Abstract
Background
Enrollment in the Stenting and Aggressive Medical Management for the Prevention of stroke in Intracranial Stenosis (SAMMPRIS) trial was halted owing to higher than expected 30-day stroke rates in the stenting arm. Improvement in peri-procedural stroke rates from angioplasty and stenting for intracranial atherosclerotic disease (ICAD) requires an understanding of the mechanisms of these events.
Objective
To identify the types and mechanisms of peri-procedural stroke after angioplasty and stenting for ICAD.
Methods
Patients that suffered a hemorrhagic or ischemic stroke or a cerebral infarct with temporary signs (CITS) within 30 days of attempted angioplasty and stenting in SAMMPRIS were identified. Study records, including case report forms, procedure notes, and imaging were reviewed. Strokes were categorized as ischemic or hemorrhagic. Ischemic strokes were categorized as perforator territory, distal embolic, or delayed stent thrombosis. Hemorrhagic strokes were categorized as subarachnoid or intraparenchymal. Causes of hemorrhage (wire perforation, vessel rupture) were recorded.
Results
Three patients suffered an ischemic stroke after diagnostic angiography. Two were unrelated to the procedure. Twenty-one patients suffered an ischemic stroke (n= 19) or CITS (n=2) within 30 days of angioplasty and stenting. Most (n=15) were perforator territory and many of these occurred after angiographically successful angioplasty and stenting of the basilar artery (n = 8). Six patients suffered subarachnoid hemorrhage (three from wire perforation) and seven a delayed intraparenchymal hemorrhage.
Conclusion
Efforts at reducing complications from angioplasty and stenting for ICAD must focus on reducing the risks of regional perforator infarction, delayed intraparenchymal hemorrhage, and wire perforation.
Keywords: Angioplasty and Stenting, Stroke, Hemorrhage
Introduction
The efficacy of angioplasty and stenting for patients with symptomatic intracranial atherosclerotic disease was recently evaluated in the Stenting and Aggressive Medical Management for the Prevention of Recurrent stroke in Intracranial Stenosis (SAMMPRIS) trial. 1 This trial is the largest prospective study to date in this population. Enrollment was stopped early owing to a higher rate of 30-day stroke and death in the stenting arm relative to aggressive medical management. Two hundred and twenty-four patients were randomized to the stenting arm and thirty-three (14.7%) suffered a symptomatic stroke within 30 days of enrollment. An additional four patients suffered an intracranial hemorrhage with symptoms lasting less than 24 hours or a cerebral infarction with temporary signs (CITS) within 30 days of the stenting procedure.2
Detailed statistical analyses of the relationship between clinical, procedural, and operator variables and the risk of 30-day adverse events have already been published.2, 3 The aims of the present study are to investigate the specific nature and mechanism of individual events, to describe the frequencies of the different mechanisms, and to describe the clinical and imaging features of each event to provide a more complete understanding of the peri-procedural events in the trial. These data will be critical for developing an understanding of how patient selection or devices could be improved to reduce the risk of perioperative stroke from angioplasty and stenting for symptomatic intracranial atherosclerotic disease (ICAD).
Methods
SAMMPRIS is an ongoing randomized, multi-center clinical trial funded by the National Institute of Neurological Disorders and Stroke.1, 4 Enrollment and randomization are complete but medical treatment and follow-up of enrolled patients is continuing until March 2013. The study design has been published.1, 4 Eligibility criteria included either transient ischemic attack (TIA) or non-disabling stroke within 30 days prior to enrollment attributable to angiographically-verified 70% to 99% stenosis of a major intracranial artery.
PTAS Procedure
The Gateway PTA Balloon Catheter and Wingspan Stent System (Boston Scientific Corporation, Stryker Neurovascular, Fremont CA) was used for PTAS in the trial. Specific aspects of the study protocol for PTAS procedure, post-procedure care, and aggressive medical management (same in both arms of the trial) have been published. 1, 5 The PTAS procedure was mandated for within three business days of randomization. A 600 mg loading dose between 6 – 24 hours before PTAS was allowed if the patient was not on daily clopidogrel (75 mg) for five days prior to PTAS. Systemic heparinization during PTAS was required with a target activated clotting time (ACT) of between 250 and 300 seconds.
Central Adjudication of Outcome after PTAS
Clinical evaluations of treated patients were required at study entry and four days (by study coordinator) and 30 days after enrollment. The patient was examined by a study neurologist and brain imaging was typically performed if a peri-procedural stroke was suspected. All potential study endpoints were adjudicated by central physician investigators blinded to treatment assignment. 1,2
Ischemic stroke was defined as a new focal neurological deficit of sudden onset that lasted at least 24 hours and was not associated with a hemorrhage on brain imaging (computed tomography (CT) or magnetic resonance imaging (MRI)). If symptoms lasted for less than 24 hours and was associated with a new infarct on brain imaging, the event was classified as a CITS. Hemorrhagic stroke was defined as parenchymal, subarachnoid, or intraventricular hemorrhage detected by CT or MRI that was associated with new neurological signs or symptoms lasting > 24 hours or a seizure. These were primary endpoints. Hemorrhagic stroke associated with symptoms or signs (excluding seizure) less than 24 hours in duration were not considered primary endpoints.
Subtype Classification of Events
The present study is a retrospective and post-hoc analysis of data collected in the trial. Patients with centrally-adjudicated 30-day ischemic stroke, symptomatic hemorrhagic stroke, asymptomatic hemorrhagic stroke or CITS after randomization to the stenting arm were identified. Study records, including case report forms, scanned documents such as procedure and progress notes and discharge or death summaries, and records of central end-point adjudication were reviewed. In addition, electronically archived (iSite, Phillips, Eindhoven Netherlands) baseline, procedural and post-procedure brain and vascular images were reviewed.
All peri-procedural (within 30 days after randomization) ischemic strokes, CITS and hemorrhagic strokes were categorized by consensus of the three primary investigators (DF, CD, MC) based on an assessment of the available imaging and clinical data. Hemorrhagic strokes were classified as subarachnoid hemorrhages (SAH) when the bleeding was predominantly subarachnoid and the presentations were evident immediately after the procedure, or as reperfusion hemorrhages when the bleeding was predominantly intraparenchymal (ICH) and within the vascular distribution of the stented vessel. Ischemic strokes were further categorized as local perforator territory (covered by the stent), distal embolic, and delayed stent thrombosis based on imaging findings and the clinical deficits. Ischemic strokes were categorized as perforator occlusions if the infarct(s) and symptoms could be localized to the distribution supplied by perforating vessels arising within the margins of the stent, or embolic if the infarct was in a territorial distribution distal to the treated lesion and the symptoms were explained by the distal infarct. If a patient had lesions on imaging in local perforator and distal territories, but the symptoms were consistent with perforator ischemia only, the event was classified as a perforator event. When more than one mechanism contributed to the clinical findings, the stroke was described as mixed. Delayed stent occlusion was diagnosed if there was imaging or other presumptive evidence of stent occlusion.
