Abstract
Recent studies suggest that DAF, a complement regulatory protein, present in lipid rafts, is utilized by Dr fimbriated E. coli for their binding and internalization. Previous studies in our laboratory have shown that nitric oxide (NO) can reduce the invasion of Dr (+) E. coli and the severity of uterine infection in pregnant rats. Also, the expression level of DAF both at the mRNA and protein levels has been shown to be reduced by NO. Therefore NO mediated down regulation of DAF appears to be an important factor in reducing the susceptibility to E. coli infection. However, it is unclear if NO can actually modulate the membrane association of DAF and therefore initial bacterial binding to cells. Here we show that NO induces the delocalization of DAF from the GM1 rich lipid rafts. Using biochemical and cell biological approaches in a uterine epithelial cell model (Ishikawa cells) we show that upon exposure to NO, DAF accumulates in caveolae. We also show that interaction of DAF with the caveolar protein, caveolin1 leads to their internalization in to endosomes. The NO induced delocalization of DAF from the lipid raft and its accumulation in caveolae are mediated through a cGMP pathway. The acute localized synthesis of NO and its influence on DAF localization may represent an important unrecognized phenomenon of host defense against Dr (+) E. coli bacteria, as well as many disease conditions that involve complement system.
Keywords: DAF, Nitric Oxide, Lipid Raft, Caveolae, Ishikawa cells
1. Introduction
In the plasma membrane, lipids particularly glycosphingolipids containing saturated hydrocarbon tails and cholesterol along with some proteins cluster together in distinct membrane domains or lipid rafts. Lipid rafts are in liquid ordered phase because of their tightly packed domains due to the saturated acyl chains of sphingolipids but have a high degree of lateral mobility because of the presence of cholesterol. Lipids and proteins enter and exit the lipid rafts in a few nanosecond time scale. Most of the proteins in the lipid rafts are appended to the membrane through a glycosylphosphatidylinositol (GPI)-anchor. These GPI-anchored proteins are involved in vital cellular processes like membrane trafficking, protein sorting and signal transduction (Varma and Mayor, 1998). Caveolae, flask shaped invaginations of the plasma membrane are a distinct subset of lipid rafts and differ from the lipid raft in having oligomerized hairpin like caveolin proteins. Unlike plasma membrane, lipid rafts are insoluble in cold non-ionic detergents, mainly Triton X-100; therefore, they are also described as Detergent Resistant Membranes (DRM) (Brown and Rose, 1992). It is suggested that lipid raft domains might play a crucial role in cellular processes by facilitating and coordinating close interactions between key molecules involved in cellular processes (Viola and Gupta, 2007).
Decay Accelerating Factor (DAF) is a lipid raft associated, glycosylinositol-anchored complement regulatory membrane protein which is constitutively expressed at high levels on all serum exposed cells including epithelial cells. DAF protects the cells from autologous complement-mediated injury by accelerating the dissociation of preformed C3/C5 convertase complexes of both the classical and alternative pathways of the complement system (Thomas and Lublin, 1993). Deficiency of DAF is linked to many disease conditions like nocturnal hemoglobinuria, autoimmune diseases and pregnancy loss due to luteal phase defect (Kaul et al., 1995;Parker, 1996). In contrast, higher level of DAF expression is associated with the malignancy of colon and endometrial cancer cells (Murray et al., 2000;Niehans et al., 1996). Apart from complement function, DAF also serves as receptor for many bacterial and viral pathogens, especially Dr-fimbriated Escherichia coli which cause urinary tract infections in humans (Goluszko et al., 1999). It has been shown that many ligands including bacteria get internalized through caveolae after binding to lipid raft associated receptors in the form of caveosomes and thus bypass the endosome pathway that ultimately leads to their degradation in lysosomes (Parton and Richards, 2003).
Nitric oxide (NO) is a gaseous molecular mediator which is produced in the process of enzymatic oxidation of L-arginine to L-citruline. Several studies have indicated that NO may interfere with lipid rafts by increasing the distance between molecules clustered in rafts or by dissociating raft constituents from the cellular cytoskeleton (Li et al., 2001;Oliferenko et al., 1999). The findings, expression of DAF is reduced by NO (Fang et al., 2004) disruption of lipid raft by the depletion of cholesterol significantly restricts the invasion of E.coli (Selvarangan et al., 2000), NO production is elevated during pregnancy and suppression of NO production leads to an increase in the severity of uterine infection (Nowicki et al., 1997), all suggest the existence of a unified mechanism of host defense mediated through NO. However, it is not clear whether actually NO alters the localization of DAF in the plasma membrane lipid raft and thus prevents the initial binding of E.coli to the cells. We postulated that NO plays an important role in separating DAF, the epithelial receptor, away from the lipid raft. To test this hypothesis we utilized several biochemical and cell biological approaches such as isolation of DRM, Proximity Ligation Assay (Gustafsdottir et al., 2005), Density gradient ultracentrifugation ect., in NO donor exposed uterine epithelial cell model (Ishikawa cells) which expresses DAF and susceptible to invasion of E. coli associated with gestational infections (Fang et al., 2004).
2. Materials and Methods
2.1. Cell culture
Ishikawa cells are a human endometrial cell line derived from a well differentiated endometrial adenocarcinoma that has been shown to mimic endometrial epithelial cells (Nishida et al., 1985). Ishikawa cells were routinely cultured in Eagle's minimum essential medium (MEM) containing 2 mM L-glutamine, 100 μg of penicillin-streptomycin/ml, and 10 mM HEPES (Invitrogen, Carlsbad, CA, U.S.A) supplemented with 10% fetal calf serum (FBS) (Gemini Bioproducts, West Sacramento, CA, U.S.A) at 37°C in a humidified 5% CO2 atmosphere.
