Abstract
Ovarian cancer is the sixth most common cancer worldwide among women in developed countries and the most lethal of all gynecologic malignancies. There is a critical need for the introduction of targeted therapies to improve outcome. Epidemiological evidence suggests a critical role for steroid hormones in ovarian tumorigenesis. There is also increasing evidence from in vitro studies that estrogen, progestin, and androgen regulate proliferation and invasion of epithelial ovarian cancer cells. Limited clinical trials have shown modest response rates; however, they have consistently identified a small subset of patients that respond very well to endocrine therapy with few side effects. We propose that it is timely to perform additional well-designed trials that should include biomarkers of response.
Introduction
Ovarian cancer is the sixth most common cancer worldwide among women in developed countries and the most lethal of all gynecologic malignancies (Jemal et al. 2011). About 90% of primary malignant ovarian tumors are epithelial carcinomas and are further classified as serous, endometrioid, clear cell, mucinous, transitional, mixed cell, or undifferentiated based on cell morphology (Cho & Shih 2009). Recent technological advances have shed light on both the cellular and the molecular biology of ovarian cancer such that it is now widely believed that `ovarian cancer' is a general term for a group of molecularly and etiologically distinct diseases that share an anatomical location (Vaughan et al. 2011). In particular, the diverse histological types of epithelial tumors are believed to be derived from different tissues. For example, high-grade serous carcinomas are believed to arise from the ovarian surface epithelium and/or the distal fallopian tube (Bell 2005, Crum et al. 2007), whereas endometrioid and clear cell carcinomas are believed to arise from endometriotic lesions (Nezhat et al. 2008). In contrast, most mucinous tumors are believed to be metastases to the ovary from the gastrointestinal tract, including the colon, appendix, and stomach (Lee & Young 2003, Kelemen & Kobel 2011, Zaino et al. 2011).
Despite the differences in the putative tissues of origin of epithelial ovarian cancers (EOC), the presence of sex steroid hormone receptors in many of these tissues of origin for ovarian cancer (Catalano et al. 2000, Akahira et al. 2002, Wada-Hiraike et al. 2006, Horne et al. 2009, Shao et al. 2011), as well as in many malignant epithelial ovarian tumors (Rao & Slotman 1991), suggests a potential role for hormones in the origin and promotion of these diseases. However, at this point in time, detailed mechanistic studies are lacking, and models to study hormone response in vitro and in vivo are very limited. Few and often small clinical trials have not resulted in any advances in the use of endocrine treatment for ovarian cancer. In this review, we will summarize and discuss epidemiological evidence and laboratory data that collectively strongly support a critical role for hormones in ovarian cancer development and progression, focusing on the steroids estrogen, progesterone, and androgen. We will also provide an overview of clinical trials targeting the receptors of these steroids. Due to space limitations, we will not discuss the role of gonadotropins in this review and ask the readers to refer to previous reviews on this topic (Zheng et al. 2007, So et al. 2008, King & Wong 2011). We will end the review with our suggestions for future directions for the field, such as the need for further mechanistic studies and well-designed clinical trials, which should include the use of biomarkers.
The epidemiology of EOC
Reproductive and hormonally related risk factors
The most consistently reported reproductive and hormonally related factors found to protect against EOC are use of oral contraceptives (OCs), increasing parity, and having a tubal ligation. In contrast, increasing age and nulliparity have been consistently shown to increase EOC risk. Other hormonally linked factors, such as hormone replacement therapy (HRT) use, infertility, endometriosis, breast-feeding, hysterectomy, and central adiposity, have shown some association with EOC, but the data are neither as strong nor as consistent as OC use, parity, and tubal ligation. Table 1 summarizes the relationship between reproductive and hormonally related risk factors and EOC.
Table 1.
Hormonally related epidemiological factors associated with EOC risk
| Strength of relationship | |
|---|---|
| Factors associated with increased EOC | |
| Reproductive factors | |
| Nulliparity | +++ |
| Exogenous hormone use | |
| Combined HRT | + |
| Estrogen-only HRT | ++ |
| Androgens | + |
| Reproductive disorders | |
| Endometriosis | ++a |
| PCOS | +/0 |
| Infertility | ++ |
| Other | |
| Age | +++ |
| Factors associated with decreased EOC risk | |
| Reproductive factors | |
| Pregnancy | − − − |
| Breast-feeding | − |
| Twinning and other non-singleton births (note 3) | − |
| Tubal ligation | − − − |
| Hysterectomy | − − |
| Exogenous hormone use | |
| Oral contraceptive use | − − − |
| High vs low progestin OCs | − |
| High vs low estrogen OCs | 0 |
| Factors not associated with EOC risk | |
| Reproductive factors | |
| Early menarche | 0 |
| Late menopause | 0 |
| Exogenous hormone use | |
| Fertility drug use | 0 |
Endometrioid and clear cell subtype only.
+, positively associated with EOC; −, negatively associated with EOC; 0, no association with EOC.
OCs, HRT, and exogenous hormones
Both prospective and case–control studies report about a 30% decrease in ovarian cancer risk with ever use of OCs (Collaborative Group on Epidemiological Studies of Ovarian Cancer 2008), and even short-term use (6 months or less) appears protective (Greer et al. 2005). Longer duration of use imparts increased protection, with a 20% decrease in risk for each 5 years of use. More recent OC use is associated with greater protection, but even after stopping its use for 30 or more years, risk is still reduced by about 15% (Collaborative Group on Epidemiological Studies of Ovarian Cancer 2008). Risk reduction appears consistent for all histological subtypes of EOC, except for possibly mucinous tumors. Estrogen dose does not appear to affect the OC–EOC association (Collaborative Group on Epidemiological Studies of Ovarian Cancer 2008) whereas some higher progestin dose formulations may confer greater protection, although that association has not been consistent (Ness et al. 2000, Schildkraut et al. 2002, Pike et al. 2004, Lurie et al. 2007).
While the data on OC use and EOC have been consistent, the data for HRT have not. However, more recent studies, including the prospective Women's Health Initiative (WHI) (Anderson et al. 2003) and the Million Women Study (Beral et al. 2007), report an increase in risk for both estrogen-only (ET) and estrogen–progestin (EPT) formulations, although the risk associated with EPT was lower than that of ET. A recent meta-analysis of 14 published studies found risk increases 22% per 5 years of ET use compared with only 10% per 5 years of EPT use, suggesting that risk differs by regimen (Pearce et al. 2009).
Exogenous androgens may be associated with EOC. One case–control study found that use of Danazol, a synthetic androgen commonly used in the treatment of endometriosis, significantly increased EOC risk (Cottreau et al. 2003), although this finding has not been replicated (Olsen et al. 2008). Ever use of testosterone (tablets, patches, troches, or cream) has been associated with a threefold increase in EOC (Olsen et al. 2008).
Childbearing and breast-feeding
Like OC use, childbearing has been consistently shown to be associated with a reduced risk of EOC in both prospective and case–control studies, with an observed protective effect similar to or greater than that observed for OC use (about 30% for the first full-term pregnancy). Moreover, increasing parity is associated with increasing protection, with each additional full-term pregnancy conferring about a 10% decrease in risk (Braem et al. 2010, Tsilidis et al. 2011). Even an incomplete pregnancy appears to provide some protection against EOC, although the magnitude of the protective effect is less than that of a full-term pregnancy (Riman et al. 1998). Finally, multiple births in a single pregnancy (giving birth to twins, triplets, etc.) may be associated with a greater risk reduction than singleton births (Whiteman et al. 2000). In contrast to childbearing, the evidence for a relationship between breast-feeding and EOC is inconsistent, although most studies show a small negative association (Riman et al. 2004, Danforth et al. 2007).
Reproductive disorders and other reproductive factors
Factors affecting childbearing have also been shown to be associated with EOC. In most studies, infertility has been associated with an increased risk, which may be greatest among women who fail to conceive (Vlahos et al. 2010). In general, infertility treatment does not appear to increase EOC risk, although the subset of treated women who remain nulliparous may be at an increased risk (Vlahos et al. 2010).
Endometriosis, defined as the presence and growth of endometrial tissue outside the uterine cavity, has also been associated with EOC. A recent pooled analysis of 13 case–control studies showed a threefold increase in the incidence of clear cell EOC and a twofold increase in endometrioid EOC among women with a self-reported history of endometriosis (Pearce et al. 2012).
An increased risk of EOC was reported by one case–control study (Schildkraut et al. 1996) among women with polycystic ovary syndrome (PCOS), a condition associated with menstrual dysfunction, infertility, obesity, the metabolic syndrome, hyperandrogenism, and insulin resistance. However, the finding was based on a small number of cases (n=7) and the association was limited to nonusers of OCs and thin women. Further case–control and prospective studies have failed to confirm this relationship (Pierpoint et al. 1998, Olsen et al. 2008, Brinton et al. 2010).
Tubal ligation has been consistently shown to be associated with reduction in EOC risk (Cibula et al. 2011). This protection appears similar in magnitude to OC use and child bearing (about 30%) and is protective in high-risk women (i.e. BRCA1/2 carriers) as well. Hysterectomy has also been shown to reduce EOC risk, although the magnitude of the association is not as great nor as consistent as that reported for tubal ligation (Riman et al. 2004). Finally, reproductive factors associated with other hormonally linked cancers, such as age at first menarche, age at menopause, and length of reproductive years, have not been consistently associated with EOC (Riman et al. 2004).
Weighing the hormone: EOC epidemiological evidence
The epidemiological relationships between reproductive and hormonally related risk factors and EOC suggest that hormones play a role in the etiology of the disease. Indeed, an early, small (31 cases and 62 controls), prospective study reported that circulating levels of androstenedione and DHEA were significantly associated with increased risk of EOC in a dose-dependent fashion, whereas levels of DHEAS, estradiol, estrone, and progesterone were not (Helzlsouer et al. 1995). However, three subsequent prospective studies, with a total of over 580 ovarian cancer cases, have failed to replicate these early positive hormone–EOC associations (Lukanova et al. 2003, Rinaldi et al. 2007, Tworoger et al. 2008). Thus, the evidence to date suggests that circulating hormone levels are not associated with EOC. Based on the existing data, however, the exact nature of the hormone–EOC link remains unclear, with some factors supporting and others refuting a direct association. Below we will describe and discuss current understanding of roles of hormones in EOC.
Estrogens
The evidence linking estrogens to EOC are mixed. Although pregnancy raises circulating estrogen levels, intraovarian levels are reduced. OCs reduce endogenous estrogen levels (Killick et al. 1987). In contrast, risk-conferring HRT raises circulating estrogen levels. Breast-feeding reduces both circulating and intraovarian estrogen levels (Rosenblatt & Thomas 1993). Endometriosis is associated with an increased local production of estradiol as well as increased expression of estrogen receptor α (ERα). Moreover, compared with levels in the early reproductive years, circulating peri-menopausal estradiol levels are higher, reflecting the period when EOC risk begins to sharply rise (Prior 2005). Together, these data suggest that estrogens may be associated with an increase in EOC risk.
Further evidence for an estrogen–EOC link comes from genetic susceptibility studies. Specifically, a large, international, pooled analysis of ten case–control studies comprising 4946 women with primary invasive EOC and 6582 controls found that women with the rs1271572 TT genotype were at a significantly increased risk of EOC compared with women with the G allele (Lurie et al. 2011). The association was even stronger among women ≤50 years. rs1271572 is located in the promoter of the ERβ gene (ESR2), where it maps to a binding region for MyoD and AP-4, previously shown to be important for expression of ERβ (Li et al. 2000). ERβ is believed to inhibit proliferation and motility of ovarian cancer cells as well as facilitate apoptosis (Bardin et al. 2004a, Cheng et al. 2004b, Treeck et al. 2007). Hence, there is a potential causal association of rs1271572 with EOC, especially among pre- and perimenopausal women, who have higher circulating estrogen concentrations compared with their postmenopausal counterparts, further supporting an association between estrogens and EOC.
However, other factors do not support an estrogen–EOC link. No prospective study has found an association between circulating estrogen levels and EOCOC use reduces endogenous estrogen levels; however, OC estrogen dose does not alter the magnitude of the observed EOC protective effect (Collaborative Group on Epidemiological Studies of Ovarian Cancer 2008). Finally, reproductive factors, such as early menarche and late menopause, which are associated with greater estrogen exposure and are linked to estrogen-associated breast and endometrial cancers, have not been consistently associated with EOC.
Progesterone and progestins
Epidemiological data suggest that progestins and progesterone may have a protective role against EOC. Progestin-containing OCs raise circulating progesterone levels about threefold. Progestin-only OCs may be as protective as EPT regimens and high-dose progestin OCs may be more protective than low-dose OCs (Rosenberg et al. 1994). Pregnancy, a consistently strong protective factor, raises progesterone levels, too. Third trimester circulating progesterone levels are more than ten times higher than luteal phase levels during the menstrual cycle. Moreover, progesterone levels in non-singleton births, which are more protective against EOC than singleton births, are higher than in singleton births (Batra et al. 1978, Haning et al. 1985). Although the risk of EOC appears increased with HRT use, regimens containing a progestin confer a lower relative risk compared with ET regimens, suggesting that the progestin component may mitigate the deleterious effect of estrogens. Ovulatory infertility, which is associated with reduced progesterone production, may increase EOC risk (Brinton et al. 1989, Rossing et al. 1994). Finally, endometriosis is associated with resistance to progesterone, which has been suggested to be due to the presence of inhibitory PR-A isoform and absence of transcription activating PR-B isoform (Attia et al. 2000).