Images and procedure reports were carefully reviewed for any evidence of mechanical or procedural causes of the hemorrhagic or ischemic event, including wire perforation, vessel rupture for hemorrhage and vessel dissection for ischemic stroke.
Tables of the data collected for each individual patient were created based on the results of these reviews (Tables 1–4). Tabulated data included age and gender, the nature of the qualifying event (stroke, TIA and major symptoms), baseline imaging findings if available, location of the symptomatic stenosis, time from qualifying event to procedure, pertinent details of the procedure, nature and timing of the complication, presumed mechanism of the event, and the 30-day outcome (modified Rankin Score, mRS).
Table 1.
Angiogram-only strokes
| Patient Numb er |
Age/ Gend er |
Qualifying Event |
Baseline Imaging |
Location of Stenotic Artery |
Timing QE to Procedure (days) |
Procedural and Stroke Details | Mechanism of Stroke Associated with Clinically Relevant Infarct |
30-day Outco me |
|---|---|---|---|---|---|---|---|---|
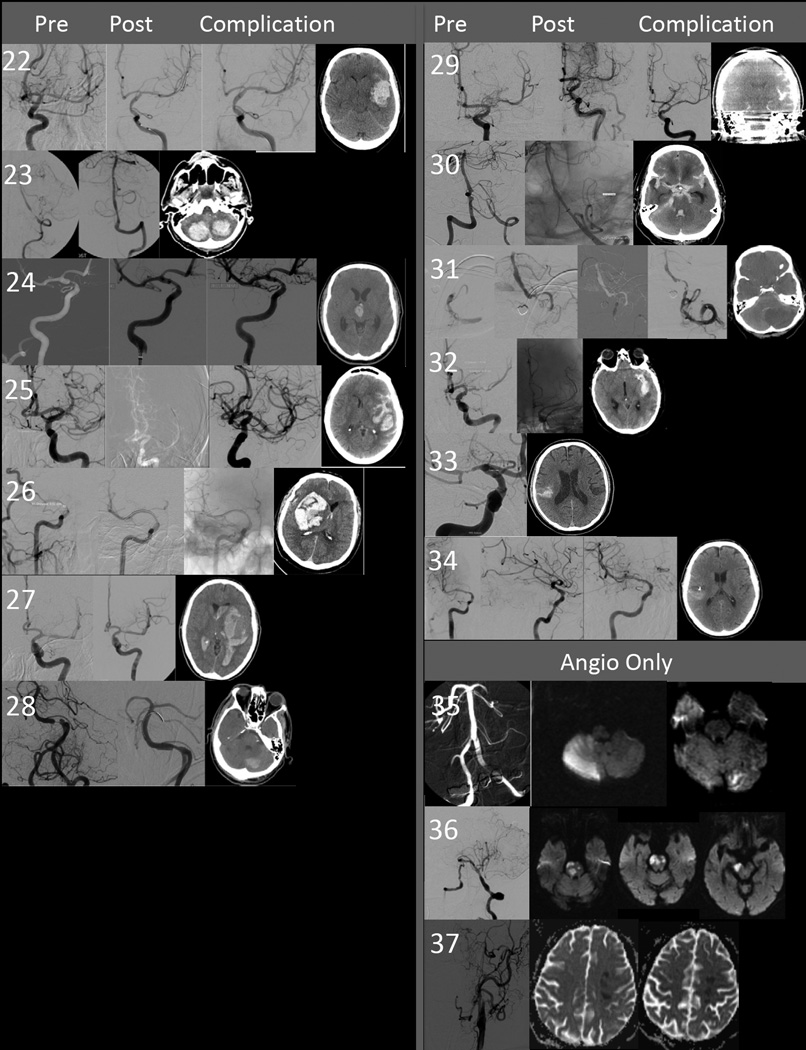
| 35 | 57/M | Stroke: Ataxia And weakness |
MR (+ infarct) |
Vertebrobasil ar junction |
7 | 80% lesion at VBJ by diagnostic catheter angiogram Angiogram under anesthesia showed significant reduction in stenosis, now <60%. New PICA evident on angio (Figure 2) New vision loss post angio. New occipital and posterior inferior cerebellar artery territory strokes on repeat MR |
Embolic Complication of angio |
mRS 4 |
| 36 | 70/F | Stroke: dysarthria, arm and leg weakness |
No baseline MR or CT submitte d |
Basilar | 5 | Diagnostic angiogram (3 days after stroke) demonstrated 98% basilar stenosis Therapeutic (for planned angioplasty) angiogram showed interval occlusion at the site of tenosis Retrograde filling from PComA. atient awoke stable Acute stroke in territory 3 days later. New infarctions by MR in pons and midbrain |
Delayed thrombo- embolic |
Died 3 days after angio |
| 37 | 74/F | Stroke: face, arm and leg weakness, aphasia |
CT (+ infarct) |
Internal carotid artery |
4 | Catheter angiogram (2 days after stroke) demonstrated 89% cavernous ICA stenosis Treatment angiogram showed complete occlusion Acute stroke in the territory 5 days later while in rehab. (Figure 2, MR ADC maps) |
Delayed thrombo- embolic |
mRS 4 |
Infarct size: 1.5 cm or less – small, 1.5 to 3.0 cm – medium. No petechial hemorrhage unless otherwise noted. Corresponding images described in the
“Procedural and Stroke Details” are shown in Figure 2 by patient number, unless indicated otherwise.
M = male; F = female; MR = magnetic resonance; CT = computed tomography; mRS = modified Rankin score; QE = Qualifying event; ADC = apparent diffusion coefficient)
Table 4.