2.2. Proximity ligation assay
Ishikawa cells sparsely grown on 16-well Lab-Tek chamber slide (Electron Microscopy Sciences, Hatfield, PA, U.S.A) were treated with NO donor DETANONOate (DTNO) (Cayman Chemicals, Ann Arbor, MI, U.S.A) at a concentration of 1mM in minimum essential medium free of L-arginine and phenol red (Atlanta Biologicals, Lawrenceville, GA, U.S.A) supplemented with 5% FBS for different time intervals. Ishikawa cells were also treated with 0.3 μM ODQ (Cayman Chemicals) and 0.1 mM 8-bromo cGMP (Sigma-Aldrich, St. Louis, MO, U.S.A) for 2 hours. The cells were then washed with PBS and fixed using 4% paraformaldehyde at room temperature for 5 minutes or permeabilized using 0.2% Tween-20 in PBS containing 1% BSA and then fixed. Fixed cells were incubated with primary antibodies for DAF (Clone IH4, courtesy of D. M. Lublin, Washington University School of Medicine, Seattle, Wash, U.S.A), Caveolin 1(rabbit polyclonal, cat No. sc-894, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A), and Ganglioside GM1 (rabbit polyclonal, cat No. ab23943, Abcam, Cambridge, MA, U.S.A) diluted 1:500 in blocking solution for 90 minutes at 37°C in a humid chamber. A negative control was prepared by incubating the cells in blocking solution without any of these primary antibodies. Proximate ligation assay was performed using Duolink II Fluorescence kit (Olink Biosciences, Uppsala, Sweden) according to the manufacturer's instructions. Briefly, after the incubation with primary antibodies, cells were washed with buffer A (0.01 M Tris, 0.15 M NaCl, and 0.05% Tween-20, pH 7.4) for 2 × 10 minutes and then incubated with rabbit plus and mouse minus PLA probes (1:5 diluted in antibody diluents) for 60 minutes at 37°C. The total reaction mixture in all the steps was 30 μl/well. Cells were again washed with buffer A for 2 × 5 minutes and incubated with ligation mixture (1:5 diluted in molecular grade water) for 30 minutes at 37°C. Cells were washed with buffer A for 2 × 2 minutes and incubated with amplification mixture (1:5 diluted in molecular grade water) for 100 minutes at 37°C. Cells were then washed twice with buffer B (0.2 M Tris, 0.1 M NaCl, pH 7.5) for 10 minutes each and rinsed with 0.01X buffer B, allowed to dry at room temperature in the dark. Slides were then mounted with cover slips using duolink II mounting medium and the images were observed under fluorescence microscope (Olympus, U-TV1 X) under 20X objective. Images were processed and red spots were counted using Image-Pro Plus software (MediaCybernetics, Silver Spring, MD, U.S.A).
2.3. Isolation of Detergent Resistant Membrane (DRM) fraction and Thin-layer chromatography for (TLC) ganglioside GM1
Confluent Ishikawa cells, were treated with 1 mM DETANONOate in minimum essential medium free of L-arginine and phenol red supplemented with 5% FBS for 2 hours. Cells were then washed with cold PBS, scraped and pelleted by centrifugation at 300 × g for 5 minutes at 4°C. Pellets were resuspended in 25 mM tris buffer (pH 7.4) containing 150 mM NaCl, 5 mM EDTA, 2% Triton-X 100, and Protease inhibitor cocktail (10 μl/ml, Sigma-Aldrich), kept on ice for 30 minutes and then centrifuged at 12,000 × g for 10 minutes at 4°C. Resulting cell lysates were then mixed with Optiprep density gradient solution (60% stock solution in sterile water, Sigma-Aldrich) in a centrifuge tube to achieve a final Optiprep concentration of 45%. This was sequentially overlaid by 35% and 5% Optiprep in lysis buffer and centrifuged at 150,000 × g for 3 hours at 4°C using SW55Ti rotor (Beckman-Coulter, Fullerton, CA). Six fractions were collected from top to bottom. The distribution of DAF, Caveolin1 and Transferrin receptor in different fractions were analyzed by western blot using monoclonal anti DAF, rabbit polyclonal anti caveolin1(1:1000) and monoclonal anti transferrin receptor (mouse monoclonal, cat No. 13-6800, Invitrogen,1:1000) antibodies. The TLC for ganglioside GM1 was performed as reported (Mutoh et al., 1995) with some modifications. Briefly, the total lipids from the density gradient fractions were extracted with chloroform and methanol, 2:1 (vol/vol). Samples obtained (10 μl) were subjected to ascending thin-layer chromatography developed in chloroform/methanol/0.02% CaCl2, 55:45:10 (vol/vol) on TLC Silica gel 60 plates (EMD biosciences, San Diego, CA, U.S.A). The identification of GM1 was accomplished by using Alexa fluor-488 conjugated cholera toxin B subunit (Molecular probes, Eugen, OR, U.S.A).