Although the epidemiological data linking progesterone with reduced EOC risk are substantial, they are not definitive. For example, the data on OC progestin dose and EOC are not consistent. Additional compelling data come from a large consortium of 12 ovarian cancer case–control studies: a functional SNP, +331/CT, in the progesterone receptor (PR) alters the relative transcription of the two PR isoforms, PR-A and PR-B. Variants associated with increased PR-B, which acts as a classical steroid receptor, in theory should be associated with decreased EOC risk. Conversely, variants associated with increased PR-A, which inhibits in part PR-B, should be associated with increased risk. However, in this large study involving 4788 cases and 7614 controls, PR variants were not found to alter EOC risk, except for possibly the endometrioid subtype (Pearce et al. 2008).
Androgens
Several pieces of epidemiological data support a role for androgens in EOC development. OCs, one of the strongest protective factors, reduce circulating androgen levels (Gaspard et al. 1983, Murphy et al. 1990, Coenen et al. 1996). Tubal ligation and hysterectomy are also associated with decreased circulating androgen levels (Laughlin et al. 2000, Davison et al. 2005, Danforth et al. 2010). The potential association between PCOS, a hyperandrogenic condition, and EOC provides further support for the androgen–EOC link. Finally, the possible increased risk associated with use of exogenous androgenic agents further supports the relationship.
Despite these epidemiological associations, the data are not conclusive. Circulating androgen levels have not been associated with increased risk in three large prospective studies. Notably, androgen levels decline with age, with the decline being greater in the earlier reproductive years than in later decades (Davison et al. 2005). This is in contrast to the age–EOC relationship wherein incidence and risk rise slowly in the early reproductive years, then sharply increase beginning at around age 40, and continue to increase through late age (Howlader et al. 2011). In addition, factors associated with androgen levels as well as those altering androgen levels have not been consistently associated with EOC. For example, PCOS has not been consistently associated with the disease and the data linking exogenous androgen use with EOC have not been confirmed. Furthermore, OC formulations that contain androgenic agents do not differ in the magnitude of their protective effect compared with non-androgenic formulations (Greer et al. 2005). Finally, a trinucleotide repeat polymorphism in exon one of the androgen receptor (AR) gene, the length of which is inversely associated with the ability of the AR–ligand complex to transactivate AR-responsive genes, has been inconsistently associated with EOC in both magnitude and direction (Spurdle et al. 2000, Menin et al. 2001, Santarosa et al. 2002, Lee et al. 2005a, Schildkraut et al. 2007, Ludwig et al. 2009).
Summary of epidemiological data and the hormone–EOC associations
The epidemiological evidence suggests a role for androgens and estrogens in the etiology of EOC. Data further support a protective role for progesterone and progestins against EOC. However, while supportive, the data are inconclusive and in some cases contradictory. One possible explanation may be that the relationship between hormones and EOC risk depends more on the intraovarian environment than on circulating hormone levels (Lukanova & Kaaks 2005). It is also possible that the timing of exposures, for example, in the pre- vs the postmenopause years, may be critical. Moreover, the interaction of lifestyle and hormonal factors may alter the hormone–EOC link. However, due to study design issues such as sample size and recall of specific details when assessing exposures that precede diagnosis by many years, such interactions are challenging to assess. Finally, the heterogeneity of EOC may contribute to the inconsistent findings linking hormonally associated reproductive, lifestyle, and host factors with the diseases. To date, most epidemiological studies have considered EOCs as a single disease, which may partially explain inconsistencies among studies as well as some negative finding. Indeed, a very recent study suggests that association between circulating levels of sex steroid hormones and EOC differ by tumor histology and invasiveness. These data suggest that the previously reported negative findings (Lukanova et al. 2003, Rinaldi et al. 2007, Tworoger et al. 2008, Modugno & Edwards 2012) may be due to treating EOCs as a single entity. In the future, it will be important to determine potential associations with histological and molecular subtypes of EOC.
In vitro and in vivo studies of hormone action in ovarian cancer
Role of ER signaling action in ovarian cancer cells
Estrogen exerts its effect through two receptors, ERα and ERβ. A number of studies have addressed the expression of both isoforms in clinical samples and their functions in cell line models. ERβ is highly expressed throughout the normal ovary, including in granulosa cells, theca cells, corpora lutea, oocytes, as well as cultures of primary ovarian surface epithelial (OSE) cells (Kuiper et al. 1996, Byers et al. 1997, Brandenberger et al. 1998, Hillier et al. 1998); however, its expression is progressively lost during ovarian cancer development and progression (Lau et al. 1999, Bardin et al. 2004b, Lazennec 2006, Chan et al. 2008). While this loss has been associated with loss at the genetic level, there is increasing evidence that lower expression of ERβ can also result from epigenetic changes, namely hypermethylation of its promoter (Geisler et al. 2008, Suzuki et al. 2008, Yap et al. 2009). Of interest, a recent study showed nuclear localization of ERβ in normal ovarian tissue, but cytoplasmic localization in the tumor tissue, which was associated with worse outcome (De Stefano et al. 2011). In contrast, ERα expression is maintained, or even increased, in a subset of ovarian tumors (Rao & Slotman 1991, Chan et al. 2008). As a result, there is an increase in the ERα/ERβ ratio with malignant progression of the ovary. There is limited knowledge about the expression of ERα/ERβ in different histological subtypes of ovarian cancer, and even less about their expression in different molecular subtypes, such as the proliferative, immunoreactive, differentiated, and mesenchymal subtypes in high-grade serous cancers, as defined by the TCGA analysis (Bell et al. 2011).
Although details are little understood, a number of studies clearly show that estrogen treatment exerts pro-proliferative action, which can be blocked with the antiestrogens tamoxifen and ICI 182 780 (Galtier-Dereure et al. 1992, Langdon et al. 1994). There is also increasing evidence for estrogen mediating increased motility and invasion of ovarian cancer cells (Hua et al. 2008, Zhu et al. 2012). In a recent in vivo study, using a mouse model in which overexpression of SV40Tag in OSE results in poorly differentiated ovarian tumors, estrogen treatment caused an earlier onset of tumors, decreased overall survival (OS) time, and a distinctive papillary histology (Laviolette et al. 2010).
To confirm that estrogen-mediated growth stimulatory effects in ovarian cancer cell lines were indeed mediated by ERα, O'Donnell et al. (2005) treated ERα/ERβ-expressing ovarian cancer cells with ERα and ERβ-specific ligands. Treatment with PPT (the ERα ligand) but not with DPN (the ERβ ligand) resulted in growth stimulation, confirming a role for ERα in the estrogen-mediated growth stimulation. Given the tumor suppressive-like activity of ERβ, it is not surprising that overexpression of ERβ can result in inhibition of ovarian cancer cell motility and invasion (Zhu et al. 2011). One might expect some growth inhibition with ERβ ligands, but such studies have yet to be performed in ovarian cancer cells.
A number of studies have identified ERα target genes in ovarian cancer cells, revealing some overlap with the estrogen response in breast cancer cells but also unique targets. Early studies have shown regulation of genes involved in proliferation, invasion and cell cycle regulation such as cathepsin (Rochefort et al. 2001), c-fos, pS2, cyclins (Albanito et al. 2007), TGFα (Simpson et al. 1998), fibulin (Clinton et al. 1996, Roger et al. 1998, Moll et al. 2002), c-myc (Chien et al. 1994), and SDF-1 (Hall & Korach 2003), PR (Langdon et al. 1998), and more recently members of the semaphorin family (Joseph et al. 2010). In addition, a number of IGFBPs have been described to be regulated by estrogen (O'Donnell et al. 2005), and levels of IGFBP3, IGFBP4, and IGFBP5 were predictive in a letrozole trial in ovarian cancer patients (Walker et al. 2007a).
A gene expression array study has been reported, in which ER-positive PEO1 cells were treated with estradiol for 24 h, and target genes were identified using a 1.2K array. Using a threefold change as cutoff, the authors identified five induced and 23 downregulated genes. The induced genes were TNFDF7, TRAP1, FOSL1, TFAP4, and cathepsin D, and among the repressed genes were cyr61, vimentin, fibronectin, IGFBP3, and several keratins (Hall & Korach 2003). The finding of repression was more dominant compared with induction, is of interest; however, given the 24-hour treatment, additional experiments are necessary to show that the identified genes are truly direct ER targets. Of note, there is one study detailing mechanism of estrogen-mediated repression of target genes, focusing on the folate receptor, in ovarian cancer cells (Kelley et al. 2003). The authors show a role for ERα and the corepressor SMRT, and a lack of involvement of coactivators, including those of the p160 family in the repression.
In contrast to breast cancer for which ligand-independent activation of ER activity has been well described, there is limited literature on such activity in ovarian cancer. One study has described ERK2-mediated phosphorylation of ERα in response to DC44 interaction with hyaluron and subsequent IQGAP1 recruitment (Bourguignon et al. 2005). There is some preliminary evidence that the observed interaction between hormone response and obesity in ovarian cancer could result from cross talk between ERα and leptin signaling. Using BG-1 cells as a model system, Choi et al. (2011) showed that treatment with ICI 182 780, a pure ER antagonist, blocked leptin-induced cell proliferation. The effect was mediated by ERα interaction between phosphorylated Stat3, which was at least in part mediated by ERK and PI3K pathways. Given the recent finding of BG-1 cells being identical to MCF-7 breast cancer cells, these data should be interpreted with caution, unless DNA fingerprinting was performed to confirm authenticity of the BG-1 cells as ovarian cancer cells (Korch et al. 2012).
Lack of response to estrogen and antiestrogen can result from primary resistance (e.g. due to lack of ER expression or activity) or can develop as secondary (i.e. acquired) resistance, for example, resulting from activation of alternative pathways. However, there has been a paucity of studies on endocrine resistance in ovarian cancer, with few exceptions. The SKOV3 cells, for example, have been described to be resistant to estrogen and antiestrogen treatment, associated with loss of PR, and overexpression of Her2 and cathepsin D (Hua et al. 1995). Subsequently, Lau et al. (1999) described a 32 bp deletion in exon 1 of ERα in SKOV3 cells, which is potentially very exciting; however, no follow-up studies have been reported. Further studies of ER target genes in vitro but also in vivo, such as in established estrogen-sensitive xenografts from PE04, OVA-5, and OVCAR-3 cells (Ritchie & Langdon 2001) and in resistance models, and mechanistic analysis of ER and coregulator recruitment, for example, using chromatin immunoprecipitation studies, are warranted to move this field forward.
Progesterone action in normal ovary and ovarian cancer cells
There is evidence that loss of PR expression is associated with increasing grade of ovarian cancer (Langdon et al. 1998, Lau et al. 1999, Akahira et al. 2002). Also, in contrast to ER, where little is known about subtype-specific expression, PR levels seem to be higher in endometrioid tumors compared with other ovarian cancers (Langdon et al. 1998). The same study reported an association between PR, stage, and outcome – PR expression was higher in low-grade tumors, and high PR expression was associated with improved survival.
A number of studies showed that progesterone treatment of OSE and ovarian cancer cells results in decreased proliferation and increased apoptosis. Rodriguez et al. (1998) performed in vivo studies, using primates, which showed that progesterone treatment resulted in a four- to six-fold increase in the number of apoptotic ovarian epithelial cells compared with control and estrogen treated monkey. The same group subsequently showed that this effect can be enhanced by treatment with NSAIDs, a concept of potential interest for the prevention of ovarian cancer (Rodriguez et al. 2012). The induction of apoptosis was associated with decreased expression of TGFβ1 and increased expression of TGFβ2 and TGFβ3, suggesting that differential regulation of TGFβ family members could play a role in progestin-mediated induction of apoptosis (Rodriguez et al. 2002). While details of underlying mechanisms are sparse, some studies have addressed this question and have shown enhanced TRAIL-mediated cell death (Syed et al. 2007), induction of p53 (Bu et al. 1997), and increased expression FasL (Syed & Ho 2003). Interestingly, in addition to the induction of apoptosis, activation of PR was also shown to induce cell cycle arrest and senescence (Takahashi et al. 2009).
As for ER, there is limited knowledge of PR downstream target genes in ovarian cancer. Syed et al. (2005) treated human OSE cells (HOSE 642, HOSE 6–8, and HOSE 12–12) and ovarian cancer cell lines (OVCA429, OVCA420, and OVCA432), previously described to be PR positive (Lau et al. 1999), with high-dose progesterone. Using a small gene expression array (n=2000 genes), the authors identified 171 progesterone-regulated genes in HOSE cells and 135 in ovarian cancer cells. They focused on ATF3, caveolin-1, DLC1, and nm23-H2, showing that these genes were indeed direct PR target genes, and given their antitumor and anti-invasive properties, the authors speculate that induction of these candidates could be related to progesterone-induced antitumor effects.
