over the 4.4 years of this study, TUG performance increased 0.40 seconds (95% CI =0.24–0.55), more than the increase in performance time from baseline or increasing kyphosis. Thus, the increasing kyphosis and worsening TUG observed might indicate an underlying decline in physical status that could be clinically important.
A recent consensus report aimed at preventing or delaying functional decline and disability in frail older people recommended targeting treatments at all of the impairments that increase risk of frailty.27 The current data suggest that increasing kyphosis predicts longer TUG, thus increasing the risk for falls, functional decline, disability, and frailty. Although age, health status, grip strength, BMI, change in hip BMD, and new vertebral fractures are associated with longer mobility time, increasing kyphosis is a significant factor that should also be considered when developing interventions aimed at preventing functional decline. Increasing kyphosis may be part of an aging syndrome associated with bone loss, fractures, and muscle weakness leading to decline in mobility, but it is important to recognize that greater kyphosis can be easily clinically identified and that effective treatments that reduce kyphosis are available.13,15,17,28 Further work is needed to identify the specific mechanisms involved in increasing kyphosis and to determine whether attenuating the expected age-related rise in level of kyphosis can slow mobility decline.
LIMITATIONS
This study has several limitations. The population was highly selected on several factors, including BMD, and 65% reported very good to excellent health. Thus, results from this study may not be generalizable to community-dwelling women overall. Five percent of the women in the FIT placebo group had incomplete data at baseline, and 13% were lost to follow-up or lacked complete data at closeout, although adjustment for covariates associated with impaired mobility and dropout increases the plausibility of the assumption that the omitted observations were missing at random. Measurement error cannot be excluded as an explanation for change in kyphosis, although the large sample size provides confidence that the effects are due to real change in kyphosis. Although the study was longitudinal, observational data cannot unequivocally demonstrate a causal association or show that interventions to reduce kyphosis would meaningfully improve mobility.
CONCLUSIONS
The current study indicates that increasing kyphosis angle was independently associated with poorer mobility. The results provide evidence that increasing kyphosis is a more sensitive marker of impairment than baseline level of kyphosis. These results should be replicated in other cohorts, and increasing kyphosis should be studied to determine whether interventions that prevent or reduce increasing kyphosis prevent mobility decline.
Acknowledgments
We wish to thank Lisa Palermo for providing access to the data.
This research was supported by the UCSF–Kaiser Building Interdisciplinary Research Careers in Women’s Health and co-funded by the National Institute of Child Health and Human Development and the Office of Research on Women’s Health (5K12 HD052163).
Sponsor’s Role: The sponsor collaborated with the investigators in the design and analysis of data for the main studies. The sponsor had no involvement in this manuscript other than reviewing it as part of the Steering Committee.
Footnotes
Conflict of Interest: Kado is a consultant for Medtronic/ Kyphon. Black has research contracts at the University of California at San Francisco (UCSF) with Merck, Roche, and Novartis. None of the other authors have a conflict of interest.
Author Contributions: Katzman: study concept, study design, and data analysis. Vittinghoff: study design and data analysis. All of the authors had access to the data and participated in interpretation of the data and preparation of the manuscript. Black designed and managed the main randomized clinical trial including data collection and analysis.
References
- 1.Balzini L, Vannucchi L, Benvenuti F, et al. Clinical characteristics of flexed posture in elderly women. J Am Geriatr Soc. 2003;51:1419–1426. doi: 10.1046/j.1532-5415.2003.51460.x. [DOI] [PubMed] [Google Scholar]
- 2.Sinaki M, Brey RH, Hughes CA, et al. Balance disorder and increased risk of falls in osteoporosis and kyphosis: Significance of kyphotic posture and muscle strength. Osteoporos Int. 2005;16:1004–1010. doi: 10.1007/s00198-004-1791-2. [DOI] [PubMed] [Google Scholar]
- 3.Ryan SD, Fried LP. The impact of kyphosis on daily functioning. J Am Geriatr Soc. 1997;45:1479–1486. doi: 10.1111/j.1532-5415.1997.tb03199.x. [DOI] [PubMed] [Google Scholar]
- 4.Hirose D, Ishida K, Nagano Y, et al. Posture of the trunk in the sagittal plane is associated with gait in community-dwelling elderly population. Clin Biomech. 2004;19:57–63. doi: 10.1016/j.clinbiomech.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Schenkman M, Shipp KM, Chandler J, et al. Relationships between mobility of axial structures and physical performance. Phys Ther. 1996;76:276–285. doi: 10.1093/ptj/76.3.276. [DOI] [PubMed] [Google Scholar]