2.4. Separation of cellular membranes
Ishikawa cells grown on 100 mm dishes were treated with DETANONOate at a concentration of 1 mM in minimum essential medium free of L-arginine and phenol red supplemented with 5% FBS for 2 hours. Cells were then washed once with cold PBS. One ml of TNE buffer (25 mM tris-HCl, pH7.4, 150 mM NaCl, 5 mM EDTA, and 5 mM DTT) was added and cells were scraped using a rubber policeman. Contents were transferred to microcentrifuge tubes, centrifuged at 300 × g for 3 minutes at 4°C and the supernatant discarded. Pellets were resuspended in 1.5 ml of TNE buffer and sonicated at 4°C with output and cycle set at 30% allowing 10 seconds between each 10 pulses. Each sonicated cell lysate was passed through a 23-gage syringe needle (3 ml maximum volume) ten times. The homogenates were centrifuged at 3000 × g for 15 minutes at 4°C. Each resulting supernatant, which consists of total cellular membranes and cytosol was ultracentrifuged at 200,000 × g for 45 minutes. Each resulting pellet which contains total cellular membranes was resuspended with 400 μl TNE buffer by passing through 23-gage syringe needle. Resulting suspension was overlaid on 10-30% Optiprep gradient prepared according to the manufacturer's instructions (axis-shield, Rodelokka, Oslo, Norway) and ultracentrifuged at 100,000 × g for 16 hours. Twenty fractions (245 μl each) were collected from top to bottom and the distribution of DAF, Caveolin1 and membrane markers were analyzed by western blot. The primary antibodies used for membrane marker proteins were Rab 5 (C8B1) for early endosomes (Rabbit monoclonal, Cat No. 3547, Cell Signaling Technology, Danvers, MA, U.S.A), Syntaxin 6 (C34B2) for trans-Golgi (Rabbit monoclonal, Cat No. 2869, Cell Signaling Technology) and Akt for plasma membrane (Rabbit polyclonal, Cat No. 9272, Cell Signaling Technology).
2.5. Western blot analysis
Protein concentrations in the six fractions were determined by Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL, U.S.A), against BSA standards. Proteins (5 μg) were separated by polyacrylamide gel electrophoresis. Electrophoresis was carried out under reducing conditions except DAF for which a non-reducing condition was employed to preserve the epitope structure required for the binding of DAF monoclonal antibody used in this study. After electrophoresis, proteins were electro transferred to PVDF membranes. Membranes were blocked with TBST (20 mM Tris, 500 mM NaCl, 1% Tween-20, pH 7.5) containing 5% fat-free milk for 1 hour at room temperature and incubated with the primary antibodies overnight at 4°C. Blots were then washed with TBST and incubated with horseradish-peroxidase conjugated secondary antibodies, goat antimouse antibody or goat antirabbit antibody for 1 hour at room temperature. The bound antibody was visualized on a blue sensitive autoradiography film using either supersignal west pico chemiluminiscence substrate (Pierce Biotechnology, Rockford, IL, U.S.A) or ECL plus western blotting detection system (GE Healthcare, Little Chalfont, UK) according to the instructions provided by the manufacturer.
2.6. Statistical analysis
GraphPad Prism (version 3) was used for statistical analysis. Comparisons between groups were made by ANOVA, followed by Bonferroni's multiple comparison tests. P value of <0.05 was considered to represent a significant difference between the compared values.
3. Results
3.1. DAF and caveolin1 accumulate in DRM after NO exposure
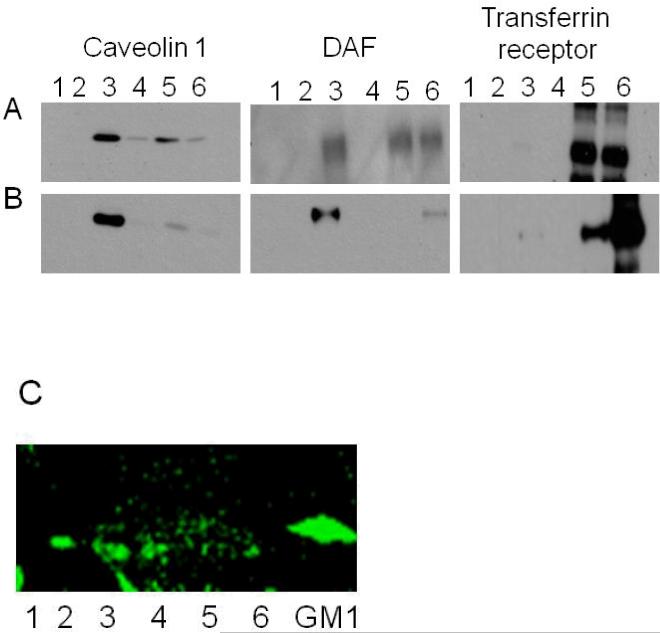
To examine the effect of acute release of nitric oxide on the localization of DAF within lipid rafts, we isolated the DRM from control as well as NO donor exposed cells. It has been well documented that detergent insoluble lipid rafts float to a low density during density gradient centrifugation. We fractionated the cell homogenates prepared in Triton-X 100 containing lysis buffer by equilibrium density gradient centrifugation on an OptiPrep density gradient. Previous studies have shown that in OptiPrep density gradient, lipid rafts migrate to the interface between 5 and 30% layers and in this study this corresponds to fraction 3. We confirmed the distribution of lipid raft in to fraction 3 by TLC of lipids extracted from the fractions. A major band in fraction 3 corresponding to ganglioside GM1 was identified after probing the TLC plate with ganglioside GM1 binding protein cholera toxin B subunit conjugated to Alexa fluor-488 (Fig. 1A). The density gradient fractionation of both control and NO exposed cell homogenates gave flocculent band at the interface between 5 and 30% OptiPrep. This indicates that lipid rafts are intact even after the exposure of the cells to NO. Under basal conditions, Caveolin1 was found predominantly in fraction 3 but was distributed to other high density fractions also (Fig. 1B). We believe that the distribution of caveolin1 in to high density fractions is due to their association with cytoskeleton because the association of lipid raft proteins with the cellular cytoskeleton is well established (Patschan et al., 2006). Upon NO treatment most of the caveolin1 migrated to fraction 3 probably due to their dissociation from cytoskeleton (Fig. 1C). DAF also showed a similar pattern (Figs. 1B,1C) suggesting accumulation of both DAF and caveolin1 in detergent resistant membrane domain. Transferrin receptor, a membrane protein was used as negative control in this experiment to show the specificity of NO action. Results presented here indicate that lipid rafts remain intact after the exposure to NO but NO enhances the mobility of rafts by liberating them from cytoskeleton and DAF containing lipid rafts appear to accumulate in caveolae.