Subarachnoid Hemorrhage
| Patient Numbe r |
Age/ Gend er |
Qualifyin g Event |
Baseline Imaging |
Locatio n of Stenotic Artery |
Parent artery diameter (per site) |
Timing and Details of Procedure |
Post Procedural details |
Mechanis m |
30-day Modifie d Rankin Scale |
|---|---|---|---|---|---|---|---|---|---|
| 29 | 54/F | TIA: Episode of aphasia for a few minutes |
MR (same day): no acute lesion, small old cortical strokes in territory |
M1 | 2.21 mm | 28 days after TIA. Subtle extravasation seen in distal inferior division near tip of exchange wire after stent placement. |
Confirmed by DSA-CT. Required decompres sive craniectomy. |
Wire perforati on |
5 |
| 30 | 74/M | Stroke: slurred speech noted on awakeni ng. Dysarthri a and right facial droop persisted more than 24 hours |
MR (same day): Acute pontine infarction |
VBJ | 2.00 mm | 11 days after stroke. No SAH identified during procedure. Lost guide catheter during exchange for stent and repositioned guide catheter after placing micro- exchange wire into SCA |
Acute SAH centered in superior vermian region on DSA-CT. Required Urgent ventriculost omy. Developed extensive brainstem infarction |
Wire Perforatio n |
5 |
| 31 | 69/F | TIA: dizziness upper extremit y numbne ss |
MR (5 months prior): Normal |
Distal VA |
4.00 mm | 22 days after TIA and diagnostic angio. Interval occlusion of distal VA. Isolated VB system with chronic contralateral VA occlusion and absent PComAs. Abciximab administered. Lesion was crossed with SL-10 catheter. Location in basilar confirmed. 3.5 × 9 mm Gateway inflated. Active extravasation seen at PICA origin (Figure). SAH persisted after reversal and 60 minutes of balloon inflation. Treated successfully with nBCA |
Long complicated hospital course |
Vessel rupture |
5 |
| 32 | 57/ M | Stroke: dysarthri a, L facial, UE, LE weaknes s |
MR (same day): acute corona radiate, subinsular infarction in the territory |
Petrous ICA |
3.90 | 24 days after stroke. Small amount of extravasation noted in distal branche near tip of exchange wire. Branch occluded with coils. |
LUE and Facial weakness, and dysarthria postop. CT shows some SAH and infarction around coils |
Wire Perforatio n |
0 |
| 33 | 50/F | TIA: Recurre nt episodes of upper extremit y weaknes s. |
MR (same day): no acute lesion. Small old cortical and subcortical infarctions in the territory. |
M1 | 2.30 mm | 4 days after TIA. Extravasation evident on angio after stent deployment. Appeared to arise from two distal MCA branches, one that had been accessed with the guidewire. This was treated with Onyx. After a delay of 15 minutes, no further extravasation was noted from the second branch. |
Post op MR showed an acute posterior division MCA infarction and sylvian SAH. Initial global aphasia but made substantial improvement |
Unknown | 2 |
| Asymptomatic SAH | |||||||||
| 34 | 76/F | TIA (CITS): 2 Syncopa l Episodes consider ed TIAs by site |
MR (2 days post): acute caudate stroke |
M1 | 3.50 mm | 9 days after CITS. No SAH identified during procedure. Unable to advance stent into M1 segment. Angioplasty repeated and second stent attempt failed. |
Acute SAH in sylvian fissure on DSA-CT. Asymptoma tic |
Unknown | 0 |
Notes: Baseline MR not required by protocol.
Figures (Figures 1 and 2) were constructed using images submitted from the site: baseline qualifying events, pre and post-procedural angiograms, and relevant representative images of the hemorrhage or infarction.
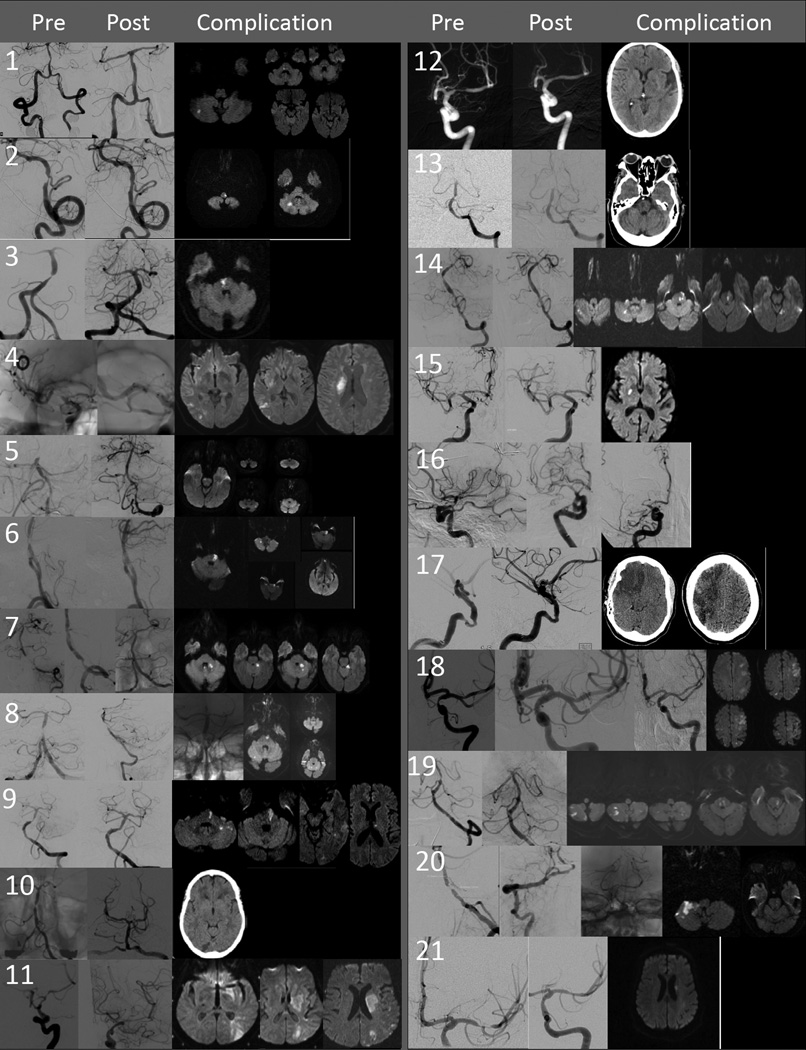
Figure 1.
Composite figure of pre and post-angioplasty and stenting angiogram, and brain imaging demonstrating the location and extent of ischemic injury for each individual patient. The number corresponds to the patient number in table 2. Descriptions of the procedure and relevant findings on the images is found in the table under the description of the heading “procedure and stroke details”
Figure 2.
Composite figure of pre and post-angioplasty and stenting angiogram, and brain imaging demonstrating the location and extent of hemorrhage or ischemic injury for each individual patient. The number corresponds to the patient number in tables 1, 3, and 4. Descriptions of the procedure and relevant findings on the images is found in the table under the description of the headings “timing and details of procedural” and “Post-procedural details”.
Results
Angiography-Only Strokes
Two hundred and twenty patients underwent attempted angioplasty and stenting. The procedure was aborted in six prior to any intervention owing to the findings on catheter angiography and in one case when attempts to cross the lesion with a wire were unsuccessful. Three of these seven patients suffered an ischemic stroke (Table 1, patients 35–37, Figure 2). One was a definite procedural embolic stroke (patient 35) in a patient randomized to stenting based on prior imaging but found to have less than 50% stenosis at the time of the planned PTAS procedure. The remaining two patients suffered strokes days later (patients 36 and 37). Both of these patients had developed an interval complete occlusion of the target vessel between enrollment and the planned intervention.