Importantly, there is some evidence that progesterone might synergize with chemotherapeutic drugs to induce apoptosis. For example, high doses of progesterone were shown to enhance cisplatin-induced apoptosis (Murdoch et al. 2008). However, this is not straightforward, as other studies have reported opposite effects. Peluso et al. (2008, 2009) for example, showed that progesterone treatment can result in decreased cisplatin-induced apoptosis. This apparent controversy might be, at least in part, due to progesterone's action on two receptors, PRA and PRB, and their relative expression in the target cells. In addition, progesterone can also bind to a protein complex containing the progesterone membrane receptor component 1 (PGMRC1/mPR). Indeed, the Pelusso laboratory has published a few studies showing an involvement of PGRMC1 in sensitivity of ovarian cancer cells to cisplatin (Peluso et al. 2008, 2009). Interestingly, PGRMC1 shares homology with cytochrome b5-related proteins rather than with hormone receptors and can bind heme (Peluso 2011). Different cellular responses to progesterone could also result from altered expression of the more recently identified membrane PRs mPRα, β, and γ. The mPRs belong to the larger progestin and adipoQ receptor gene family (mPRα – PAQR7; mPRβ – PARQ8; and mPRγ – PARQ5), and while they are not members of the classical G-protein family, they can activate G-proteins and affect cAMP levels. They bind progesterone with high affinity, but do not interact with synthetic progestin R5020, or the PR antagonist RU486. They are abundantly expressed in ovarian cancer cells including those that lack expression of classical PR, and in clinical specimens representing all major histological subtypes (Romero-Sánchez et al. 2008, Charles et al. 2010). Charles et al. (2010) showed that progesterone alone did not affect cAMP levels in ES-2 and SKOV3 cells; however, it enhanced isoproterenol-induced and β1,2-adrenergic receptor-mediated increases in cAMP levels. This resulted in activation of JNK1/2 and p38 MAPK activity and subsequently induction of pro-apoptotic genes like Bax.
Thus, the effects of progesterone in ovarian cancer cells are mediated by the classical PR, by PGRMCs, and finally by mPRs, through both genomic and non-genomic actions (for review, see Peluso (2007)). This signaling is undoubtedly very complex, and while potentially attractive as a clinical target, significantly more research needs to be done before we can begin to understand intricate details of progesterone action in ovarian cancer.
Limited understanding of AR action in ovarian cancer cell lines
The AR is expressed in both normal ovary and ovarian cancer. Edmondson et al. (2002) have shown that OSE cells express AR and respond to androgen with increased proliferation and attenuated apoptosis. Epithelial cells, especially those within inclusion cysts arising after ovulation, appear to be exposed to high levels of androgen (Risch 1998). Ovarian cancer cells also express 17β-hydroxysteroid dehydrogenase (17β-HSD) converting androstenedione (which is a weak androgen) to testosterone (Blomquist et al. 2002, Chura et al. 2009). Importantly, there are also a number of studies showing overexpression of AR in ovarian cancer (Kühnel et al. 1987, Chadha et al. 1993, Ilekis et al. 1997, Lau et al. 1999, Lee et al. 2005b).
Although sparse, there is evidence from in vitro studies suggesting that androgens affect gene expression, growth, invasion, and survival in ovarian cancer cell lines. Shi et al. (2011) have shown that androgen's effect on survival of ovarian cancer cells was associated with increased expression, activity, and phosphorylation of telomerase. They also showed an androgen-mediated degradation of the cell cycle inhibitor p27 (Shi et al. 2011). Androgen was reported to stimulate DNA synthesis/S-phase fraction in low-passage OSE culture (Syed et al. 2001, Edmondson et al. 2002), and in ovarian cancer cells (Sheach et al. 2009); however, other studies failed to observe an effect of androgens on growth (Karlan et al. 1995). Finally, activation of AR has been shown to stimulate ovarian cancer cell invasion (Gogoi et al. 2008, Ligr et al. 2011).
There is evidence for cross talk between androgen signaling and other signaling pathways. For example, Evangelou et al. (2000) showed that dihydrotestosterone (DHT) treatment of Hey and SKOV-3 ovarian cancer cells, and of ascites-derived OVCAS-16 cells, prevented growth inhibitory effect of TGF-β, while DHT alone had no effect on growth. This effect of DHT was associated with downregulation of TGF-β1 and TGF-β2 receptors. Interestingly, the same group went on to show similar effects of DHT on blocking TGF-β-mediated growth inhibition in cells isolated from ovarian surface epithelium of women undergoing oophorectomy for non-ovarian indications or with a germline BRCA mutation (Evangelou et al. 2003).
To the best of our knowledge, there is only one study reporting a genome-wide analysis of androgen target genes. Sheach et al. (2009) identified more than 100 AR target genes in OVCAR3 cells with the majority being related to transcription, proliferation, and G-protein signaling. The G-proteins that were significantly induced by androgen treatment in OVCA3 cells included GNAI3, ELKS, GSTPI, RERG, Rab25, Rab45, and Rab35. The induction of Rab25 is of special interest as this small GTPase has previously been shown to proliferation, survival, and invasion of ovarian cancer cells (Fan et al. 2006) and to be overexpressed in aggressive ovarian cancers (Cheng et al. 2004a).
Coregulator proteins in ovarian cancer
Although not a major focus of this review, there is little doubt that coregulator proteins – activating or repressing steroid receptors – do play a role in ovarian tumorigenesis. SRC3/AIB1 was shown to be amplified in up to 25% of ovarian cancer (Bautista et al. 1997, Tanner et al. 2000). The coregulator p44/Mep50/WDR77 increases activities of ER and AR and stimulates proliferation and invasion of ovarian cancer cells in the presence of estrogen or androgen (Ligr et al. 2011). It shows strong cytoplasmic localization in normal ovarian surface, and fallopian tube epithelia, while it is mainly in the nucleus in invasive ovarian carcinoma. The coactivator ARA70 was highly expressed in invasive ovarian tumors but not in the normal ovary (Shaw et al. 2001). And finally, corepressor proteins have also been described to be highly expressed in ovarian cancer – Havrilesky showed that 35% of tumors expressed NCoR, and 71% expressed NCoR2/SMRT (Havrilesky et al. 2001). Given the known critical role of coregulator proteins in sensitivity and resistance to hormones, future studies on their expression and function in ovarian cancer development and progression are warranted.
Summary: basic science of hormone response in ovarian cancer
Steroid hormone signaling in ovarian cancer is complex yet little understood. It is imperative that we develop and use in vitro as well as in vivo models representing the different histological and molecular ovarian cancer subtypes to decipher signaling pathways and downstream target genes of the steroid receptors before we can improve efficacy of endocrine treatment in ovarian cancer.
Clinical trials involving hormonal therapy
Introduction
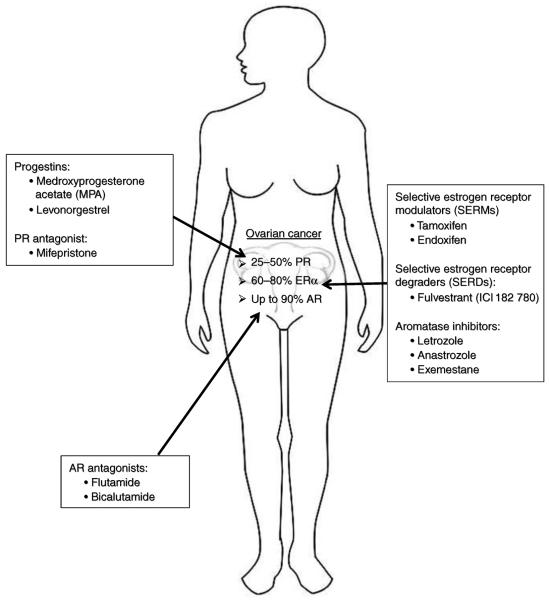
Targeting hormone receptors has been a successful therapeutic strategy in endocrine-sensitive tumors such as breast, prostate, and endometrial cancers (Moreau et al. 2006, Decruze & Green 2007, Poole & Paridaens 2007). There is clear evidence that EOC is a hormone-responsive cancer, at least in a subset of tumors. Nuclear receptors are widely expressed in EOC: ER is expressed in 61–79% of EOC, with highest expression in serous and endometrioid subtypes (Glavind & Grove 1990, Rao & Slotman 1991, Lindgren et al. 2001, Lee et al. 2005b). PR is expressed in ~25–50% of all EOC, but is as high as 91% in endometrioid subtypes (Fujimura et al. 2001, Lindgren et al. 2001, Lee et al. 2005b), and expression of AR is as high as 90% (Kühnel et al. 1987). Despite the high expression of endocrine-responsive receptors, hormonal therapy has only a minor role in the treatment of EOC. Therapy targeting the ER, for example, has been employed since the 1960s, but variable clinical responses have limited their usefulness in ovarian cancer (Langdon & Smyth 2008). Given that hormonal therapy is a relative nontoxic anticancer therapy, which is easy to administer and that it is well tolerated, the question is why hormonal therapy is not used more widely in ovarian cancer? Can it be made more effective? Below we summarize and discuss endocrine therapies in ovarian cancer, again focusing on ER, PR, and AR (also depicted and summarized in Fig. 1).
Figure 1.
Steroid receptors as clinical targets in ovarian cancer. Estimates of % tumors expressing PR, ERα, or AR (without considering subtypes of ovarian cancer due to lack of information). Also listed are approved drugs or drugs used in previous or currently ongoing ovarian cancer trials.
Targeting the ER in ovarian cancer
Tamoxifen, a selective ER modulator (SERM), that produces antiestrogen effects through competitive inhibition of ER has been used with variable results for the treatment of ovarian cancer. The majority of clinical trials involving tamoxifen were small phase II trials of patients with heavily pretreated recurrent disease. A prospective Gynecologic Oncology Group (GOG) study by Hatch et al. (1991), evaluated the response to tamoxifen in patients with recurrent or persistent disease following primary treatment. One hundred and five patients were treated with tamoxifen 20 mg twice a day after frontline chemotherapy. Study participants had an 18% objective response rate (RR), with 10% having a complete response (CR). Reanalysis of this study, focusing on patients with platinum-refractory disease, showed an objective RR of 13% (Markman et al. 1996). Of note, the objective RRs to second-line platinum-based therapy range from <10 to >40% (Markman & Bookman 2000). A similar objective RR of 17% (5/29) was seen in patients with unknown ER/PR status treated with tamoxifen after failure of cytotoxic therapy in three phase II clinical trials published by the Mid-Atlantic Oncology Program in 1993 (Ahlgren et al. 1993). Therefore, hormonal therapy is well within the response range of chemotherapeutic agents but considerably easier to administer and with fewer side effects. Unfortunately, few of these trials report ER status of the ovarian tumors. One study reported that patients with ER+ tumors had higher RRs to tamoxifen treatment than ER− tumors, although this difference was not statistically significant (Hatch et al. 1991). In another small study, all patients with stable disease (SD) were ER positive (Schwartz et al. 1982). ER status did not correlate with response in two other trials; however, ER status was known in only one-fourth to one-third of patients enrolled (Shirey et al. 1985, Weiner et al. 1987).
In 2010, the GOG published the results of a phase II trial of tamoxifen vs thalidomide in women with biochemical recurrence of EOC, fallopian tube, or primary peritoneal carcinoma based on rising CA125 after CR to frontline platinum/taxane therapy (Hurteau et al. 2010). The interim analysis did not show any difference in the benefit of thalidomide relative to tamoxifen, and the study was stopped early. At a median follow-up of 31 months, the progression-free survival (PFS) was 3.2 and 4.5 months while median survival was 24.0 and 33.2 months for thalidomide and tamoxifen arms respectively. Thalidomide was associated with an increased risk (HR=1.76, 95% CI=1.16–2.68) compared with tamoxifen. Also, there was significantly less toxicity associated with tamoxifen treatment, and more patients in this treatment arm received ≥3 cycles. As this trial did not contain a third arm (no treatment or another active agent), the possible interpretations of the tamoxifen results are limited. However, they are very encouraging and indicate that additional study of tamoxifen (and other endocrine treatments) in this patient population, i.e. asymptomatic patients with biochemical evidence of recurrent ovarian cancer, is warranted. Markman et al. (2004a) examined the evidence behind this management strategy with a retrospective review of 56 women with asymptomatic recurrent ovarian or primary peritoneal cancer treated with 20–40 mg Tamoxifen daily before initiation of cytotoxic chemotherapy. The median duration of treatment was 3 months, but 42% of patients were on Tamoxifen for ≥6 months and 19% for ≥12 months. The most common reasons for stopping single-agent tamoxifen were a continued rise of serum CA125, progression of disease on CT scan or physical exam, or the development of cancer-related symptoms. No standard of care exists for the management of asymptomatic recurrent disease, and therefore, trials testing hormonal therapy in this setting seem warranted.
While investigations continue to examine the utility of hormonal therapy, contemporary studies publishing randomized and nonrandomized trials evaluating the use of tamoxifen in EOC are lacking. A Cochrane review published in 2010 attempted to identify randomized and nonrandomized studies of more than ten patients with recurrent ovarian cancer treated with tamoxifen (Williams et al. 2010). Surprisingly, the search of articles published between 2002 and 2009 yielded no trials that satisfied the inclusion criteria. Publications during that period consisted only of observational data from single-arm studies of women treated with tamoxifen. Additionally, no data on tamoxifen's effect on symptom control, quality of life, or prolongation of life were available from the uncontrolled, non-comparative trials that were screened as part of this Cochrane review. A Cochrane review using the same inclusion criteria as the more recent publication did identify 11 nonrandomized series, one nonrandomized phase II study, and two randomized trials from 1997 to 2002 (Williams 2001; see Table 2). In total, 60 of the 623 (9.6%) women treated with tamoxifen had an objective response and SD of 4 weeks or more was seen in 131/411 (31.9%) women from eight studies. Due to lack of data, duration of response, survival, symptoms palliation, and quality of life were not assessed.