- 6.Huang MH, Barrett-Connor E, Greendale GA, et al. Hyperkyphotic posture and risk of future osteoporotic fractures: The Rancho Bernardo study. J Bone Miner Res. 2006;21:419–423. doi: 10.1359/JBMR.051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ball JM, Cagle P, Johnson BE, et al. Spinal extension exercises prevent natural progression of kyphosis. Osteoporos Int. 2009;20:481–489. doi: 10.1007/s00198-008-0690-3. [DOI] [PubMed] [Google Scholar]
- 8.Ensrud KE, Black DM, Harris F, et al. Correlates of kyphosis in older women. The Fracture Intervention Trial Research Group. J Am Geriatr Soc. 1997;45:682–687. doi: 10.1111/j.1532-5415.1997.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 9.Cortet B, Roches E, Logier R, et al. Evaluation of spinal curvatures after a recent osteoporotic vertebral fracture. Joint Bone Spine. 2002;69:201–208. doi: 10.1016/s1297-319x(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 10.Takeda N, Kobayashi T, Atsuta Y, et al. Changes in the sagittal spinal alignment of the elderly without vertebral fractures: A minimum 10-year longitudinal study. J Orthop Sci. 2009;14:748–753. doi: 10.1007/s00776-009-1394-z. [DOI] [PubMed] [Google Scholar]
- 11.Schneider DL, von Muhlen D, Barrett-Connor E, et al. Kyphosis does not equal vertebral fractures: The Rancho Bernardo study. J Rheumatol. 2004;31:747–752. [PubMed] [Google Scholar]
- 12.Sinaki M, Itoi E, Rogers JW, et al. Correlation of back extensor strength with thoracic kyphosis and lumbar lordosis in estrogen-deficient women. Am J Phys Med Rehabil. 1996;75:370–374. doi: 10.1097/00002060-199609000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Greendale GA, Huang MH, Karlamangla AS, et al. Yoga decreases kyphosis in senior women and men with adult-onset hyperkyphosis: Results of a randomized controlled trial. J Am Geriatr Soc. 2009;57:1569–1579. doi: 10.1111/j.1532-5415.2009.02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katzman WB, Sellmeyer DE, Stewart AL, et al. Changes in flexed posture, musculoskeletal impairments, and physical performance after group exercise in community-dwelling older women. Arch Phys Med Rehabil. 2007;88:192–199. doi: 10.1016/j.apmr.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Benedetti MG, Berti L, Presti C, et al. Effects of an adapted physical activity program in a group of elderly subjects with flexed posture: Clinical and instrumental assessment. J Neuroeng Rehabil. 2008;5:32. doi: 10.1186/1743-0003-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoi E, Sinaki M. Effect of back-strengthening exercise on posture in healthy women 49 to 65 years of age. Mayo Clin Proc. 1994;69:1054–1059. doi: 10.1016/s0025-6196(12)61372-x. [DOI] [PubMed] [Google Scholar]
- 17.Pfeifer M, Begerow B, Minne HW. Effects of a new spinal orthosis on posture, trunk strength, and quality of life in women with postmenopausal osteoporosis: A randomized trial. Am J Phys Med Rehabil. 2004;83:177–186. doi: 10.1097/01.phm.0000113403.16617.93. [DOI] [PubMed] [Google Scholar]
- 18.Katzman WB, Vittinghoff E, Kado DM. Age-related hyperkyphosis, independent of spinal osteoporosis, is associated with impaired mobility in older community-dwelling women. Osteoporos Int. 2010 May 18; doi: 10.1007/s00198-010-1265-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bischoff HA, Stahelin HB, Monsch AU, et al. Identifying a cut-off point for normal mobility: A comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing. 2003;32:315–320. doi: 10.1093/ageing/32.3.315. [DOI] [PubMed] [Google Scholar]
- 20.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 21.Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 22.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896–903. [PubMed] [Google Scholar]
- 23.Black DM, Reiss TF, Nevitt MC, et al. Design of the Fracture Intervention Trial. Osteoporos Int. 1993;3(3 Suppl):S29–S39. doi: 10.1007/BF01623005. [DOI] [PubMed] [Google Scholar]
- 24.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27(2 Suppl):S178–S189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 25.Bohannon RW. Reference values for the Timed Up and Go test: A descriptive meta-analysis. J Geriatr Phys Ther. 2006;29:64–68. doi: 10.1519/00139143-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Wang CY, Yeh CJ, Hu MH. Mobility-related performance tests to predict mobility disability at 2-year follow-up in community-dwelling older adults. Arch Gerontol Geriatr. 2009 Nov 26; doi: 10.1016/j.archger.2009.11.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: A consensus report. J Am Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 28.Bautmans I, Van Arken J, Van Mackelenberg M, et al. Rehabilitation using manual mobilization for thoracic kyphosis in elderly postmenopausal patients with osteoporosis. J Rehabil Med. 2010;42:129–135. doi: 10.2340/16501977-0486. [DOI] [PubMed] [Google Scholar]