Procedural Ischemic Strokes
Twenty-one ischemic events (19 ischemic strokes and 2 CITS) occurred in the remaining 213 patients (Table 2, Patients 1 −21, Figure 1). Of the 21 events, 14 were evident on emerging from general anesthesia (patients 1 – 14). Eleven of the fourteen primarily involved local perforators (and resulted in clinical syndromes attributable to the perforator infarction), two were embolic (patients 7 and 10) and one was mixed embolic and perforator territory owing to acute intraprocedural stent thrombosis (patient 11, post angio). Technical difficulties were noted in two of the 11 perforator territory strokes that were evident immediately after the procedure (patient 1 (small V4 dissection flap) and patient 8 (difficult vertebral artery access). Post-angioplasty angiographic images were unremarkable in all 11 cases of perforator infarction. Distal embolic occlusion was evident on the post-angioplasty images in one case (patient 7, missing left superior cerebellar artery on post angioplasty angiogram). This patient also received no plavix load and ACTs were not recorded during the procedure.
Table 2.
Acute Ischemic Stroke
| Patient | Age/ Gender |
Qualifying Event |
Baseline Imaging |
Location of Stenotic Artery and % stenosis |
Timing QE to Procedure (days) |
Procedural and Stroke Details | Mechanism of Stroke Associated with Clinically Relevant Infarct |
30-day Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 80/F | TIA: slurred speech, ataxia, vertigo, LUE and LLE numbness |
MR: no acute/ subacute infarcts. Old R pontine infarct |
Basilar; 71% |
11 | Small V4 dissection, otherwise uncomplicated procedure 24 hours after procedure: diplopia,slurred speech, gait instability. MR showed: new infarct in R pons(perforators), small R cerebellar infarcts (probable embolus to jailed AICA) and small bilateral occipital lobes (distal emboli). |
Occlusion of local perforators to pons |
mRS 1 (1 at study entry) |
| 2 | 58/M | Stroke: vertical diplopia, dizziness, RUE and RLE ataxia, gait ataxia, nausea, vomiting, and dysarthria |
CT - Acute / Subacute bilateral cerebellar and R occipital infarcts |
L VA; 70% | 9 | Uncomplicated procedure. Decreased pain sensation right arm and leg, left face numbness after procedure. MR showed left lateral medullary infarct (perforator) and small R cerebellar infarct (maybe subacute from qualifying event versus embolus to R cerebellum supplied by jailed L PICA – supplies both PICA territories). |
Occlusion of local perforator to medulla |
mRS 1 (1 at study entry) |
| 3 | 67/M | TIAs: cortical blindness, dizziness, nausea, vomiting, facial tingling, LUE and LLE numbness |
MR – No acute infarcts. Old L pontine infarct. |
Basilar; 80% |
4 | Uncomplicated procedure. New diploplia, dysarthria, L sided weakness, L arm numbness. MR – isolated acute R pontine infarct |
Occlusion of local perforators to pons |
mRS 2 (0 at study entry) |
| 4 | 60/F | Stroke: perioral numbness, L face, arm and leg numbness and left hemiparesis. |
No MR or CT submitted but records indicate “R basal ganglia” infarct |
R M1; 69% |
6 | Uncomplicated procedure. Pre and post angio images are unsubtracted (Figure 1) New left face and arm weakness. MR shows new infarct in R basal ganglia, internal capsule, and corona radiate (perforators), and infarcts of insula and temporal/ parietal lobes (distal emboli) |
Occlusion of Local Lenticulostriate perforators |
mRS 3 (0 at study entry) |
| 5 | 65/F | Stroke: non- specific bilateral blurry vision, dizziness, nausea, vomiting, LUE weakness and headache |
MR - Acute / Subacute bilateral cerebellar and Rmedial temporal lobe infarct |
L VA – Basilar Junction; 89% |
10 | Uncomplicated procedure. Pre image (Figure 1) shows no flow beyond AICAs and microcatheter through stenosis Patient awoke from anesthesia with encephalopathy, the following morning increased LUE weakness. MR shows new R pontine infarct (perforator) and bilateral cerebellar infarcts (proximal PICA and distal AICA/SCA emboli) and small R temporal and R occipital infarcts (distal emboli, not shown) |
Occlusion of local perforators to pons |
mRS 4 (3 at study entry) |
| 6 | 78/M | Stroke: confusion, bilateral blurry vision, dizziness, R face/UE/LE numbness and weakness, RUE/LE ataxia, gait ataxia, oscillopsia, dysarthria |
CT – No infarcts |
Basilar; 80% |
7 | Uncomplicated procedure. Worsening symptoms after stenting including newly reported L arm and leg ataxia, left arm weakness, and R face numbness. MR shows L pontine infarct (perforator), small R cerebellar infarct (probable embolus to jailed R AICA), and small L thalamic infarct (distal embolus) |
Occlusion of local perforators to pons |
mRS 4 (2 at study entry) |
| 7 | 55/M | TIA: bilateral blurred vision, dysarthria, ataxia |
CT - 1 month earlier showed no infarcts. No brain imaging after TIA. |
L VA; 81% | 22 | No plavix load AND ACT not monitored (protocol deviation). Occlusion of the left SCA during procedure (Figure 1, post image) LUE and LLE dysmetria after stenting. MR shows L cerebellar infarct (embolus to L SCA) and small inferior vermian infarction (embolic, L AICA supplies midline inferior vermis) |
Distal embolism | mRS 1 (0 at study entry) |
| 8 | 74/M | TIA: 2 episodes on the same day consisting of dizziness and gait ataxia lasting 1–2 minutes |
MR – no infarcts |
Basilar; 87% |
22 | Challenging procedure with difficult access. Slurred speech, horizontal diploplia, R arm/leg/face weakness hours after the procedure. MR shows infarct in L pons (perforators), small bilateral cerebellar infarcts (proximal PICA and distal SCA emboli), and small R occipital infarct (distal embolus, (not shown)) |
Occlusion of local perforators to pons |
mRS 2 (1 at study entry – not stroke related) |
| 9 | 59/M | Stroke: bilateral blurry vision, diploplia, dysarthria, disorientation, and headache |
MR – acute / subacute infarcts in both occipital lobes, both cerebellar hemispheres and L medial temporal lobe |
Basilar; 74% |
13 | Uncomplicated procedure. Slurred speech, R hemiparesis. MR shows new infarct in L pons (perforator), L cerebellar infarct (embolus to jailed L AICA), and small, left thalamic infarct (distal embolus) |
Occlusion of local perforators to pons |
mRS 3 (1 at study entry) |