Table 2.
Response rates of tamoxifen (TAM) in persistent or recurrent EOC
| Number of patients responding (%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| References | Trial | n | Drug dosage | CR | PR | SD | PD | Other |
| Marth et al. (1997) | Nonrandomized phase II | 65 | TAM 30–40 mg PO daily | 2 (3) | 2 (3) | 50 (77) | 11 (17) | Recurrent disease, refractory ≥1 chemotherapy regime |
| Gennatas et al. (1996) | Nonrandomized series | 50 | TAM 40 mg PO daily | 2 (4) | 26 (52) | NA | NA | 50% previously untreated, 50% refractory or progressive disease |
| Jager et al. (1995) | Unblinded randomized controlled trial | 33 | TAM 30 mg PO daily | 0 | 0 | 2 (6) | NA | Progressive disease |
| Van Der Velden et al. (1995) | Nonrandomized series | 30 | TAM 40 mg PO daily | 2 (7) | NA | 10 (33) | NA | Refractory or recurrent disease |
| Ahlgren et al. (1993) | Nonrandomized series | 29 | TAM 80 mg PO×30 d, then 20 mg PO daily | 2 (7) | 7 (24) | 18 (62) | 6 (21) | Prior cytotoxic chemotherapy |
| Losa et al. (1993) | Randomized trial comparing hormonal therapy (168 patients in three arms) | NA | TAM 40 mg PO daily | 0 | 1 | 22 | 32 | Prior multi-agent cytotoxic chemotherapy |
| Hatch et al. (1991) | Nonrandomized phase II | 105 | TAM 40 mg PO daily | 10 (10) | 8 (8) | 40 (38) | 47 (45) | Recurrent or persistent disease |
| Osborne et al. (1988) | Nonrandomized series | 51 | TAM 100mg/m2 PO over 24 h then 40 mg PO daily | 0 | 1 (2) | 0 | 50 (98) | Disease refractory to ≥1 chemotherapy regime |
| Weiner et al. (1987) | Nonrandomized series | 31 | TAM 160mg/m2 PO×7d, then 20 mg PO daily | 1 (3) | 2 (6) | 6 (19) | 22 (71) | Prior cytotoxic chemotherapy |
| Slevin et al. (1986) | Nonrandomized series | 22 | TAM 20 mg PO daily | 0 | 0 | 1 (5) | 21 (95) | Disease refractory to ≥1 chemotherapy regime |
| Hamerlynck et al. (1985b) | Nonrandomized series | 36 | TAM 40 mg PO daily | 0 | 2 (6) | 7 (19) | NA | Prior cytotoxic chemotherapy |
| Landoni et al. (1985) | Nonrandomized series | 19 | TAM 40 mg PO daily | 0 | 0 | 7 (37) | NA | Prior cytotoxic chemotherapy |
| Shirey et al. (1985) | Nonrandomized series | 23 | TAM 20–40 mg PO daily | 0 | 0 | 19 (83) | NA | Disease refractory to ≥1 chemotherapy regime |
| Schwartz et al. (1982) | Nonrandomized series | 13 | TAM 20 mg PO daily, increase to 40 mg PO daily if disease progression | 0 | 1 (8) | 4 (31) | 8 (62) | Rapidly advancing recurrent disease |
PO, oral; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NA, not available.
The majority of women who develop ovarian cancer are postmenopausal at the time of diagnosis. In postmenopausal women, the major source of circulating estrogen is from the peripheral conversion (in skin and adipose tissue) of androstenedione by the enzyme aromatase. Additionally, aromatase expression has been shown in malignant ovarian epithelial cells resulting in intratumoral production of estrogen (Cunat et al. 2005). The use of aromatase inhibitors (AI), such as letrozole and anastrozole, for the treatment of ER+ breast cancer has been a great success, and several studies have investigated the use of these agents in the treatment of recurrent or persistent ovarian cancer (see Table 3). Similar to other hormonal therapies, the results of AI treatment are variable. In seven phase II trials, with known ER status, the complete and partial RRs were 0–4 and 0–11% respectively (Bowman et al. 2002, del Carmen et al. 2003, Papadimitriou et al. 2004, Gourley et al. 2006, Verma et al. 2006, Smyth et al. 2007, Ramirez et al. 2008). However, benefit from AI therapy can be measured by prevention of tumor progression in addition to tumor regression. In a phase II trial evaluating the utility of letrozole in a selected group of ER+ patients with recurrent ovarian cancer, a 17% RR was seen by CA125 criteria and 26% of patients had SD at 6 months of treatment (Smyth et al. 2007). Response to AI therapy correlated with ERα expression with those patients having the highest expression of ERα having the greatest RR, 33%, compared with 0% RR in those with low expression. In another study, response to letrozole was associated with increased tumor production of aromatase (Walker et al. 2007b). The ideal use of AIs may be following initial cytoreductive surgery and adjuvant chemotherapy for stabilization of any residual disease, potentially serving to prolong PFS; however, a trial comparing DFS in ER+ patients treated with AIs vs placebo is still warranted before such therapy can be justified.
Table 3.
Response rates of aromatase inhibitors in persistent or recurrent EOC (all are phase II trials)
| Number of patients responding (%) |
|||||||
|---|---|---|---|---|---|---|---|
| References | n | Drug | CR | PR | SD | PD | ER status |
| Bowman et al. (2002) | 54 | Letrozole | 0 | 5 (9) | 14 (26) | 30 (56) | Mixed ER−/ER+ tumors |
| Papadimitriou et al. (2004) | 27 | Letrozole | 1 (4) | 3 (11) | 5 (19) | 18 (67) | Mixed ER−/ER+ tumors |
| Gourley et al. (2006) | 33 | Letrozole | 0 | 3 (9) | 14 (42) | 16 (49) | NA |
| Smyth et al. (2007) | 42 | Letrozole | 0 | 7 (17) | 11 (26) | NA | ER+ |
| Ramirez et al. (2008) | 33 | Letrozole | 0 | 1 (3) | 7 (21) | 23 (70) | ER+ |
| Del Carmen et al. (2003) | 53 | Anastrozole | 0 | 1 (2) | 22 (42) | 30 (57) | Mixed ER−/ER+ tumors |
| Verma et al. (2006) | 22 | Exemestane | 0 | 0 | 8 (36) | NA | NA |
More recently, the pure antiestrogen fulvestrant (ICI 182 870) was tested in a phase II study. Multi-recurrent ER+ EOC were treated with single-agent fulvestrant until intolerance or disease progression (Argenta et al. 2009). Of the 26 patients in the study, there was one CR (4%), one PR (4%), and nine patients had SD (35%). While the median time to progression was 2 months, two patients remained on treatment for >250 days. The drug was well tolerated and there were no grade 3 or 4 toxicities. Similar to the AI trials, the authors of this study suggest that the clinical utility of this agent may be optimized by its use early in the adjuvant treatment setting or as long-term consolation therapy after primary disease remission. Sustained benefit in selected patients suggests that a subset of patients may exist that may prove to benefit substantially from this approach. It is most critical to be able to identify this group of patients that is most likely to respond. There are a few studies that have attempted to identify biomarkers for endocrine treatment response in ovarian cancer (Bowman et al. 2002, Smyth et al. 2007, Walker et al. 2007a) but such studies are clearly in their infancy and will need to be expanded.
PR ligand in EOC
Several large clinicopathological studies have shown that PR expression is associated with an increased OS in EOC (Münstedt et al. 2000, Lee et al. 2005b, Tangjitgamol et al. 2009). One study showed that PR expression was also associated with improved response to chemotherapy (Tangjitgamol et al. 2009). These findings, together with in vitro studies suggesting that PR induces apoptosis (and potentially senescence) in ovarian cancer cells, strongly suggest modulation of PR levels and/or activity as a form of endocrine treatment of EOC. Niwa et al. (2008) reported their findings of the effect of combination medroxyprogesterone acetate (MPA) with primary adjuvant chemotherapy in advanced EOC in 2008. Both PFS and OS were significantly longer in the patients treated with combination MPA and platinum-based chemotherapy compared with the control group. These effects were more pronounced in the group with higher PR expression.
Zheng et al. (2007) recently reviewed the utility of PR ligands in ovarian cancer treatment by examining 13 clinical trials that included 432 patients with recurrent or refractory ovarian cancer treated with megestrol acetate or MPA. Ten patients (2.3%) had CR, 21 (4.9%) had a partial response, and 47 (10.9%) had SD. The authors concluded that the efficacy of progestational agents in recurrent EOC has not been established based on the currently available literature (trials are summarized in Table 3).
The antiprogestin, mifepristone, has also been used in the treatment of platinum-resistant ovarian cancer. In 2000, Rocereto et al. (2000) reported an overall RR of 26.5%, with 8.8% (3/34) having a CR, including one patient that remained without evidence of disease for over 3 years. These promising results could not be reproduced in a recent phase II trial in patients with recurrent or persistent ovarian, peritoneal, and fallopian tube cancers, in which only one response was seen among the 22 patients (Rocereto et al. 2010). Efforts are also ongoing testing mifepristone specifically in endometrioid adenocarcinomas (Ramondetta et al. 2009).
The in vitro data, and the promising evidence of treatment response in subset of patients, strongly suggest that further investigation of progestins, either alone or in combination with chemotherapy, is warranted. Given recent preclinical data, and basic science findings, one can expect to see drugs that target different PR isoforms (PR-A vs PR-B) and that might target other progesterone binding receptors (i.e. mPRs and PGRMCs).
Role for targeting AR in ovarian cancer?
In vitro studies have shown that androgen increases proliferation of normal human OSE cells and human ovarian cancer cells (Syed et al. 2001, Edmondson et al. 2002), making targeting AR a promising treatment strategy. There is increasing evidence that AR is not only a great target in prostate cancer but in other hormone-driven tumors, such as breast cancer (Nahleh 2008). However, the use of antiandrogens in the management of ovarian cancer has been limited to several small clinical trials in the recurrent setting. Two trials from the late 1990s evaluated the use of this flutamide, a nonsteroidal drug with antiandrogen properties, in recurrent ovarian cancer; Vassilomanolakis et al. (1997) reported a RR and disease stabilization of 4.3 and 8.7% respectively, while Tumolo et al. (1994) had slightly higher RRs and SD at 6.3 and 28%. These phase II trials were small, including only 24 and 32 patients respectively. A more recent trial, published in 2007, examined the use of bicalutamide, an antiandrogen, with goserelin, a GNRH agonist, in women with EOC who were in their second or higher disease remission (Levine et al. 2007). The use of these agents did not appear to prolong PFS in this group of patients, and there was no association between AR repeat number, genotype, or haplotype and PFS. Like for ER and PR-targeting trials, the identification of biomarkers of response will be critical. Given recent study by Elattar et al. (2012), nuclear expression of AR might be a viable biomarker for androgen sensitivity. DHT treatment of primary ovarian cancer cultures established from ascitic fluid resulted in increased in S-phase, and this was strongly associated with nuclear AR levels. Thus, in summary, AR modulation as a viable treatment option has not been validated at this time; however, additional studies potentially including novel drugs such as abiraterone, and including the use of biomarkers, are needed.
Combination therapies
PR can be induced with estrogen (and with tamoxifen in situation where it functions as an agonist), and several trials have studied the utility of combination hormonal therapy targeting this cross talk. Although no clinical response was seen with combination tamoxifen and progesterone (Belinson et al. 1987, Jakobsen et al. 1987), a significant clinical response was seen in one trial of 65 women with refractory ovarian cancer treated with sequential ethinyl estradiol and medroxyprogesterone, with a RR of 14% and SD in 20% of patients (Freedman et al. 1986). These findings were later confirmed with a similar although smaller clinical trial (Fromm et al. 1991).
Several small phase II trials have looked at the addition of goserelin to tamoxifen, again in the recurrent EOC setting. One trial reported one CR (3.8%), two PR (7.7%), and ten patients with SD (38.5%) with a median progression-free interval (PFI) of 4 months and OS of 13.6 months for the entire cohort (Hasan et al. 2005). A similar trial published 6 years earlier had similar findings with a PFI of 5 months and OS of 8 months (Hofstra et al. 1999).
Few trials have examined the utility of combining hormonal therapy to chemotherapy vs chemotherapy alone in the adjuvant setting for advanced ovarian cancer. There was no difference in progression-free or OS in 100 women with stage III or IV EOC randomized to receive combination cisplatin/doxorubicin with or without the addition of tamoxifen (Patel & Rothenberg 1994). However, tamoxifen was only administered during chemotherapy (median time: 36 weeks), and >54% of patients had residual tumor ≥2 cm. Additionally, hormone receptor status was only determined in 72% of patients. The same clinical question was asked in the recurrent disease setting. In one trial with 50 patients with recurrent disease, treatment with a platinum agent and tamoxifen showed a RR of 50% (Benedetti Panici et al. 2001). The high RRs in platinum-resistant disease lead other investigators to conduct similar trials. However, similar results have yet to be replicated, and in one trial of 14 patients with platinum-resistant disease, there were no objective responses to combination carboplatin/tamoxifen (Johnson et al. 1993, Markman et al. 2004b).