Fig. 1. DAF and Caveolin1 accumulate in Detergent Resistant Membrane fraction (DRM) after NO exposure.
Ishikawa cells grown to 80% confluency were treated with 1 mM DETANONOate for 2 hours. Representative western blots of (A) non-treated cells and (B) cells exposed to DETANONOate. Cell lysates were prepared in lysis buffer containing 2% Triton-X 100, overlaid with OptiPrep density gradient and fractionated by ultracentrifugation. Six fractions were collected from top to bottom. DRM float to fraction 3 after centrifugation. (C) Thin layer chromatography of six fractions probed with Alexa fluor-488 conjugated cholera toxin-B subunit to visualize the distribution of lipid raft marker ganglioside GM1. Bands corresponding to the positive control GM1, occur in fractions 2, 3 and 4 with major band associated with fraction 3.
3.2. Nitric oxide induces the separation of DAF from Ganglioside GM1 rich lipid rafts and its accumulation in caveolae
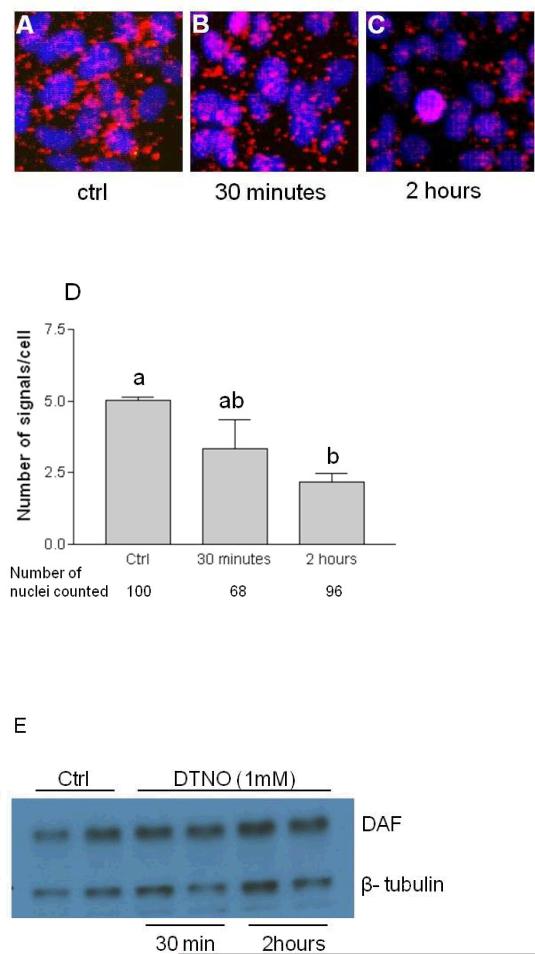
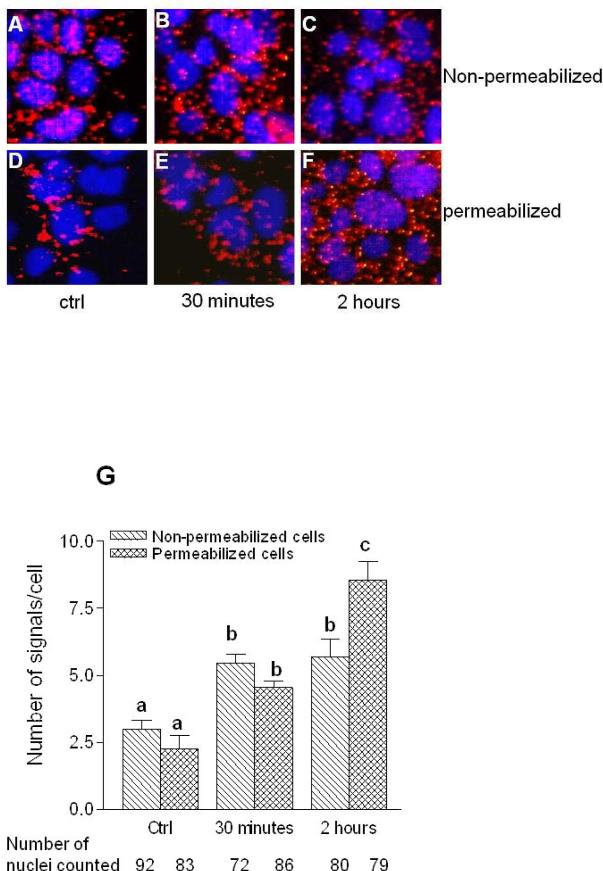
In order to determine whether lipid raft as a whole migrate to caveolae or DAF alone depart the lipid raft and accumulate in caveolae we studied the proximity between DAF, GM1 and caveolin1 proteins using PCR based proximate ligation assay (PLA). First, we validated the PLA assay using primary antibodies against DAF, GM1 and caveolin1. The associations between any two of these proteins produced fluorescent red spots. When the primary antibodies were omitted in the assay red spots were not produced indicating the specificity of the assay (Fig. 2). The number of DAF and GM1 molecules which were in close association were decreased by the exposure of the cells to NO donor as revealed by the number of dots per cell in PLA assay (Figs. 3A-C) indicating separation of DAF from GM1. This separation of DAF from GM1 started as early as 30 minutes after the exposure to NO but significant reduction (p<0.05 compared to control) was observed after 2 hours of exposure (Fig. 3D). The expression of DAF remained unchanged during this time course (Fig. 3E) suggesting that reduced association between DAF and GM1 is not due to less number of DAF molecules but due to its migration out of the lipid raft. On the other hand the proximity between DAF and caveolin1 was increased by NO (Figs. 4A-C) indicating an enhanced interaction between DAF and caveolin1. To further examine whether the increased interaction between DAF and caveolin1 results in their internalization, we performed PLA assay in cells which were either non permeable for primary antibodies or permeabilized by treating with the detergent Tween-20. When the PLA assay was performed in cells which were not detergent permeabilized for primary antibodies a 1.9 fold increase in signals per cell was observed within 30 minutes and no further increase occurred up to 2 hours. In permeabilized cells signals per cell increased significantly within 30 minutes, continued to increase and reached 2.3 fold more compared to control (Fig. 4G) after 2 hours. Overall these results indicate that NO induced an acute accumulation of DAF in caveolae on the plasm membrane. But the accumulation of DAF in caveolae appears to reach equilibrium as there was no further accumulation with longer exposure time. However, signals per cell in permeabilized cells which represent cytoplasmic surface as well as organelle associated DAF and caveolin1 interactions increased steadily in a time dependent manner. Results presented here show that when stimulated by NO, DAF migrates out of the GM1 lipid rafts, interacts with caveolin1 and enters the cytoplasmic phase of the plasma membrane.