| 10 | 71/F | Stroke: dizziness, gait ataxia, weakness of L UE and LLE |
MR - no acute / subacute infarcts. Old L pontine and L thalamic infarcts |
Basilar; 77% |
4 | Uncomplicated Difficulty reading/vision loss after procedure. L homonymous hemianopia CT shows right occipital infarction |
Distal embolism | mRS 1 (2 at study entry) |
| 11 | 61/F | Stroke: aphasia, confusion, RUE and RLE weakness |
No MR submitted for central review. Site review -“ subcortical ischemia in LMCA territory” |
Proximal L M1; 72% |
14 | Post-Stent placement L ACA occluded and multiple distal L MCA emboli (not shown) New right hemiplegia. MR shows large confluent infarct involving caudate, putamen, and capsule (perforator) and infarcts of the L frontal, L parietal and L temporal lobe (distal emboli). |
Combined – occlusion of local lenticulostriate perforators and distal embolism |
mRS 5 (3 at study entry) |
| 12 | 68/F | TIA: speech arrest and RUE weakness lasting 10–15 minutes. Several episodes of R arm weakness and aphasia 2 months prior, one of which “associated with small acute infarct”. |
MR – acute L frontal and L corona radiate/ centrum semi-ovale infarcts (so this patient’s presenting TIA was actually a cerebral infarct with temporary signs (CITS) |
L M1; 68% |
18 | Uncomplicated procedure. Post-procedure mild dysarthria, word finding difficulty and RUE drift. CT shows new infarcts in L putamen and internal capsule |
Occlusion of local lenticulostriate perforators |
mRS 1 (2 at study entry – not related to qualifying event) |
| 13 | 64/F | TIA: 1st episode 5 minutes of vertigo, 2nd episode 2 hours of gait ataxia and dysarthria. |
MR - no acute/ subacute infarcts but did show old infarcts in bilateral pons, R thalamus, R parietal and R putamen / capsule |
Basilar; 66% |
4 | Uncomplicated procedure per report. Intubated for 48 hours, POD#2 New LUE and LE weakness. CT shows evolving right pontine infarct |
Occlusion of local perforators to pons |
mRS 2 (1 at study entry) |
| 14 | 46/M | TIA: R sided blurry vision, RUE and RUE ataxia, dysarthria, and peri-oral numbness |
MRI –acute /subacute infarcts |
Basilar; 75% |
3 | Uncomplicated procedure. Post-op: dizziness, dysarthria, nystagmus, no motor deficit. CT showed new R cerebellar infarct 6 days later right face weakness, dysarthria worse, ataxia. MR shows infarct in L pons (perforators), small R cerebellar infarct (emboli to jailed R AICA), and L mesial temporal infarct (distal embolus) |
Occlusion of local perforators to pons |
mRS 2 (0 at study entry) |
|
Delayed Stroke |
||||||||
| 15 | 74/F | Stroke: L face weakness and LUE numbness |
MR – acute /subacute infarcts in R frontal and parietal lobes |
R M1; 75% |
11 | Uncomplicated procedure. Awoke stable L arm weakness 36 hours later. MR showed a small R putamen/internal capsule (perforator) |
Occlusion of local lenticulostriate perforators |
mRS 1 (0 at study entry) |
| 16 | 71/F | Stroke: L facial and LLE weakness |
MR – acute /subacute infarcts of the R caudate and R frontal and parietal lobes |
R ICA; 70% |
4 | Delayed filling of insular branches noted at baseline that persisted post-stent. Systemic integrelin started 4 days after stent developed left hemiparesis (0/5 LUE, 3/5 LLE), R gaze preference, mild neglect, L homonymous hemianopia. Repeat angiogram showed stent occlusion (Final angio in Figure). |
Delayed Stent Thrombosis |
mRS 4 (0 at study entry) |
| 17 | 58/F | Stroke (followed by TIA) : left homonymous hemianopia, dysarthria, left face numbness and L face weakness |
MR – acute infarcts of R frontal, parietal and temporal lobes |
R ICA; 75% |
6 | Angioplasty X 2 and stent. Uncomplicated. Awoke stable. 6 days later, R sided headache, left hemiplegia. CT at outside hospital showed R hyperdense MCA sign (not shown) and large infarcts of R frontal, parietal and temporal lobe |
Probable Delayed Stent Thrombosis |
Not done at 30 days but mRS 3 at 90 days (3 at study entry) |
| 18 | 74/F | Stroke: aphasia, confusion and RLE weakness |
CT – acute / subacute infarcts of L frontal and parietal lobes |
L ICA; 69% |
10 | Stent required post-dilation Thrombus forming on stentand in A1 segment at delayed angiogram (10 mins) administered IV abciximab. Persisted at 20 minuntes, gave IA tPA (5.4 mg). Neurological baseline after extubated Pt found down by husband 4 days after procedure with R hemiplegia and aphasia. Stent open on repeat angiogram. MR showed extensive L MCA, ACA and R ACA embolic lesions |
Distal emboli | mRS 4 (2 at study entry) |
| 19 | 77/F | Stroke: presented with dizziness and loss of consciousness. Exam showed gait ataxia, RUE and R LE weakness, and R face and R UE numbness |
MR – acute /subacute infarcts of bilateral cerebellum and R medulla |
Basilar; 78% |
4 | Difficult access (radial approach) otherwise uncomplicated 6 days after procedure, Diplopia, bilateral UE ataxia, nystagmus, R face sensory loss, L leg numbness. MR showed new bilateral pontine infarcts (perforators) and small bilateral cerebellar infarcts (emboli to jailed bilateral AICA) |
Occlusion of local perforators to pons plus emboli to jailed bilateral ICAs |
mRS 4 (3 at study entry) |
| CITS | ||||||||
| 20 | 77/ M | Stroke: vertigo, dysarthria, R hemiparesis, confusion. Good response to tPA with residual mild dysarthria and R leg weakness |
MR – no acute / subacute infarcts |
Basilar; 89% |
Uncomplicated. Right brachial approach Transient weakness of R arm night of procedure that resolved by next morning. MR showed large R cerebellar infarct (jailed AICA embolism) and L small pontine infarcts (perforators) |
Embolus to jailed AICA |
mRS 0 0 at 30 days (mRS 0 at study entry) |
|
| 21 | 61/F | Stroke: aphasia and R face weakness |
MR – acute /subacute L frontal and parietal infarcts |
L M1; 70% |
Uncomplicated 3 weeks post procedure, transient “new L hemisphere symptoms”. MR showed new small L corona radiata infarct |
Occlusion of local perforators |
mRS 3 at 30 days (2 at study entry) |
Infarct size: 1.5 cm or less – small, 1.5 to 3.0 cm – medium. No petechial hemorrhage unless otherwise noted. Corresponding images pre and post angioplasty and stenting and follow up brain imaging showing the ischemic infarctions described in the “Procedural and Stroke Details” are shown in Figure 1 by patient number, unless indicated otherwise.