Randomized trials of combination progestin therapy and chemotherapy vs chemotherapy alone have also been plagued by small patient sample size, unknown tumor hormone receptor status, and heavily pretreated disease. The development of chemoresistant disease is a big clinical problem, and while still little understood, increased expression of P-glycoprotein, a transmembrane energy-dependent drug efflux pump, contributes to resistance in some tumor models (Johnson et al. 1993, Patel & Rothenberg 1994). Although several preclinical models showed the ability of progestins to overcome P-glycoprotein-mediated multidrug resistance (Yang et al. 1989, Fleming et al. 1992, Wang et al. 1994, Panasci et al. 1996, Tansan et al. 1997), there were no objective responses observed in 44 patients with paclitaxel-refractory ovarian cancer treated with combination paclitaxel and megestrol acetate (Panasci et al. 1996). Unfortunately, response to combination MPA-cytotoxic chemotherapy as first-line therapy also showed no difference in RR or survival compared with chemotherapy alone in 71 patients with advanced ovarian cancer (Tansan et al. 1997).
Finally, a number of biological agents are being studied for the treatment of ovarian cancer, some in combination with hormonal therapy. There is recent in vitro evidence that tamoxifen resistance may be related to EGFR overexpression in solid tumors like breast (Shou et al. 2004) and that combination treatment with gefitinib (Iressa), a signal transduction inhibitor of EGFR tyrosine kinase, and tamoxifen was more effective than either agent alone (Hiscox et al. 2004). Additionally, there is evidence that EGFR overexpression is seen in 30–70% of cases of platinum-resistant ovarian tumors (Scambia et al. 1995, Bartlett et al. 1996). Therefore, a phase II trial of combination tamoxifen and gefitinib was conducted in 56 patients with ovarian cancer refractory or resistant to platinum-and taxane-based therapy. Although no tumor responses were seen, 16 patients had SD (Wagner et al. 2007). Gefitinib has also been used in combination therapy with the AI anastrozole; in patients with asymptomatic Müllerian cancer, there was a RR of 3% (1/35 patients) (Krasner 2007). Although this number of responders is disappointingly low, the authors comment that the single responder had a durable CR and an additional patient had SD for more than 600 days.
Ongoing clinical trials
At the time of writing of this review, we identified one clinical trial involving the use of hormonal modulation for the treatment of ovarian cancer, which is actively enrolling patients. NCT01273168 is a phase I clinical trial sponsored by the National Cancer Institute (NCI) of endoxifen, a potent and active metabolite of tamoxifen that may have clinical utility as an antiestrogen (Wu et al. 2009). It is administered to adults with hormone receptor-positive solid tumors including breast, dermoid, and gynecologic tumors that did not respond to standard treatment. Study participants will take daily oral endoxifen in escalating doses at 28-day intervals to determine safety, tolerability, pharmacokinetics, and determine the maximum tolerated dose. Additionally, an investigational radiolabeled imaging agent, 16-α-[(18)F]-fluoro-17β estradiol, will be used with positron emission tomography (PET) to determine in situ tumor ER activity before and after treatment with endoxifen. Another NCI trial, NCT00445887, is not examining the role of hormonal therapy in the treatment of ovarian cancer but instead is evaluating the effect of oral levonorgestrel on prevention of ovarian carcinoma in high-risk patients.
Several other clinical trials in recurrent ovarian cancer patients have completed enrollment; however, results have not yet been published, including NCT00003865, assessing toremifene citrate, a SERM, and NCT00181688, a phase II trial of combination of AI anastrozole and gefitinib.
Conclusion: endocrine treatment in ovarian cancer
In summary, there is strong in vitro and in vivo evidence, and epidemiological data showing that estrogens (and potentially other steroids) regulate ovarian carcinogenesis. In addition, ER is highly expressed in subsets of ovarian tumors. Clinical trials testing endocrine therapy in ovarian cancer to date have been poorly designed and not properly evaluated to allow solid conclusions. Many did not select on ER status, and few correlated ER status with response. While the overall RR to hormonal therapy in ovarian cancer appears to be modest, there are clearly subgroups of patients that respond very well. As hormonal agents have the benefit of easy administration, low cost, and data on long-term effects of prolonged use, we must identify such subgroups of patients! Thus, the identification of biomarkers predicting endocrine treatment response and their introduction into clinical practice for both selection of patients for enrollment in clinical trials and subsequently for personalized endocrine treatment are critical. To accomplish such goals, we must continue to investigate mechanism of action of hormones in ovarian cancer, we must perform additional well-designed clinical trials using biomarkers, and we must collect tissue.
Table 4.
Response rates to progestins (MA and MPA) and antiprogestins in persistent or recurrent EOC (all are phase II trials)
| Responders (%) |
|||||
|---|---|---|---|---|---|
| References | n (patients) | Drug | CR | PR | SD |
| Mangioni et al. (1981) | 33 | MPA | 0 | 5 (15) | 2 (6) |
| 30 | MPA | 0 | 0 | 2 (7) | |
| Slayton et al. (1981) | 19 | MPA | 0 | 0 | 1 (5) |
| Aabo et al. (1982) | 27 | MPA | 0 | 1 (4) | 0 |
| Tropé et al. (1982) | 25 | MPA | 0 | 1 (4) | 9 (36) |
| Geisler (1985) | 23 | MA | 7 (30) | 4 (17) | 0 |
| Hamerlynck et al. (1985a) | 41 | MPA | 0 | 1 (2) | 7 (17) |
| Sikic et al. (1986) | 47 | MA | 1 (2) | 3 (6) | 5 (11) |
| Belinson et al. (1987) | 33 | MA | 0 | 0 | 12 (39) |
| Ahlgren et al. (1993) | 32 | MA | 0 | 0 | 13 (41) |
| Malfetano et al. (1993) | 24 | MPA | 0 | 1 (4) | 9 (38) |
| Veenhof et al. (1994) | 54 | MA | 0 | 1 (2) | 9 (17) |
| Wiernik (1998) | 30 | MA | 0 | 0 | 0 |
| Rocereto et al. (2000) | 44 | Mifepristone | 3 (9) | 6 (17) | NA |
| Wilailak et al. (2001) | 36 | MA | 3 (8) | 4 (11) | NA |
| Rocereto et al. (2010) | 22 | Mifepristone | 0 | 1 | 3 |
MA, megestrol acetate; MPA, medroxyprogesterone; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NA, not available.
Acknowledgments
Funding
Ms C L Andersen is supported by a T32 training grant from the Molecular Pharmacology Training Program at the University of Pittsburgh.
Footnotes
Declaration of interest The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Aabo K, Pedersen AG, Haid I, Dombernowsky P. High-dose medroxyprogesterone acetate (MPA) in advanced chemotherapy-resistant ovarian carcinoma: a phase II study. Cancer Treatment Reports. 1982;66:407–408. [PubMed] [Google Scholar]
- Ahlgren JD, Ellison NM, Gottlieb RJ, Laluna F, Lokich JJ, Sinclair PR, Ueno W, Wampler GL, Yeung KY, Alt D, et al. Hormonal palliation of chemoresistant ovarian cancer: three consecutive phase II trials of the Mid-Atlantic Oncology Program. Journal of Clinical Oncology. 1993;11:1957–1968. doi: 10.1200/JCO.1993.11.10.1957. [DOI] [PubMed] [Google Scholar]
- Akahira J, Suzuki T, Ito K, Kaneko C, Darnel AD, Moriya T, Okamura K, Yaegashi N, Sasano H. Differential expression of progesterone receptor isoforms A and B in the normal ovary, and in benign, borderline, and malignant ovarian tumors. Japanese Journal of Cancer Research. 2002;93:807–815. doi: 10.1111/j.1349-7006.2002.tb01323.x. doi:10.1111/j.1349-7006.2002. tb01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Andò S, et al. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Research. 2007;67:1859–1866. doi: 10.1158/0008-5472.CAN-06-2909. doi:10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pettinger M, Liu J, McNeeley SG, Lopez AM, Women's Health Initiative Investigators Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women's Health Initiative randomized trial. Journal of the American Medical Association. 2003;290:1739–1748. doi: 10.1001/jama.290.13.1739. doi:10.1001/jama.290.13.1739. [DOI] [PubMed] [Google Scholar]
- Argenta PA, Thomas SG, Judson PL, Downs LS, Jr, Geller MA, Carson LF, Jonson AL, Ghebre R. A phase II study offulvestrant in the treatment of multiply-recurrent epithelial ovarian cancer. Gynecologic Oncology. 2009;113:205–209. doi: 10.1016/j.ygyno.2009.01.012. doi:10.1016/j.ygyno.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. Journal of Clinical Endocrinology and Metabolism. 2000;85:2897–2902. doi: 10.1210/jcem.85.8.6739. doi:10.1210/jc.85.8.2897. [DOI] [PubMed] [Google Scholar]
- Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERβ expression as a common step in estrogen-dependent tumor progression. Endocrine-Related Cancer. 2004a;11:537–551. doi: 10.1677/erc.1.00800. doi:10.1677/erc.1.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin A, Hoffmann P, Boulle N, Katsaros D, Vignon F, Pujol P, Lazennec G. Involvement of estrogen receptor β in ovarian carcinogenesis. Cancer Research. 2004b;64:5861–5869. doi: 10.1158/0008-5472.CAN-04-0552. doi:10.1158/0008-5472.CAN-04-0552. [DOI] [PubMed] [Google Scholar]
- Bartlett JM, S P, Langdon BJ, Simpson M, Stewart D, Katsaros P, Sismondi S, Love WN, Scott AR, Williams AM, et al. The prognostic value of epidermal growth factor receptor mRNA expression in primary ovarian cancer. British Journal of Cancer. 1996;73:301–306. doi: 10.1038/bjc.1996.53. doi:10.1038/bjc.1996.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra S, Sjoberg NO, Aberg A. Human placental lactogen, estradiol-17β, and progesterone levels in the third trimester and their respective values for detecting twin pregnancy. American Journal of Obstetrics and Gynecology. 1978;131:69–72. doi: 10.1016/0002-9378(78)90476-3. [DOI] [PubMed] [Google Scholar]
- Bautista S, Vallès H, Walker RL, Anzick S, Zeillinger R, Meltzer P, Theillet C. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. doi:10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- Belinson JL, McClure M, Badger G. Randomized trial of megestrol acetate vs. megestrol acetate/tamoxifen for the management of progressive or recurrent epithelial ovarian carcinoma. Gynecologic Oncology. 1987;28:151–155. doi: 10.1016/0090-8258(87)90208-3. doi:10.1016/0090-8258(87)90208-3. [DOI] [PubMed] [Google Scholar]
- Bell DA. Origins and molecular pathology of ovarian cancer. Modern Pathology. 2005;18(Suppl 2):S19–S32. doi: 10.1038/modpathol.3800306. doi:10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- Bell D, Berchuck A, Birrer M, Chien J, Cramer D, Dao F, Dhir R, DiSaia P, Gabra H, Glenn P, et al. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. doi:10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti Panici P, Greggi S, Amoroso M, Scambia G, Battaglia FA, Gebbia V, Salerno G, Paratore MP, Mancuso S. A combination of platinum and tamoxifen in advanced ovarian cancer failing platinum-based chemotherapy: results of a phase II study. International Journal of Gynecological Cancer. 2001;11:438–444. doi: 10.1046/j.1525-1438.2001.01059.x. doi:10.1046/j.1525-1438.2001.01059.x. [DOI] [PubMed] [Google Scholar]
- Beral V, Million Women Study Collaborators. Bull D, Green J, Reeves G. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet. 2007;369:1703–1710. doi: 10.1016/S0140-6736(07)60534-0. doi:10.1016/S0140-6736(07)60534-0. [DOI] [PubMed] [Google Scholar]