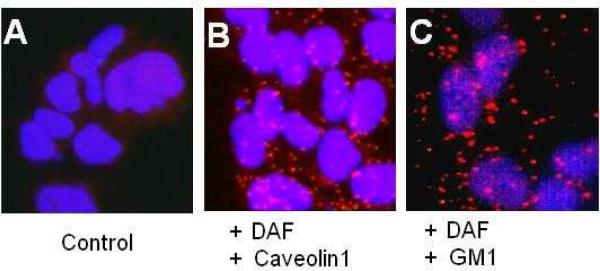
Fig. 2. The proximity Ligation Assay produces red fluorescent spots if two proteins are in close association.
Ishikawa cells were sparsely grown on 16-well Lab-Tek chamber slide for 48 h and then subjected to proximate ligation assay. A) PLA assay without primary antibodies B) PLA assay with primary antibodies against DAF and caveolin1 C) PLA assay with primary antibodies against DAF and ganglioside GM1.
Fig. 3. The proximity between DAF and Ganglioside GM1 decreases after NO exposure.
Ishikawa cells were sparsely grown on 16-well Lab-Tek chamber slide for 48 h and then treated with 1 mM DETANONOate for different time periods. Representative images of Proximity ligation assay for DAF and GM1 in paraformaldehyde fixed non-permeabilized cells. (A) non-exposed cells (B) exposed to DETANONOate for 30 minutes (C) exposed to DETANONOate for 2 h. (D) Number of signals per cell for DAF and GM1 proximity are plotted. Number of signals per cell decreased significantly after 2 h exposure to NO (p<0.05 compared to control). (E) Western blot of control and DETANONOate exposed cell lysates probed with anti-CD55 antibody and anti β-tublin antibody as loading control. Data shown are the means ± S.E.M. of six independent experiments. Different letters above each column in the bar graph indicate significant differences. Statistical analysis was done by one-way ANOVA with Bonferroni's multiple comparison test.
Fig. 4. The proximity between DAF and Caveolin1 increases after NO exposure.
Ishikawa cells were sparsely grown on 16-well Lab-Tek chamber slide for 48 h and then treated with 1 mM DETANONOate for different time periods. Representative images of Proximity ligation assay for DAF and Caveolin1 performed before (upper panel, A-C) and after (lower panel, D-F) permeabilizing the paraformaldehyde fixed cells. Cells were either not exposed (A and D) or exposed to DETANONOate for 30 minutes (B and E) or exposed to DETANONOate for 2 h (C and F). (G) Number of signals per cell in control and NO exposed cells are plotted. Number of signals per cell in non-permeabilized cells increased significantly within 30 minutes (p<0.05 versus control) and remained same after 2 h exposure to NO (p<0.01versus control). Number of signals per cell in permeabilized cells increased significantly within 30 minutes (p<0.05 versus control) and then further increased after 2 h exposure to NO (p<0.01 versus 30 minutes exposure and p<0.001 versus control). Data shown are the means ± S.E.M. of six independent experiments. Different letters above each column in bar graph indicate significant differences. Statistical analysis was done by one-way ANOVA with Bonferroni's multiple comparison test.