M = male; F = female; MR = magnetic resonance; CT = computed tomography; mRS = modified Rankin score; QE = Qualifying event; AICA = anterior inferior cerebellar artery; MCA = middle cerebral artery; ACA = anterior cerebral artery; M1 – first segment of the MCA, A1 – first segment of the ACA; VA = vertebral artery; L = left; R =right; PICA = posterior inferior cerebellar artery; UE = upper extremity; LE = lower extremity; IA = intra-arterial; % stenosis = central reading
Five patients had a delayed ischemic event (patients 15 −19). All delayed events occurred within 6 days of the procedure. One of these events was a local perforator stroke only (patient 15, at 36 hours). One patient had a complete stent thrombosis at four days (patient 16), one had a probable stent thrombosis at 6 days (patient 17), one had extensive distal emboli at 4 days (patient 18), and one had mixed perforator and distal embolic strokes at 6 days (patient 19).
Both of the CITS events were related to local perforator or branch (jailed AICA) vessel occlusions (patients 20 and 21). One was in the early post-operative period and the other occurred three weeks after the procedure.
Delayed Intraparenchymal Hemorrhage
Seven patients had primarily parenchymal hemorrhage (IPH) (Table 3, patients 22–28, Figure 2). Six were endpoint events with symptoms lasting more than 24 hours: one was noted 2 hours after the procedure (patient 27) and five were noted when neurological signs developed several hours after PTAS. In the 7 patients with IPH, baseline brain imaging showed infarcts in five (two presenting with ischemic strokes (patients 25 and 28) and three presenting with CITS (patients 23, 24 and 27). The remaining two had either a normal CT (patient 22) or no imaging (patient 26) at baseline. All IPHs were distributed within the vascular territory of the treated artery. Of the six symptomatic IPHs, four were fatal, one resulted in a modified Rankin score (mRS) of 5, and one resulted in a mRS of 2 at 90 days. One patient had a cerebellar hemorrhage with transient post-operative nausea but no symptoms at 24 hours (patient 28).
Table 3.
Intraparenchymal Hemorrhage
| Patient Numb er |
Age/ Gend er |
Qualifyin g Event |
Baseline Imaging |
Location of Stenotic Artery |
Parent artery diamete r (per site) |
Timing and Details of Procedure |
Post-Procedural details | 30-day Modified Rankin Scale |
|---|---|---|---|---|---|---|---|---|
| 22 | 59/F | TIA: recurrent episodes of aphasia and weakness |
CT (3 days later): Normal |
M1 | 2.90 mm | 4 days after TIA. Uncomplicated. Extubated on table, normal neurological examination |
9 hours post procedure developed aphasia and hemiplegia. CT showed hemorrhage in revascularized territory. Went to OR for evacuation. |
5 |
| 23 | 70/M | TIA (CITS): mild pontine stroke 6 weeks prior, then transient dysarthria TIA |
MR (same day as CITS): small medullar and pontine, two medium cerebellar acute infarctions |
VA | 3.00 mm | 6 days after CITS. Uncomplicated. Isolated VB system with occluded contralateral vertebral artery distal to PICA. Extubated in angio with unchanged baseline neurological examination on arrival to ICU |
Hours (time not documented) after procedure in ICU became drowsy, then acutely hypertensive and lost brainstem reflexes. CT showed bilateral cerebellar IPH, with secondary IVH and SAH and obstructive hydrocephalus (not shown). Went to OR emergently for decompressive craniectomy |
Died 11 days after procedure |
| 24 | 46/M | TIA (CITS): Upper extremity weakness |
MR (1 day post CITS): small parietal and corona radiata, medium frontal acute infarctions |
Petrous ICA |
4.38 mm | 20 days after CITS. Uncomplicated. Fetal PCA |
2 days post procedure. Sudden onset weakness and confusion at home after discharge. CT showed thalamic IPH (in territory of fetal PCA) with ventricular extension. Required with ventricular extension. Required urgent urgent EVD. |
1 |
| 25 | 76/F | Stroke: Word- finding diffiulties |
MR (same day): small frontal and medium corona radiate acute infarctions. Old small putamen infarct with petechial hemorrhage |
M1 | 2.18 | 26 days after stroke. Uncomplicated. Extubated with unchanged baseline neurological examination |
12 hours after procedure became acutely hemiparetic and aphasic. Urgent CT showed large ICH with local SAH in the revascularized territory. Family withdrew care. |
Died 3 days after procedu re |
| 26 | 52/M | TIA: Face, arm, leg weakness and dysarthria |
None available |
Supracli noid ICA |
3.03 | 28 days after TIA. Uncomplicated. Extubated in angio with unchanged normal neurological examination |
7 hours after procedure. CT showed 7 cm ICH with herniation and shift. |
Died 1 day after procedu re |
| 27 | 60/F | TIA (CITS): Face and arm weakness, aphasia |
MR (same day): small frontal, temporal and caudate, medium temporal and parietal infarctions. |
M1 | 2.20 | 8 days after CITS. Uncomplicated. Extubated with normal baseline neurological examination |
2 hours after procedure. Became acutely hypertensive with neurological decline. CT showed large ICH in territory. Progressed rapidly to brain death. |
Died 1 day after procedu re |
| Asymptomatic | ||||||||
| 28 | 73/M | Stroke: Diploplia, ataxia, arm and leg weakness |
MR (sme day): small occipital, thalamic and bilateral cerebellar acute infarctions |
Basilar | 3.48 | 3 days after stroke. Uncomplicated. |
12 hours after procedure. Transient nausea. No new deficit. CT showed 3.5 cm cerebellar ICH |
1 |
Infarct size: 1.5 cm or less – small, 1.5 to 3.0 cm – medium. No petechial hemorrhage unless otherwise noted . The pre and post angioplasty and stenting angiographic images corresponding to the procedure description are shown in Figure 2 by patient number. Representative CT images showing the location and extent of hemorrhage (described in the post-procedure detail column) are shown in Figure 2 as well, unless otherwise indicated. M = male; F = female; MR = magnetic resonance; CT = computed tomography; mRS = modified Rankin score; QE = Qualifying event;
Procedural Subarachnoid Hemorrhage
Subarachnoid hemorrhage (SAH) occurred in six patients (Table 4, patients 29–34, Figure 2). Three patients had wire perforations that were evident during the procedure (patients 29, 30, and 32). The clinically adjudicated endpoint for patient 32 was an ischemic stroke: the perforation was successfully treated with coil embolization of the injured arterial branch. This resulted in a clinical ischemic stroke (adjudicated and reported as ischemic stroke in the original primary analysis). A fourth patient (patient 33) had acute SAH during the procedure but the etiology could not be ascertained. One patient with interval occlusion between randomization and the endovascular procedure was treated with angioplasty alone (patient 31, protocol violation). This resulted in a vessel rupture (post plasty angio showing active extravastion). One final patient (patient 34) had an asymptomatic SAH identified on post-operative CT. No cause was determined.