- Blomquist CH, Bonenfant M, McGinley DM, Posalaky Z, Lakatua DJ, Tuli-Puri S, Bealka DG, Tremblay Y. Androgenic and estrogenic 17β-hydroxysteroid dehydrogenase/17-ketosteroid reductase in human ovarian epithelial tumors: evidence for the type 1, 2 and 5 isoforms. Journal of Steroid Biochemistry and Molecular Biology. 2002;81:343–351. doi: 10.1016/s0960-0760(02)00117-6. doi:10.1016/S0960-0760(02)00117-6. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Gilad E, Rothman K, Peyrollier K. Hyaluronan-CD44 interaction with IQGAP1 promotes Cdc42 and ERK signaling, leading to actin binding, Elk-1/estrogen receptor transcriptional activation, and ovarian cancer progression. Journal of Biological Chemistry. 2005;280:11961–11972. doi: 10.1074/jbc.M411985200. doi:10.1074/jbc.M411985200. [DOI] [PubMed] [Google Scholar]
- Bowman A, Gabra H, Langdon SP, Lessells A, Stewart M, Young A, Smyth JF. CA125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: identification of an endocrine-sensitive subgroup. Clinical Cancer Research. 2002;8:2233–2239. [PubMed] [Google Scholar]
- Braem MG, Onland-Moret NC, van den Brandt PA, Goldbohm RA, Peeters PH, Kruitwagen RF, Schouten LJ. Reproductive and hormonal factors in association with ovarian cancer in the Netherlands cohort study. American Journal of Epidemiology. 2010;172:1181–1189. doi: 10.1093/aje/kwq264. doi:10.1093/aje/kwq264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenberger AW, Tee MK, Jaffe RB. Estrogen receptor α (ER-α) and β (ER-β) mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: down-regulation of ER-β in neoplastic tissues. Journal of Clinical Endocrinology and Metabolism. 1998;83:1025–1028. doi: 10.1210/jcem.83.3.4788. doi:10.1210/jc.83.3.1025. [DOI] [PubMed] [Google Scholar]
- Brinton LA, Melton LJ, III, Malkasian GD, Jr, Bond A, Hoover R. Cancer risk after evaluation for infertility. American Journal of Epidemiology. 1989;129:712–722. doi: 10.1093/oxfordjournals.aje.a115186. [DOI] [PubMed] [Google Scholar]
- Brinton LA, Moghissi KS, Westhoff CL, Lamb EJ, Scoccia B. Cancer risk among infertile women with androgen excess or menstrual disorders (including polycystic ovary syndrome) Fertility and Sterility. 2010;94:1787–1792. doi: 10.1016/j.fertnstert.2009.10.012. doi:10.1016/j.fertnstert.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu SZ, Yin DL, Ren XH, Jiang LZ, Wu ZJ, Gao QR, Pei G. Progesterone induces apoptosis and up-regulation of p53 expression in human ovarian carcinoma cell lines. Cancer. 1997;79:1944–1950. doi: 10.1002/(sici)1097-0142(19970515)79:10<1944::aid-cncr15>3.0.co;2-v. doi:10.1002/(SICI)1097-0142(19970515)79:10<1944∷AID-CNCR15>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Byers M, Kuiper GG, Gustafsson JA, Park-Sarge OK. Estrogen receptor-β mRNA expression in rat ovary: down-regulation by gonadotropins. Molecular Endocrinology. 1997;11:172–182. doi: 10.1210/mend.11.2.9887. doi:10.1210/me.11.2.172. [DOI] [PubMed] [Google Scholar]
- del Carmen MG, Fuller AF, Matulonis U, Horick NK, Goodman A, Duska LR, Penson R, Campos S, Roche M, Seiden MV. Phase II trial of anastrozole in women with asymptomatic Müllerian cancer. Gynecologic Oncology. 2003;91:596–602. doi: 10.1016/j.ygyno.2003.08.021. doi:10.1016/j.ygyno.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Catalano MG, Pfeffer U, Raineri M, Ferro P, Curto A, Capuzzi P, Corno F, Berta L, Fortunati N. Altered expression of androgen-receptor isoforms in human colon-cancer tissues. International Journal of Cancer. 2000;86:325–330. doi: 10.1002/(sici)1097-0215(20000501)86:3<325::aid-ijc4>3.0.co;2-g. doi:10.1002/(SICI)1097-0215(20000501)86:3<325∷AID-IJC4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Chadha S, Rao BR, Slotman BJ, van Vroonhoven CC, van der Kwast TH. An immunohistochemical evaluation of androgen and progesterone receptors in ovarian tumors. Human Pathology. 1993;24:90–95. doi: 10.1016/0046-8177(93)90067-q. doi:10.1016/0046-8177(93)90067-Q. [DOI] [PubMed] [Google Scholar]
- Chan KK, Wei N, Liu SS, Xiao-Yun L, Cheung AN, Ngan HY. Estrogen receptor subtypes in ovarian cancer: a clinical correlation. Obstetricia et Gynecologica. 2008;111:144–151. doi: 10.1097/01.AOG.0000296715.07705.e9. doi:10.1097/01.AOG.0000296715.07705.e9. [DOI] [PubMed] [Google Scholar]
- Charles NJ, Thomas P, Lange CA. Expression of membrane progesterone receptors (mPR/PAQR) in ovarian cancer cells: implications for progesterone-induced signaling events. Hormones & Cancer. 2010;1:167–176. doi: 10.1007/s12672-010-0023-9. doi:10.1007/s12672-010-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, Liu J, Smith-McCune K, Lu KH, Fishman D, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nature Medicine. 2004a;10:1251–1256. doi: 10.1038/nm1125. doi:10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- Cheng J, Lee EJ, Madison LD, Lazennec G. Expression of estrogen receptor β in prostate carcinoma cells inhibits invasion and proliferation and triggers apoptosis. FEBS Letters. 2004b;566:169–172. doi: 10.1016/j.febslet.2004.04.025. doi:10.1016/j.febslet.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Chien CH, Wang FF, Hamilton TC. Transcriptional activation of c-myc proto-oncogene by estrogen in human ovarian cancer cells. Molecular and Cellular Endocrinology. 1994;99:11–19. doi: 10.1016/0303-7207(94)90140-6. doi:10.1016/0303-7207(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Cho KR, Shih I. Ovarian cancer. Annual Review of Pathology. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. doi:10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Lee KT, Leung PC. Estrogen receptor α pathway is involved in leptin-induced ovarian cancer cell growth. Carcinogenesis. 2011;32:589–596. doi: 10.1093/carcin/bgq276. doi:10.1093/carcin/bgq276. [DOI] [PubMed] [Google Scholar]
- Chura JC, Ryu HS, Simard M, Poirier D, Tremblay Y, Brooker DC, Blomquist CH, Argenta PA. Steroid-converting enzymes in human ovarian carcinomas. Molecular and Cellular Endocrinology. 2009;301:51–58. doi: 10.1016/j.mce.2008.07.015. doi:10.1016/j.mce.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Cibula D, Widschwendter M, Májek O, Dusek L. Tubal ligation and the risk of ovarian cancer: review and meta-analysis. Human Reproduction Update. 2011;17:55–67. doi: 10.1093/humupd/dmq030. doi:10.1093/humupd/dmq030. [DOI] [PubMed] [Google Scholar]
- Clinton GM, Rougeot C, Derancourt J, Roger P, Defrenne A, Godyna S, Argraves WS, Rochefort H. Estrogens increase the expression of fibulin-1, an extracellular matrix protein secreted by human ovarian cancer cells. PNAS. 1996;93:316–320. doi: 10.1073/pnas.93.1.316. doi:10.1073/pnas.93.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen CM, Thomas CM, Borm GF, Hollanders JM, Rolland R. Changes in androgens during treatment with four low-dose contraceptives. Contraception. 1996;53:171–176. doi: 10.1016/0010-7824(96)00006-6. doi:10.1016/0010-7824(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Epidemiological Studies of Ovarian Cancer. Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303–314. doi: 10.1016/S0140-6736(08)60167-1. doi:10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- Cottreau CM, Ness RB, Modugno F, Allen GO, Goodman MT. Endometriosis and its treatment with danazol or lupron in relation to ovarian cancer. Clinical Cancer Research. 2003;9:5142–5144. [PubMed] [Google Scholar]
- Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clinical Medicine & Research. 2007;5:35–44. doi: 10.3121/cmr.2007.702. doi:10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunat S, Rabenoelina F, Daurès JP, Katsaros D, Sasano H, Miller WR, Maudelonde T, Pujol P. Aromatase expression in ovarian epithelial cancers. Journal of Steroid Biochemistry and Molecular Biology. 2005;93:15–24. doi: 10.1016/j.jsbmb.2004.10.021. doi:10.1016/j.jsbmb.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. Breastfeeding and risk of ovarian cancer in two prospective cohorts. Cancer Causes & Control. 2007;18:517–523. doi: 10.1007/s10552-007-0130-2. doi:10.1007/s10552-007-0130-2. [DOI] [PubMed] [Google Scholar]
- Danforth KN, Eliassen AH, Tworoger SS, Missmer SA, Barbieri RL, Rosner BA, Colditz GA, Hankinson SE. The association of plasma androgen levels with breast, ovarian and endometrial cancer risk factors among postmenopausal women. International Journal of Cancer. 2010;126:199–207. doi: 10.1002/ijc.24709. doi:10.1002/ijc.24709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. Journal of Clinical Endocrinology and Metabolism. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. doi:10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- Decruze SB, Green JA. Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. International Journal of Gynecological Cancer. 2007;17:964–978. doi: 10.1111/j.1525-1438.2007.00897.x. doi:10.1111/j.1525-1438.2007.00897.x. [DOI] [PubMed] [Google Scholar]
- De Stefano I, Zannoni GF, Prisco MG, Fagotti A, Tortorella L, Vizzielli G, Mencaglia L, Scambia G, Gallo D. Cytoplasmic expression of estrogen receptor β (ERβ) predicts poor clinical outcome in advanced serous ovarian cancer. Gynecologic Oncology. 2011;122:573–579. doi: 10.1016/j.ygyno.2011.05.025. doi:10.1016/j.ygyno.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Edmondson RJ, Monaghan JM, Davies BR. The human ovarian surface epithelium is an androgen responsive tissue. British Journal of Cancer. 2002;86:879–885. doi: 10.1038/sj.bjc.6600154. doi:10.1038/sj.bjc.6600154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elattar A, Warburton KG, Mukhopadhyay A, Freer RM, Shaheen F, Cross P, Plummer ER, Robson CN, Edmondson RJ. Androgen receptor expression is a biological marker for androgen sensitivity in high grade serous epithelial ovarian cancer. Gynecologic Oncology. 2012;124:142–147. doi: 10.1016/j.ygyno.2011.09.004. doi:10.1016/j.ygyno.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Evangelou A, Jindal SK, Brown TJ, Letarte M. Down-regulation of transforming growth factor β receptors by androgen in ovarian cancer cells. Cancer Research. 2000;60:929–935. [PubMed] [Google Scholar]
- Evangelou A, Letarte M, Jurisica I, Sultan M, Murphy KJ, Rosen B, Brown TJ. Loss of coordinated androgen regulation in nonmalignant ovarian epithelial cells with BRCA1/2 mutations and ovarian cancer cells. Cancer Research. 2003;63:2416–2424. [PubMed] [Google Scholar]
- Fan Y, Xin XY, Chen BL, Ma X. Knockdown of RAB25 expression by RNAi inhibits growth of human epithelial ovarian cancer cells in vitro and in vivo. Pathology. 2006;38:561–567. doi: 10.1080/00313020601024037. doi:10.1080/00313020601024037. [DOI] [PubMed] [Google Scholar]
- Fleming GF, Amato JM, Agresti M, Safa AR. Megestrol acetate reverses multidrug resistance and interacts with P-glycoprotein. Cancer Chemotherapy and Pharmacology. 1992;29:445–449. doi: 10.1007/BF00684845. doi:10.1007/BF00684845. [DOI] [PubMed] [Google Scholar]
- Freedman RS, Saul PB, Edwards CL, Jolles CJ, Gershenson DM, Jones LA, Atkinson EN, Dana WJ. Ethinyl estradiol and medroxyprogesterone acetate in patients with epithelial ovarian carcinoma: a phase II study. Cancer Treatment Reports. 1986;70:369–373. [PubMed] [Google Scholar]
- Fromm GL, Freedman RS, Fritsche HA, Atkinson EN, Scott W. Sequentially administered ethinyl estradiol and medroxyprogesterone acetate in the treatment of refractory epithelial ovarian carcinoma in patients with positive estrogen receptors. Cancer. 1991;68:1885–1889. doi: 10.1002/1097-0142(19911101)68:9<1885::aid-cncr2820680906>3.0.co;2-i. doi:10.1002/1097-0142(19911101)68:9<1885∷AID-CNCR2820680906>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Hidaka T, Kataoka K, Yamakawa Y, Akada S, Teranishi A, Saito S. Absence of estrogen receptor-α expression in human ovarian clear cell adenocarcinoma compared with ovarian serous, endometrioid, and mucinous adenocarcinoma. American Journal of Surgical Pathology. 2001;25:667–672. doi: 10.1097/00000478-200105000-00016. doi:10.1097/00000478-200105000-00016. [DOI] [PubMed] [Google Scholar]
- Galtier-Dereure F, Capony F, Maudelonde T, Rochefort H. Estradiol stimulates cell growth and secretion of procathepsin D and a 120-kilodalton protein in the human ovarian cancer cell line BG-1. Journal of Clinical Endocrinology and Metabolism. 1992;75:1497–1502. doi: 10.1210/jcem.75.6.1464654. doi:10.1210/jc.75.6.1497. [DOI] [PubMed] [Google Scholar]