3.3. DAF-Caveolin1 interaction leads to their internalization to early endosomes
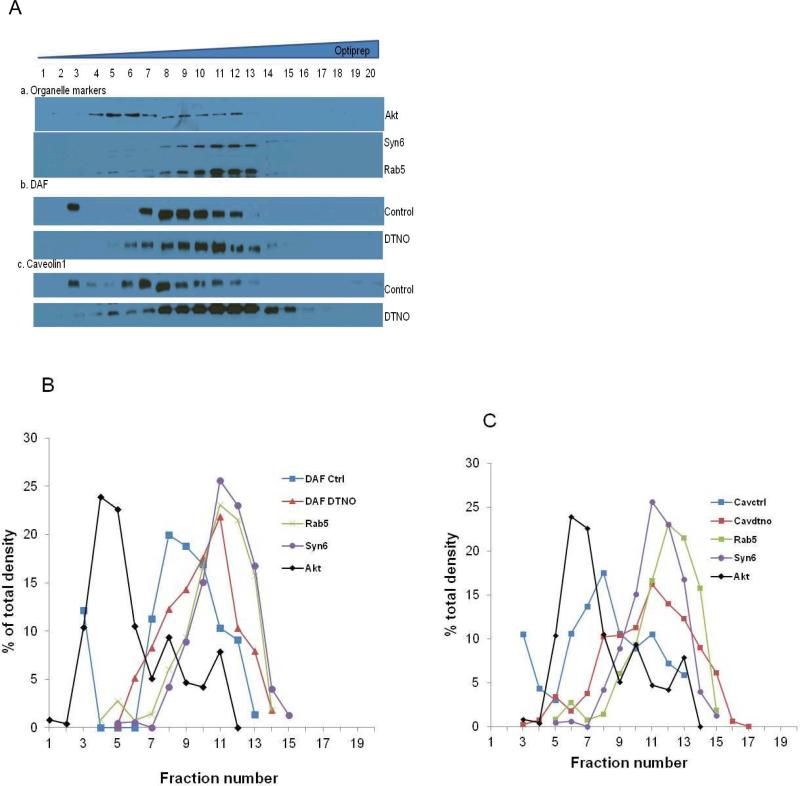
To further study the fate of DAF in the cytoplasmic phase we isolated the total membranes from cell lysates and subjected them to ultracentrifugation on OptiPrep density gradient. The western blot of 20 fractions obtained from the density gradient revealed an altered distribution of DAF and Caveolin1 in the lysates from the NO exposed cells (Fig. 5A). In control cells DAF and Caveolin1 were concentrated in the lighter fractions (5 to 9) at the top of the gradient (Figs. 5B, 5C). These fractions were also abundant in Akt, a plasma membrane marker. When the cells were exposed to NO for 2 hours both DAF and Caveolin1 peaks shifted to the right towards the heavier fractions (10 to 14) on the gradient. These fractions were also rich in Rab5, an early endosome marker and Syntaxin6, a marker for endosomes as well as trans Golgi network. The data presented here support the hypothesis that NO stimulates the caveolin dependent internalization of DAF into endosome like vesicles which ultimately fuses with trans Golgi network.
Fig. 5. DAF and caveolin1 accumulate in endosomes after NO exposure.
Ishikawa cells grown to 80% confluency were exposed to 1 mM DETANONOate for 2 h before cell harvest. Total cellular membranes were prepared by the ultracentrifugation of the cell lysate at 200,000 × g for 45 minutes. The cellular membranes were then separated by ultracentrifugation on 10 to 30% Optiprep density gradient at 100,000 × g for 16 h. Twenty fractions of 245 μl each were collected from top to bottom in the order of increasing density. (A) Distribution of DAF, caveolin1 and organelle markers Akt (plasma membrane), Rab5 (early endosomes), Syntaxin6 (Golgi bodies) were analyzed by western blot. (B) Densitometric analysis of relative abundance of DAF and organelle markers in different fractions of non-treated and NO exposed cells expressed as % of total density. (C) Densitometric analysis of relative abundance of caveolin1 and organelle markers in different fractions expressed as % of total density.
3.4. cGMP mediates the NO induced accumulation of DAF in caveolae
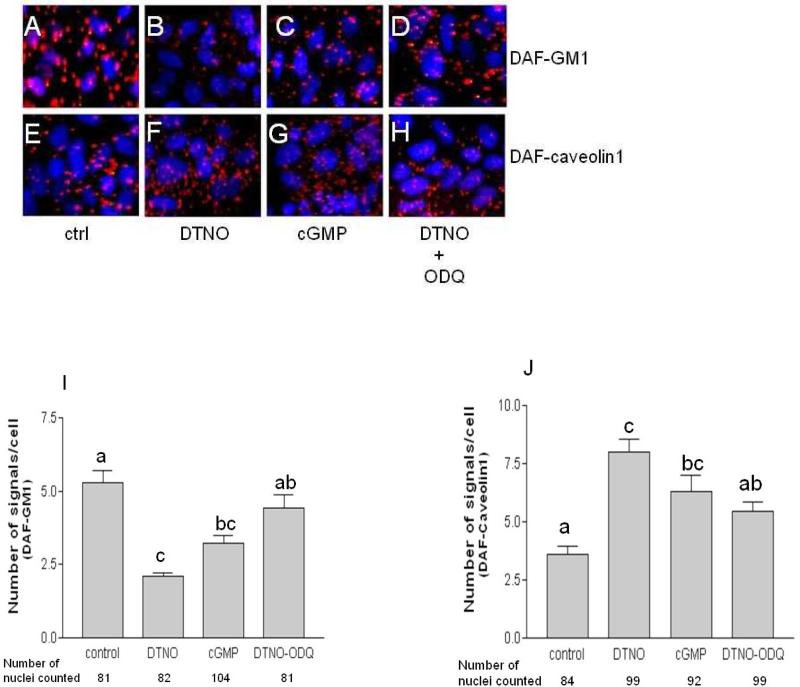
The cellular events regulated by NO occur in cGMP dependent pathways and cGMP independent pathways like nitration and S-nitrosylation. To assess whether migration of DAF is mediated by cGMP we used pharmacological inhibitor of soluble guanylyl cyclase, ODQ and cGMP analogue 8-bromo cGMP. Like DETANONOate, the 8-bromo cGMP reduced the number of signals per cell in PLA assay for DAF and GM1 (Figs. 6A-D) significantly compared to control cells (Fig. 6I). Consistent with this observation, the effect of DETANONOate was reversed by ODQ. On the other hand, the NO induced increase in number of signals per cell for DAF and Caveolin1 association was imitated by 8-bromo cGMP and reversed by ODQ (Figs. 6E-H and J). The results clearly show that the NO stimulated dissociation of DAF from GM1 lipid rafts and its accumulation in caveolae occur in a cGMP dependent manner.