Discussion
Angioplasty and stenting of intracranial arteries is an emerging, but as yet unproven, therapy for stroke risk reduction for patients with recently symptomatic ICAD. The SAMMPRIS study was the first randomized trial of this procedure for ICAD. Angioplasty and stenting in this trial was associated with a higher than expected rate of perioperative stroke. Reduction or prevention of these complications will be required for this procedure to be proven safe and effective. The purpose of this study was to perform a detailed, post-hoc review of all complications that occurred in the stenting arm, in an effort to categorize and attribute the mechanism of stroke for each patient.
The most frequent types of stroke observed in the SAMMPRIS trial were local perforator ischemic strokes (n=12), primary intraparenchymal hemorrhage (n=7), and subarachnoid hemorrhage (n=6). Distal symptomatic embolic stroke as an immediate complication of angioplasty and stenting was uncommon.
Local perforator stroke after angioplasty and/or stenting has been described in prior case series. Prior investigators have reported an association of a higher risk of procedural ischemic stroke with posterior versus anterior circulation lesions after angioplasty and stenting.6–9 Jiang and colleagues reported a 13% (9/69) 30-day risk for stroke after angioplasty and stenting of symptomatic basilar artery stenoses in a review of outcomes from a large single institution series.10 However, this study did not categorize these strokes as either embolic or local perforator. Perforator occlusion by the displaced or disrupted atheromatous debris (snow-plowing) has been postulated as the mechanism for regional perforator stroke after stenting.11 Potential approaches to reduce this risk of this phenomenon may include performing angioplasty alone rather than angioplasty and stenting 11 or using high-resolution MR vessel wall imaging for treatment planning or patient selection. 2, 12, 13 Even if the risk of perforator stroke after stenting could be reduced, aggressive risk factor management for patients with evidence of unstable or ruptured plaques adjacent to perforator-rich vessel segments may remain more effective than mechanical intervention.
Parenchymal hemorrhage occurred with a higher than expected frequency, compared to prior case series. In the U.S. Wingspan Registry, Fiorella and colleagues reported five major events in 78 patients.14 Two were vessel perforations resulting in death, one was an IPH, and two were embolic ischemic strokes.9 In the NIH wingspan registry, Zaidat and colleagues reported two IPHs in 129 patients.15 In SAMMPRIS, Fiorella, et al, reported that delayed IPH was independently associated with higher baseline percent stenosis as well as the combination of a high procedural ACT (> 300 seconds) and a loading dose of clopidogrel. The mechanism of IPH post stenting in SAMMPRIS patients is uncertain, but the relationship between severe stenosis and hemorrhage suggests the possibility of hyperperfusion or autoregulatory dysfunction as a mechanism. 16 However, the time course and clinical features are different from the typical hyperperfusion syndrome seen after extracranial carotid revascularization. 17
The etiology of the subarachnoid hemorrhages was clear in most of the cases. These could be attributed to wire perforation in three and vessel rupture in one. The vessel rupture occurred in a patient with a completely occluded segment (interval occlusion between enrollment and the angioplasty procedure) and was a protocol violation. These events were potentially avoidable. The Wingspan system requires several exchanges of catheters over a 300 cm exchange wire. The wire tip may move into small distal branches and perforate for a number of technical reasons. One is lack of attention to detail by the operator. Another is related to inadequate guide catheter support leading to abrupt movement of the system. These complications could potentially be reduced with experience, although no relationship between hemorrhage and physician experience was found in the SAMMPRIS trial. 3 These complications may also be reduced with angioplasty alone or angioplasty and stenting with devices that do not require exchanges.
The SAMMPRIS protocol did not include any tests of platelet function. The study policy disallowed resistance testing after randomization to avoid the use of antiplatelet agents or doses that were not specified by the protocol. It is unknown whether the patients with ischemic events were more resistant to aspirin or clopidogrel, or if the patients with hemorrhagic events were less resistant to the antiplatelet regimen.
This retrospective study is subject to a number of limitations, most significantly that of the attribution of stroke mechanism. This process was post-hoc, based on review of available images and clinical data, and admittedly inexact. For ischemic stroke, stroke mechanism was based primarily on brain imaging and clinical symptoms. Many of the patients that were categorized as perforator stroke also had distal lesions on DWI. While it is likely that many of these represent emboli from the procedure, the clinical relevance of asymptomatic DWI lesions is unclear. 18 Parenchymal hemorrhage was separated from subarachnoid hemorrhage based on review of imaging and the timing of the clinical event. It is possible, however, that some of the parenchymal hemorrhages were related to wire perforation.
Conclusion
The most frequent causes of peri-operative stroke in the SAMMPRIS trial were perforator-territory ischemic stroke, reperfusion hemorrhage, and subarachnoid hemorrhage. Future trials of intracranial angioplasty and stenting will need to reduce these events in order to establish the safety and efficacy of this procedure.
Supplementary Material
Acknowledgments
Funding:
The SAMMPRIS trial was funded by a research grant (U01 NS058728) from the US Public Health Service National Institute of Neurological Disorders and Stroke (NINDS). In addition, the following Clinical and Translational Science Awards, funded by the National Institutes of Health, provided local support for the evaluation of patients in the trial: Medical University of South Carolina (UL1RR029882), University of Florida (UL1RR029889), University of Cincinnati (UL1RR029890), and University of California, San Francisco (UL1RR024131).
Corporate Support: Stryker Neurovascular (formerly Boston Scientific Neurovascular) provided study devices and supplemental funding for third party device distribution, site monitoring and study auditing. This research is also supported by the Investigator-Sponsored Study Program of AstraZeneca that donates rosuvastatin (Crestor) to study patients.
Financial Support and Industry Affiliations:
Drs. Fiorella, Derdeyn, Turan, Janis, and Chimowitz, Michael Lynn M.S, and Bethany Lane RN serve on the Executive Committee of the SAMMPRIS trial which is funded by the National Institute of Neurological Disorders and Stroke (grant number: U01 NS058728). All receive salary support from the SAMMPRIS grant. All other authors were investigators in SAMMPRIS and were reimbursed from the SAMMPRIS grant for their effort. The following investigators report additional support:
Colin Derdeyn MD receives grant support from the NINDS (P50 55977; R01 NS051631). He is also on the Scientific Advisory Board for W.L Gore and Associates and is the Chair of the Scientific Advisory Board for Pulse Therapeutics.
David Fiorella MD, PhD has received institutional research support from Seimens Medical Imaging and Microvention, consulting fees from Codman/Johnson and Johnson, NFocus, W.L. Gore and Associates, and EV3/Covidien, and royalties from Codman/Johnson and Johnson. He has received honoraria from Scientia and has ownership interest in CVSL and Vascular Simulations.