- Gaspard UJ, Romus MA, Gillain D, Duvivier J, Demey-Ponsart E, Franchimont P. Plasma hormone levels in women receiving new oral contraceptives containing ethinyl estradiol plus levonorgestrel or desogestrel. Contraception. 1983;27:577–590. doi: 10.1016/0010-7824(83)90023-9. doi:10.1016/0010-7824(83)90023-9. [DOI] [PubMed] [Google Scholar]
- Geisler HE. The use of high-dose megestrol acetate in the treatment of ovarian adenocarcinoma. Seminars in Oncology. 1985;12(Suppl 1):20–22. [PubMed] [Google Scholar]
- Geisler JP, Buller E, Manahan KJ. Estrogen receptor α and β expression in a case matched series of serous and endometrioid adenocarcinomas of the ovary. European Journal of Gynaecological Oncology. 2008;29:126–128. [PubMed] [Google Scholar]
- Gennatas C, et al. Phase II trial of tamoxifen in patients with advanced epithelial ovarian cancer. ASCO Annual Meeting. 1996 [Google Scholar]
- Glavind K, Grove A. Estrogen and progesterone receptors in epithelial ovarian tumours. APMIS. 1990;98:916–920. doi: 10.1111/j.1699-0463.1990.tb05015.x. doi:10.1111/j.1699-0463.1990.tb05015.x. [DOI] [PubMed] [Google Scholar]
- Gogoi R, Kudla M, Gil O, Fishman D. The activity of medroxyprogesterone acetate, an androgenic ligand, in ovarian cancer cell invasion. Reproductive Sciences. 2008;15:846–852. doi: 10.1177/1933719108323446. doi:10.1177/1933719108323446. [DOI] [PubMed] [Google Scholar]
- Gourley C, Smith JF, Mackean M, Stevenson A, et al. Phase II study of letrozole in estrogen receptor (ER) positive relapsed epithelial ovarian cancer (EOC) Journal of Clinical Oncology. 2006;24 Abstract 5026. [Google Scholar]
- Greer JB, Modugno F, Allen GO, Ness RB. Androgenic progestins in oral contraceptives and the risk of epithelial ovarian cancer. Obstetrics and Gynecology. 2005;105:731–740. doi: 10.1097/01.AOG.0000154152.12088.48. doi:10.1097/01.AOG.0000154152.12088.48. [DOI] [PubMed] [Google Scholar]
- Hall JM, Korach KS. Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Molecular Endocrinology. 2003;17:792–803. doi: 10.1210/me.2002-0438. doi:10.1210/me.2002-0438. [DOI] [PubMed] [Google Scholar]
- Hamerlynck JV, Maskens AP, Mangioni C, van der Burg ME, Wils JA, Vermorken JB, Rotmensz N. Phase II trial of medroxyprogesterone acetate in advanced ovarian cancer: an EORTC Gynecological Cancer Cooperative Group Study. Gynecologic Oncology. 1985a;22:313–316. doi: 10.1016/0090-8258(85)90045-9. doi:10.1016/0090-8258(85)90045-9. [DOI] [PubMed] [Google Scholar]
- Hamerlynck JV, Vermorken JB, Van der Burgh ME. Tamoxifen therapy in advanced ovarian cancer: a phase II study. Proceedings of the American Society of Clinical Oncology. 1985b [Google Scholar]
- Haning RV, Jr, Kiggens AJ, Leiheit TL. Maternal serum progesterone, 17β-estradiol and estriol are increased in pregnancies which follow treatment with human menopausal gonadotropins: effects of multiple gestation and maternal endocrine status. Journal of Steroid Biochemistry. 1985;22:823–829. doi: 10.1016/0022-4731(85)90292-4. doi:10.1016/0022-4731(85)90292-4. [DOI] [PubMed] [Google Scholar]
- Hasan J, Ton N, Mullamitha S, Clamp A, McNeilly A, Marshall E, Jayson GC. Phase II trial of tamoxifen and goserelin in recurrent epithelial ovarian cancer. British Journal of Cancer. 2005;93:647–651. doi: 10.1038/sj.bjc.6602752. doi:10.1038/sj.bjc.6602752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch KD, Beecham JB, Blessing JA, Creasman WT. Responsiveness of patients with advanced ovarian carcinoma to tamoxifen. A Gynecologic Oncology Group study of second-line therapy in 105 patients. Cancer. 1991;68:269–271. doi: 10.1002/1097-0142(19910715)68:2<269::aid-cncr2820680209>3.0.co;2-o. doi:10.1002/1097-0142(19910715)68:2<269∷AID-CNCR2820680209>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Havrilesky LJ, McMahon CP, Lobenhofer EK, Whitaker R, Marks JR, Berchuck A. Relationship between expression of coactivators and corepressors of hormone receptors and resistance of ovarian cancers to growth regulation by steroid hormones. Journal of the Society for Gynecologic Investigation. 2001;8:104–113. doi:10.1016/S1071-5576(01)00093-4. [PubMed] [Google Scholar]
- Helzlsouer KJ, Alberg AJ, Gordon GB, Longcope C, Bush TL, Hoffman SC, Comstock GW. Serum gonadotropins and steroid hormones and the development of ovarian cancer. Journal of the American Medical Association. 1995;274:1926–1930. doi:10.1001/jama.1995.03530240036037. [PubMed] [Google Scholar]
- Hillier SG, Anderson RA, Williams AR, Tetsuka M. Expression of oestrogen receptor α and β in cultured human ovarian surface epithelial cells. Molecular Human Reproduction. 1998;4:811–815. doi: 10.1093/molehr/4.8.811. doi:10.1093/molehr/4.8.811. [DOI] [PubMed] [Google Scholar]
- Hiscox S, Morgan L, Barrow D, Dutkowskil C, Wakeling A, Nicholson RI. Tamoxifen resistance in breast cancer cells is accompanied by an enhanced motile and invasive phenotype: inhibition by gefitinib (`Iressa', ZD1839) Clinical & Experimental Metastasis. 2004;21:201–212. doi: 10.1023/b:clin.0000037697.76011.1d. doi:10.1023/B:CLIN.0000037697.76011.1d. [DOI] [PubMed] [Google Scholar]
- Hofstra LS, Mourits MJ, de Vries EG, Mulder NH, Willemse PH. Combined treatment with goserelin and tamoxifen in patients with advanced chemotherapy resistant ovarian cancer. Anticancer Research. 1999;19:3627–3630. [PubMed] [Google Scholar]
- Horne AW, King AE, Shaw E, McDonald SE, Williams AR, Saunders PT, Critchley HO. Attenuated sex steroid receptor expression in fallopian tube of women with ectopic pregnancy. Journal of Clinical Endocrinology and Metabolism. 2009;94:5146–5154. doi: 10.1210/jc.2009-1476. doi:10.1210/jc.2009-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, editors. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: 2011. http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site. [Google Scholar]
- Hua W, Christianson T, Rougeot C, Rochefort H, Clinton GM. SKOV3 ovarian carcinoma cells have functional estrogen receptor but are growth-resistant to estrogen and antiestrogens. Journal of Steroid Biochemistry and Molecular Biology. 1995;55:279–289. doi: 10.1016/0960-0760(95)00187-5. doi:10.1016/0960-0760(95)00187-5. [DOI] [PubMed] [Google Scholar]
- Hua K, Feng W, Cao Q, Zhou X, Lu X, Feng Y. Estrogen and progestin regulate metastasis through the PI3K/AKT pathway in human ovarian cancer. International Journal of Oncology. 2008;33:959–967. [PubMed] [Google Scholar]
- Hurteau JA, Brady MF, Darcy KM, McGuire WP, Edmonds P, Pearl ML, Ivanov I, Tewari KS, Mannel RS, Zanotti K, et al. Randomized phase III trial of tamoxifen versus thalidomide in women with biochemical-recurrent-only epithelial ovarian, fallopian tube or primary peritoneal carcinoma after a complete response to first-line platinum/taxane chemotherapy with an evaluation of serum vascular endothelial growth factor (VEGF): A Gynecologic Oncology Group Study. Gynecologic Oncology. 2010;119:444–450. doi: 10.1016/j.ygyno.2010.08.002. doi:10.1016/j.ygyno.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Ilekis JV, Connor JP, Prins GS, Ferrer K, Niederberger C, Scoccia B. Expression of epidermal growth factor and androgen receptors in ovarian cancer. Gynecologic Oncology. 1997;66:250–254. doi: 10.1006/gyno.1997.4764. doi:10.1006/gyno.1997.4764. [DOI] [PubMed] [Google Scholar]
- Jager W, Sauerbrei W, Beck E, Maassen V, Stumpfe M, Meier W, Kuhn W, Janicke F. A randomized comparison of triptorelin and tamoxifen as treatment of progressive ovarian cancer. Anticancer Research. 1995;15:2639–2642. [PubMed] [Google Scholar]
- Jakobsen A, Bertelsen K, Sell A. Cyclic hormonal treatment in ovarian cancer. A phase-II trial. European Journal of Cancer and Clinical Oncology. 1987;23:915–916. doi: 10.1016/0277-5379(87)90335-x. doi:10.1016/0277-5379(87)90335-X. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. doi:10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Ozols RF, Hamilton TC. Mechanisms of drug resistance in ovarian cancer. Cancer. 1993;71(2 Suppl):644–649. doi: 10.1002/cncr.2820710224. [DOI] [PubMed] [Google Scholar]
- Joseph D, Ho SM, Syed V. Hormonal regulation and distinct functions of semaphorin-3B and semaphorin-3F in ovarian cancer. Molecular Cancer Therapeutics. 2010;9:499–509. doi: 10.1158/1535-7163.MCT-09-0664. doi:10.1158/1535-7163.MCT-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlan BY, Jones J, Greenwald M, Lagasse LD. Steroid hormone effects on the proliferation of human ovarian surface epithelium in vitro. American Journal of Obstetrics and Gynecology. 1995;173:97–104. doi: 10.1016/0002-9378(95)90176-0. doi:10.1016/0002-9378(95)90176-0. [DOI] [PubMed] [Google Scholar]
- Kelemen LE, Kobel M. Mucinous carcinomas of the ovary and colorectum: different organ, same dilemma. Lancet Oncology. 2011;12:1071–1080. doi: 10.1016/S1470-2045(11)70058-4. doi:10.1016/S1470-2045(11)70058-4. [DOI] [PubMed] [Google Scholar]
- Kelley KM, Rowan BG, Ratnam M. Modulation of the folate receptor α gene by the estrogen receptor: mechanism and implications in tumor targeting. Cancer Research. 2003;63:2820–2828. [PubMed] [Google Scholar]
- Killick S, Eyong E, Elstein M. Ovarian follicular development in oral contraceptive cycles. Fertility and Sterility. 1987;48:409–413. doi: 10.1016/s0015-0282(16)59407-2. [DOI] [PubMed] [Google Scholar]
- King ER, Wong KK. In: Steroid Hormones and Ovarian Cancer. Steroids - Clinical Aspect. Abduljabbar H, editor. 2011. ISBN: 978-953-307-705-5, InTech. [Google Scholar]
- Korch C, Spillman MA, Jackson TA, Jacobsen BM, Murphy SK, Lessey BA, Jordan VC, Bradford AP. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecologic Oncology. 2012 doi: 10.1016/j.ygyno.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasner C. Aromatase inhibitors in gynecologic cancers. Journal of Steroid Biochemistry and Molecular Biology. 2007;106:76–80. doi: 10.1016/j.jsbmb.2007.05.026. doi:10.1016/j.jsbmb.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Kühnel R, de Graaff J, Rao BR, Stolk JG. Androgen receptor predominance in human ovarian carcinoma. Journal of Steroid Biochemistry. 1987;26:393–397. doi: 10.1016/0022-4731(87)90106-3. doi:10.1016/0022-4731(87)90106-3. [DOI] [PubMed] [Google Scholar]
- Kuiper G, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-A. Cloning of a novel receptor expressed in rat prostate and ovary. PNAS. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. doi:10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landoni F, Regallo M, Vassena L, Bonazzi C. Antiestrogen as last-line treatment in epithelial ovarian cancer. Chemioterapia. 1985 [Google Scholar]
- Langdon SP, Smyth JF. Hormone therapy for epithelial ovarian cancer. Current Opinion in Oncology. 2008;20:548–553. doi: 10.1097/CCO.0b013e3283063912. doi:10.1097/CCO.0b013e3283063912. [DOI] [PubMed] [Google Scholar]
- Langdon SP, Hirst GL, Miller EP, Hawkins RA, Tesdale AL, Smyth JF, Miller WR. The regulation of growth and protein expression by estrogen in vitro: a study of 8 human ovarian carcinoma cell lines. Journal of Steroid Biochemistry and Molecular Biology. 1994;50:131–135. doi: 10.1016/0960-0760(94)90019-1. doi:10.1016/0960-0760(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Langdon SP, Gabra H, Bartlett JM, Rabiaz GJ, Hawkins RA, Tesdale AL, Ritchie AA, Miller WR, Smyth JF. Functionality of the progesterone receptor in ovarian cancer and its regulation by estrogen. Clinical Cancer Research. 1998;4:2245–2251. [PubMed] [Google Scholar]
- Lau KM, Mok SC, Ho SM. Expression of human estrogen receptor-α and -β, progesterone receptor, and androgen receptor mRNA in normal and malignant ovarian epithelial cells. PNAS. 1999;96:5722–5727. doi: 10.1073/pnas.96.10.5722. doi:10.1073/pnas.96.10.5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Mühlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. Journal of Clinical Endocrinology and Metabolism. 2000;85:645–651. doi: 10.1210/jcem.85.2.6405. doi:10.1210/jc.85.2.645. [DOI] [PubMed] [Google Scholar]