Fig. 6. The NO induced changes in proximity between DAF-GM1 and DAF-caveolin1 is mediated through cGMP.
Ishikawa cells were sparsely grown on 16-well Lab-Tek chamber slide for 48 h and then treated with 1 mM DETANONOate, 0.1 mM 8brcGMP and 0.3 μM ODQ for 2 h. Representative images of Proximity ligation assay for DAF and GM1 (A-D), DAF and Caveolin1 (E-H). Cells were either not exposed (A and E) or exposed to DETANONOate (B and F) or exposed to 8brcGMP (C and G) or exposed to ODQ (D and H). (I) Number of signals per cell for DAF and GM1 proximity in control and treated cells are plotted. Signals per cell decreased by DETANONOate and cGMP treatment (p<0.001 and p<0.01 respectively compared to control) and reversed by ODQ treatment (p<0.001 compared to DETANONOate treated cells). (J) Number of signals per cell for DAF and caveolin1 proximity in control and treated cells are plotted. Signals per cell increased by DETANONOate and cGMP treatment (p<0.001 and p<0.05 respectively compared to control) and reversed by ODQ treatment (p<0.05 compared to DETANONOate treated cells). Data shown are the means ± S.E.M. of six independent experiments. Different letters on each column in bar graphs indicate significant difference. Statistical analysis was done by one-way ANOVA with Bonferroni's multiple comparison test.
4. Discussion
We investigated the effect of NO on the localization of DAF in lipid rafts in human endometrial cell line. Upon exposure of cells to NO, lipid rafts remain intact but DAF and caveolin1 were redistributed and accumulated in detergent resistant domain of the cell membrane. The co migration of DAF and caveolin1 in to DRM suggests the accumulation of DAF in caveolae which was further evidenced by PLA assay. The PLA assay is a PCR based immuno-technique in which the proximity or the interaction between two proteins is assessed based on the circularization of two oligonucleotide probes attached to secondary antibodies. The probes circularize only if two proteins are in close association. Rolling circle amplification produces concatemers which are then detected through hybridization of fluorescence-labeled small complementary oligonucleotides (Gustafsdottir et al., 2005). The PLA assay revealed that the association between DAF and GM1 decreased whereas association between DAF and caveolin1 increased upon NO exposure in a time dependent manner. It also revealed that the association between DAF and caveolin1 in the entire cell as opposed to membrane associated increase by NO in a time dependent manner suggesting the internalization of DAF-Caveolin1 complex. This was further substantiated by the density gradient separation of cellular membranes that revealed the accumulation of DAF and Caveolin1 in endosome-Golgi rich fractions of the lysate from the NO exposed cells. Overall, the results demonstrate that NO alters the localization of DAF and its availability on the cell surface.
Caveolae and lipid rafts have been shown to be involved in a specialized internalization pathway of many ligands and pathogens like cholera toxin, SV40, E.coli ,Chlamydia trachomatis as well as many GPI-anchored proteins (Nichols, 2002;Norkin et al., 2001;Shin et al., 2000). This pathway is involved in plasma membrane to Golgi trafficking and is distinctly different from internalization through clathrin-coated pits, and thus putatively linked to the plasma membrane remodeling. Specialized membrane vesicles named as caveosomes are proposed to be involved in caveolae mediated endocytosis. The internalization of receptors and ligands through caveolae requires a trigger in the form of a ligand binding or antibody mediated cross-linking of the receptors or activation of a phosphorylation cascade (Pelkmans and Helenius, 2002). To the best of our knowledge the data presented here report for the first time NO as a novel trigger for the caveolae mediated internalization.
We previously reported that nitric oxide reduces the severity of uterine Dr(+)E. coli infection in pregnant rats and that the mechanism is the reduced bacterial invasion due to the down regulation of DAF expression (Fang et al., 2004;Nowicki et al., 1997). The significant reduction in DAF protein occurs 24 hours after the NO exposure. Several in vitro and in vivo studies have indicated that adhesion of E. coli to the endometrial epithelial cells occurs immediately after the contact and reaches saturation in about 60(Nishikawa, 1985;Nishikawa and Baba, 1985). In this context, rapid retrieval of preexisting DAF from the cell surface to prevent the initial binding of E. coli could be an additional, immediate host response against Dr fimbriated E. coli. It is also of interest to study whether NO induced internalization of DAF makes the infected cells susceptible to complement attack in order to clear the infection. DAF being a receptor for several other microorganisms including viral pathogens and a key regulator of complement system, the present findings add a wealth of knowledge in understanding and intervention of many diseases.
Acknowledgements
We gratefully acknowledge the grant support from NIH [HL72620], [HD57013] and [HL58144] to C. Yallampalli.