Michael J. Lynn, MS receives grant support from the National Eye Institute. He is the principal investigator of the Coordinating Center for Infant Aphakia Treatment Study (EY013287) and a co-investigator on the Core Grant for Vision Research (EY006360).
Harry J. Cloft, MD, PhD has received research support for the SAPPHIRE Carotid Stent registry.
Bethany F. Lane RN has received consulting fees from Microvention Terumo.
Tanya N. Turan, MD is a past recipient of funding from the American Academy of Neurology (AAN) Foundation Clinical Research Training Fellowship and is the current recipient of a K23 grant from NIH/NINDS (1 K23 NS069668-01A1). She has also served as an expert witness in medical legal cases.
Scott Janis PhD is a program director at the National Institute of Neurological Disorders and Stroke.
Marc Chimowitz, MBChB has received research grants from NINDS to fund the WASID trial (1 R01 NS36643) and to fund other research on intracranial stenosis (1 K24 NS050307 and 1 R01 NS051688). He currently serves on the stroke adjudication committee of an industry funded osteoporosis drug trial (Merck and Co., Inc.) and on the DSMB of another industry funded patent foramen ovale closure trial (W.L Gore and Associates) and is compensated for those activities. He has also served as an expert witness in medical legal cases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental content: SAMMPRIS Trial Investigators
The authors present a very straightforward analysis of the data acquired for the SAMMPRIS trial. Primarily a descriptive study, this analysis describes with some additional detail the potential etiologies for periprocedural complications in angioplasty and stenting. Interestingly, as previously described, the authors suggest that posterior circulation (in particular, basilar artery) procedures carry the majority of complication risk secondary to vessel perforation, perforator obstruction and hemorrhagic conversion. Thus, this retrospective analysis reiterates the significant risks incurred in posterior circulation atheromatous disease and emphasizes the need for proper patient education with a realistic discussion of risks and benefits to these patients and families. Unfortunately, there is no clear discussion as to potential modifications that should or could be made in techniques or in devices.
The authors provide an excellent platform from which to begin the “deep dive” in data analysis of not only their own practice data, but for future studies that will help practitioners in this field better understand patient selection and complication avoidance for patients with intracranial (specifically posterior circulation) atheromatous disease refractory to medical management.
Charles J. Prestigiacomo, Newark, New Jersey
References
- 1.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiorella D, Derdeyn CP, Lynn MJ, et al. Detailed Analysis of Periprocedural Strokes in Patients Undergoing Intracranial Stenting in Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Stroke. 2012 doi: 10.1161/STROKEAHA.112.661173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derdeyn CP, Fiorella D, Lynn MJ, et al. Impact of operator and site experience on outcomes after angioplasty and stenting in the SAMMPRIS trial. Journal of NeuroInterventional Surgery. 2012 doi: 10.1136/neurintsurg-2012-010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chimowitz MI, Lynn MJ, Turan TN, et al. Design of the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis trial. J Stroke Cerebrovasc Dis. 2011;20:357–368. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turan TN, Lynn MJ, Nizam A, et al. Rationale, Design, and Implementation of Aggressive Risk Factor Management in the Stenting and Aggressive Medical Management for Prevention of Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Trial. Circulation Cardiovascular quality and outcomes. 2012;5:e51–e60. doi: 10.1161/CIRCOUTCOMES.112.966911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahab F, Lynn MJ, Kasner SE, et al. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology. 2009;72:2014–2019. doi: 10.1212/01.wnl.0b013e3181a1863c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang WJ, Du B, Hon SFK, et al. Do patients with basilar or vertebral artery stenosis have a higher stroke incidence poststenting? journal of NeuroInterventional Surgery. 2010;2:50–54. doi: 10.1136/jnis.2009.000356. [DOI] [PubMed] [Google Scholar]
- 8.Gröschel K, Schnaudigel S, Pilgram SM, Wasser K, Kastrup A. A Systematic Review on Outcome After Stenting for Intracranial Atherosclerosis. Stroke. 2009;40:e340–e347. doi: 10.1161/STROKEAHA.108.532713. [DOI] [PubMed] [Google Scholar]
- 9.Jiang WJ, Srivastava T, Gao F, Du B, Dong KH, Xu XT. Perforator stroke after elective stenting of symptomatic intracranial stenosis. Neurology. 2006;66:1868–1872. doi: 10.1212/01.wnl.0000219744.06992.bb. [DOI] [PubMed] [Google Scholar]
- 10.Jiang W-J, Du B, Hon SFK, et al. Do patients with basilar or vertebral artery stenosis have a higher stroke incidence poststenting? Journal of NeuroInterventional Surgery. 2010;2:50–54. doi: 10.1136/jnis.2009.000356. [DOI] [PubMed] [Google Scholar]
- 11.Levy EI, Hanel RA, Boulos AS, et al. Comparison of periprocedure complications resulting from direct stent placement compared with those due to conventional and staged stent placement in the basilar artery. Journal of Neurosurgery. 2003;99:653–660. doi: 10.3171/jns.2003.99.4.0653. [DOI] [PubMed] [Google Scholar]
- 12.Vergouwen MD, Silver FL, Mandell DM, Mikulis DJ, Krings T, Swartz RH. Fibrous cap enhancement in symptomatic atherosclerotic basilar artery stenosis. Archives of neurology. 2011;68:676. doi: 10.1001/archneurol.2011.89. [DOI] [PubMed] [Google Scholar]
- 13.Shi M, Wang S, Zhou H, Cheng Y, Feng J, Wu J. Wingspan stenting of symptomatic middle cerebral artery stenosis and perioperative evaluation using high-resolution 3 Tesla MRI. Journal of Clinical Neuroscience. 2012;19:912–914. doi: 10.1016/j.jocn.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 14.Fiorella D, Levy EI, Turk AS, et al. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007;38:881–887. doi: 10.1161/01.STR.0000257963.65728.e8. [DOI] [PubMed] [Google Scholar]
- 15.Zaidat OO, Klucznik R, Alexander MJ, et al. The NIH registry on use of the Wingspan stent for symptomatic 70–99% intracranial arterial stenosis. Neurology. 2008;70:1518–1524. doi: 10.1212/01.wnl.0000306308.08229.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Mook WNKA, Rennenberg RJMW, Schurink GW, et al. Cerebral hyperperfusion syndrome. The Lancet Neurology. 2005;4:877–888. doi: 10.1016/S1474-4422(05)70251-9. [DOI] [PubMed] [Google Scholar]
- 17.Ogasawara K, Sakai N, Kuroiwa T, et al. Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg. 2007;107:1130–1136. doi: 10.3171/JNS-07/12/1130. [DOI] [PubMed] [Google Scholar]
- 18.Derdeyn CP. Diffusion-weighted imaging as a surrogate marker for stroke as a complication of cerebrovascular procedures and devices. AJNR Am J Neuroradiol. 2001;22:1234–1235. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.