Abbreviations
- DETANONOate
(Z)-1-[N-(2-aminoethyl)-N-(2-aminoethyl)amino]diazen-1-ium-1,2-diolate
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
Reference List
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Fang L, Nowicki BJ, Urvil P, Goluszko P, Nowicki S, Young SL, Yallampalli C. Epithelial invasion by Escherichia coli bearing Dr fimbriae is controlled by nitric oxide-regulated expression of CD55. Infect Immun. 2004;72:2907–2914. doi: 10.1128/IAI.72.5.2907-2914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goluszko P, Selvarangan R, Popov V, Pham T, Wen JW, Singhal J. Decay-accelerating factor and cytoskeleton redistribution pattern in HeLa cells infected with recombinant Escherichia coli strains expressing Dr family of adhesins. Infect Immun. 1999;67:3989–3997. doi: 10.1128/iai.67.8.3989-3997.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsdottir SM, Schallmeiner E, Fredriksson S, Gullberg M, Soderberg O, Jarvius M, et al. Proximity ligation assays for sensitive and specific protein analyses. Anal Biochem. 2005;345:2–9. doi: 10.1016/j.ab.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Kaul A, Nagamani M, Nowicki B. Decreased expression of endometrial decay accelerating factor (DAF), a complement regulatory protein, in patients with luteal phase defect. Am J Reprod Immunol. 1995;34:236–240. doi: 10.1111/j.1600-0897.1995.tb00947.x. [DOI] [PubMed] [Google Scholar]
- Li H, Brodsky S, Basco M, Romanov V, De Angelis DA, Goligorsky MS. Nitric oxide attenuates signal transduction: possible role in dissociating caveolin-1 scaffold. Circ Res. 2001;88:229–236. doi: 10.1161/01.res.88.2.229. [DOI] [PubMed] [Google Scholar]
- Murray KP, Mathure S, Kaul R, Khan S, Carson LF, Twiggs LB, Martens MG, Kaul A. Expression of complement regulatory proteins-CD 35, CD 46, CD 55, and CD 59-in benign and malignant endometrial tissue. Gynecol Oncol. 2000;76:176–182. doi: 10.1006/gyno.1999.5614. [DOI] [PubMed] [Google Scholar]
- Mutoh T, Tokuda A, Miyadai T, Hamaguchi M, Fujiki N. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc Natl Acad Sci U S A. 1995;92:5087–5091. doi: 10.1073/pnas.92.11.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BJ. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat Cell Biol. 2002;4:374–378. doi: 10.1038/ncb787. [DOI] [PubMed] [Google Scholar]
- Niehans GA, Cherwitz DL, Staley NA, Knapp DJ, Dalmasso AP. Human carcinomas variably express the complement inhibitory proteins CD46 (membrane cofactor protein), CD55 (decay-accelerating factor), and CD59 (protectin). Am J Pathol. 1996;149:129–142. [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Kasahara K, Kaneko M, Iwasaki H, Hayashi K. [Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors]. Nippon Sanka Fujinka Gakkai Zasshi. 1985;37:1103–1111. [PubMed] [Google Scholar]
- Nishikawa Y. Adherence of Escherichia coli in pathogenesis of endometritis and effects of estradiol examined by scanning electron microscopy. Infect Immun. 1985;47:318–321. doi: 10.1128/iai.47.1.318-321.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa Y, Baba T. In vitro adherence of Escherichia coli to endometrial epithelial cells of rats and influence of estradiol. Infect Immun. 1985;50:506–509. doi: 10.1128/iai.50.2.506-509.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin LC, Woflstrom SA, Stuart ES. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp Cell Res. 2001;266:229–238. doi: 10.1006/excr.2001.5202. [DOI] [PubMed] [Google Scholar]
- Nowkicki B, Fang L, Singhal J, Nowicki S, Yallampalli C. Lethal outcome of uterine infection in pregnant but not in nonpregnant rats and increased death rate with inhibition of nitric oxide. Am J Reprod Immunol. 1997;38:309–312. doi: 10.1111/j.1600-0897.1997.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Oliferenko S, Paiha K, Harder T, Gerke V, Schwarzler C, Schwarz H, et al. Analysis of CD44-containing lipid rafts: Recruitment of annexin II and stabilization by the actin cytoskeleton. J Cell Biol. 1999;146:843–854. doi: 10.1083/jcb.146.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CJ. Molecular basis of paroxysmal nocturnal hemoglobinuria. Stem Cells. 14:396–411. doi: 10.1002/stem.140396. [DOI] [PubMed] [Google Scholar]
- Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 1996;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. 2003. [DOI] [PubMed] [Google Scholar]
- Patshcan S, Li H, Brodsky S, Sullivan D, De Aangelis DA, Patschan D, et al. Probing lipid rafts with proximity imaging: actions of proatherogenic stimuli. Am J Physiol Heart Circ Physiol. 2006;290:H2210–H2219. doi: 10.1152/ajpheart.01112.2005. [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–320. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- Selvarangan R, Goluszko P, Popov V, Singhal J, Pham T, Lublin DM, et al. Role of decay-accelerating factor domains and anchorage in internalization of Dr-fimbriated Escherichia coli. Infect Immun. 2000;68:1391–1399. doi: 10.1128/iai.68.3.1391-1399.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHin JS, Gao Z, Abraham SN. Involvement of cellular caveolae in bacterial entry into mast cells. Science. 2000;289:785–788. doi: 10.1126/science.289.5480.785. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Lublin DM. Identification of 5′-flanking regions affecting the expression of the human decay accelerating factor gene and their role in tissue-specific expression. J Immunol. 1993;150:151–160. [PubMed] [Google Scholar]
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Viola A, Gupta N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nat Rev Immunol. 2007;7:889–896. doi: 10.1038/nri2193. [DOI] [PubMed] [Google Scholar]