- Laviolette LA, Garson K, Macdonald EA, Senterman MK, Courville K, Crane CA, Vanderhyden BC. 17β-Estradiol accelerates tumor onset and decreases survival in a transgenic mouse model of ovarian cancer. Endocrinology. 2010;151:929–938. doi: 10.1210/en.2009-0602. doi:10.1210/en.2009-0602. [DOI] [PubMed] [Google Scholar]
- Lazennec G. Estrogen receptor β, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Letters. 2006;231:151–157. doi: 10.1016/j.canlet.2005.01.021. doi:10.1016/j.canlet.2005.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KR, Young RH. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. American Journal of Surgical Pathology. 2003;27:281–292. doi: 10.1097/00000478-200303000-00001. doi:10.1097/00000478-200303000-00001. [DOI] [PubMed] [Google Scholar]
- Lee E, Hsu C, Haiman CA, Razavi P, Horn-Ross PL, Van Den Berg D, Bernstein L, Le Marchand L, Henderson BE, Setiawan VW, et al. Genetic variation in the progesterone receptor gene and ovarian cancer risk. American Journal of Epidemiology. 2005a;161:442–451. doi: 10.1093/aje/kwi064. doi:10.1093/aje/kwi010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Rosen DG, Zhu C, Silva EG, Liu J. Expression of progesterone receptor is a favorable prognostic marker in ovarian cancer. Gynecologic Oncology. 2005b;96:671–677. doi: 10.1016/j.ygyno.2004.11.010. doi:10.1016/j.ygyno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Levine D, Park K, Juretzka M, Esch J, Hensley M, Aghajanian C, Lewin S, Konner J, Derosa F, Spriggs D, et al. A phase II evaluation of goserelin and bicalutamide in patients with ovarian cancer in second or higher complete clinical disease remission. Cancer. 2007;110:2448–2456. doi: 10.1002/cncr.23072. doi:10.1002/cncr.23072. [DOI] [PubMed] [Google Scholar]
- Li LC, Yeh CC, Nojima D, Dahiya R. Cloning and characterization of human estrogen receptor β promoter. Biochemical and Biophysical Research Communications. 2000;275:682–689. doi: 10.1006/bbrc.2000.3363. doi:10.1006/bbrc.2000.3363. [DOI] [PubMed] [Google Scholar]
- Ligr M, Patwa RR, Daniels G, Pan L, Wu X, Li Y, Tian L, Wang Z, Xu R, Wu J, et al. Expression and function of androgen receptor coactivator p44/Mep50/WDR77 in ovarian cancer. PLoS ONE. 2011;6:e26250. doi: 10.1371/journal.pone.0026250. doi:10.1371/journal.pone.0026250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren P, Bäckström T, Mählck CG, Ridderheim M, Cajander S. Steroid receptors and hormones in relation to cell proliferation and apoptosis in poorly differentiated epithelial ovarian tumors. International Journal of Oncology. 2001;19:31–38. [PubMed] [Google Scholar]
- Losa G, Landoni F, Pellegrino A, Parma G, Miceli D, Maneo A, Marzola M. Treatment of advanced ovarian cancer by hormone. International Journal of Gynecological Cancer. 1993;3(suppl 1):66. [Google Scholar]
- Ludwig AH, Murawska M, Panek G, Timorek A, Kupryjanczyk J. Androgen, progesterone, and FSH receptor polymorphisms in ovarian cancer risk and outcome. Endocrine-Related Cancer. 2009;16:1005–1016. doi: 10.1677/ERC-08-0135. doi:10.1677/ERC-08-0135. [DOI] [PubMed] [Google Scholar]
- Lukanova A, Kaaks R. Endogenous hormones and ovarian cancer: epidemiology and current hypotheses. Cancer Epidemiology, Biomarkers & Prevention. 2005;14:98–107. [PubMed] [Google Scholar]
- Lukanova A, Lundin E, Akhmedkhanov A, Micheli A, Rinaldi S, Zeleniuch-Jacquotte A, Lenner P, Muti P, Biessy C, Krogh V, et al. Circulating levels of sex steroid hormones and risk of ovarian cancer. International Journal of Cancer. 2003;104:636–642. doi: 10.1002/ijc.10990. doi:10.1002/ijc.10990. [DOI] [PubMed] [Google Scholar]
- Lurie G, Thompson P, McDuffie KE, Carney ME, Terada KY, Goodman MT. Association of estrogen and progestin potency of oral contraceptives with ovarian carcinoma risk. Obstetrics and Gynecology. 2007;109:597–607. doi: 10.1097/01.AOG.0000255664.48970.e6. doi:10.1097/01.AOG.0000255664.48970.e6. [DOI] [PubMed] [Google Scholar]
- Lurie G, Wilkens LR, Thompson PJ, Shvetsov YB, Matsuno RK, Carney ME, Palmieri RT, Wu AH, Pike MC, Pearce CL, et al. Estrogen receptor β rs1271572 polymorphism and invasive ovarian carcinoma risk: pooled analysis within the Ovarian Cancer Association Consortium. PLoS ONE. 2011;6:e20703. doi: 10.1371/journal.pone.0020703. doi:10.1371/journal.pone.0020703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfetano J, Beecham JB, Bundy BN, Hatch KD. A phase II trial of medroxyprogesterone acetate in epithelial ovarian cancers. A Gynecologic Oncology Group study. American Journal of Clinical Oncology. 1993;16:149–151. doi: 10.1097/00000421-199304000-00014. doi:10.1097/00000421-199304000-00014. [DOI] [PubMed] [Google Scholar]
- Mangioni C, Franceschi S, La Vecchia C, D'Incalci M. High-dose medroxyprogesterone acetate (MPA) in advanced epithelial ovarian cancer resistant to first- or second-line chemotherapy. Gynecologic Oncology. 1981;12:314–318. doi: 10.1016/0090-8258(81)90131-1. doi:10.1016/0090-8258(81)90131-1. [DOI] [PubMed] [Google Scholar]
- Markman M, Bookman MA. Second-line treatment of ovarian cancer. Oncologist. 2000;5:26–35. doi: 10.1634/theoncologist.5-1-26. doi:10.1634/theoncologist.5-1-26. [DOI] [PubMed] [Google Scholar]
- Markman M, Iseminger KA, Hatch KD, Creasman WT, Barnes W, Dubeshter B. Tamoxifen in platinum-refractory ovarian cancer: a Gynecologic Oncology Group Ancillary Report. Gynecologic Oncology. 1996;62:4–6. doi: 10.1006/gyno.1996.0181. doi:10.1006/gyno.1996.0181. [DOI] [PubMed] [Google Scholar]
- Markman M, Webster K, Zanotti K, Rohl J, Belinson J. Use of tamoxifen in asymptomatic patients with recurrent small-volume ovarian cancer. Gynecologic Oncology. 2004a;93:390–393. doi: 10.1016/j.ygyno.2004.01.035. doi:10.1016/j.ygyno.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Markman M, Webster K, Zanotti K, Peterson G, Kulp B, Belinson J. Phase 2 trial of carboplatin plus tamoxifen in platinum-resistant ovarian cancer and primary carcinoma of the peritoneum. Gynecologic Oncology. 2004b;94:404–408. doi: 10.1016/j.ygyno.2004.05.004. doi:10.1016/j.ygyno.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Marth C, Sorheim N, Kaern J, Trope C. Tamoxifen in the treatment of recurrent ovarian carcinoma. International Journal of Gynecological Cancer. 1997;7:256–261. doi:10.1046/j.1525-1438.1997.00463.x. [Google Scholar]
- Menin C, Banna GL, De Salvo G, Lazzarotto V, De Nicolo A, Agata S, Montagna M, Sordi G, Nicoletto O, Chieco-Bianchi L, et al. Lack of association between androgen receptor CAG polymorphism and familial breast/ovarian cancer. Cancer Letters. 2001;168:31–36. doi: 10.1016/s0304-3835(01)00473-6. doi:10.1016/S0304-3835(01)00473-6. [DOI] [PubMed] [Google Scholar]
- Modugno F, Edwards RP. Ovarian cancer: prevention, detection, and treatment of the disease and its recurrence. Molecular mechanisms and personalized medicine meeting report. International Journal of Gynecological Cancer. 2012;22:S45–S57. doi: 10.1097/IGC.0b013e31826bd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll F, Katsaros D, Lazennec G, Hellio N, Roger P, Giacalone PL, Chalbos D, Maudelonde T, Rochefort H, Pujol P. Estrogen induction and overexpression of fibulin-1C mRNA in ovarian cancer cells. Oncogene. 2002;21:1097–1107. doi: 10.1038/sj.onc.1205171. doi:10.1038/sj.onc.1205171. [DOI] [PubMed] [Google Scholar]
- Moreau JP, Delavault P, Blumberg J. Luteinizing hormone-releasing hormone agonists in the treatment of prostate cancer: a review of their discovery, development, and place in therapy. Clinical Therapeutics. 2006;28:1485–1508. doi: 10.1016/j.clinthera.2006.10.018. doi:10.1016/j.clinthera.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Münstedt K, Steen J, Knauf AG, Buch T, von Georgi R, Franke FE. Steroid hormone receptors and long term survival in invasive ovarian cancer. Cancer. 2000;89:1783–1791. doi: 10.1002/1097-0142(20001015)89:8<1783::aid-cncr19>3.0.co;2-d. doi:10.1002/1097-0142(20001015)89:8<1783::AID-CNCR19>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Van Kirk EA, Isaak DD, Shen Y. Progesterone facilitates cisplatin toxicity in epithelial ovarian cancer cells and xenografts. Gynecologic Oncology. 2008;110:251–255. doi: 10.1016/j.ygyno.2008.03.021. doi:10.1016/j.ygyno.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A, Cropp CS, Smith BS, Burkman RT, Zacur HA. Effect of low-dose oral contraceptive on gonadotropins, androgens, and sex hormone binding globulin in nonhirsute women. Fertility and Sterility. 1990;53:35–39. [PubMed] [Google Scholar]
- Nahleh Z. Androgen receptor as a target for the treatment of hormone receptor-negative breast cancer: an unchartered territory. Future Oncology. 2008;4:15–21. doi: 10.2217/14796694.4.1.15. doi:10.2217/14796694.4.1.15. [DOI] [PubMed] [Google Scholar]
- Ness RB, Grisso JA, Klapper J, Schlesselman JJ, Silberzweig S, Vergona R, Morgan M, Wheeler JE. Risk of ovarian cancer in relation to estrogen and progestin dose and use characteristics of oral contraceptives. SHARE Study Group. Steroid Hormones and Reproductions. American Journal of Epidemiology. 2000;152:233–241. doi: 10.1093/aje/152.3.233. doi:10.1093/aje/152.3.233. [DOI] [PubMed] [Google Scholar]
- Nezhat F, Datta MS, Hanson V, Pejovic T, Nezhat C, Nezhat C. The relationship of endometriosis and ovarian malignancy: a review. Fertility and Sterility. 2008;90:1559–1570. doi: 10.1016/j.fertnstert.2008.08.007. doi:10.1016/j.fertnstert.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Niwa K, Onogi K, Wu Y, Mori H, Harrigan RC, Tamaya T. Clinical implication of medroxyprogesterone acetate against advanced ovarian carcinoma: a pilot study. European Journal of Gynaecological Oncology. 2008;29:252–255. [PubMed] [Google Scholar]
- O'Donnell AJ, Macleod KG, Burns DJ, Smyth JF, Langdon SP. Estrogen receptor-α mediates gene expression changes and growth response in ovarian cancer cells exposed to estrogen. Endocrine-Related Cancer. 2005;12:851–866. doi: 10.1677/erc.1.01039. doi:10.1677/erc.1.01039. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Green AC, Nagle CM, Jordan SJ, Whiteman DC, Bain CJ, Webb PM, Australian Cancer Study Group (Ovarian Cancer) the Australian Ovarian Cancer Study Group Epithelial ovarian cancer: testing the `androgens hypothesis'. Endocrine-Related Cancer. 2008;15:1061–1068. doi: 10.1677/ERC-08-0075. doi:10.1677/ERC-08-0075. [DOI] [PubMed] [Google Scholar]
- Osborne RJ, Malik ST, Slevin ML, Harvey VJ, Spona J, Salzer H, Williams CJ. Tamoxifen in refractory ovarian cancer: the use of a loading dose schedule. British Journal of Cancer. 1988;57:115–116. doi: 10.1038/bjc.1988.22. doi:10.1038/bjc.1988.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasci L, Jean-Claude BJ, Vasilescu D, Mustafa A, Damian S, Damian Z, Georges E, Liu Z, Batist G, Leyland-Jones B. Sensitization to doxorubicin resistance in breast cancer cell lines by tamoxifen and megestrol acetate. Biochemical Pharmacology. 1996;52:1097–1102. doi: 10.1016/0006-2952(96)00456-x. doi:10.1016/0006-2952(96)00456-X. [DOI] [PubMed] [Google Scholar]
- Papadimitriou CA, Markaki S, Siapkaras J, Vlachos G, Efstathiou E, Grimani I, Hamilos G, Zorzou M, Dimopoulos MA. Hormonal therapy with letrozole for relapsed epithelial ovarian cancer. Long-term results of a phase II study. Oncology. 2004;66:112–117. doi: 10.1159/000077436. doi:10.1159/000077436. [DOI] [PubMed] [Google Scholar]
- Patel NH, Rothenberg ML. Multidrug resistance in cancer chemotherapy. Investigational New Drugs. 1994;12:1–13. doi: 10.1007/BF00873229. doi:10.1007/BF00873229. [DOI] [PubMed] [Google Scholar]
- Pearce CL, Wu AH, Gayther SA, Bale AE, Australian Cancer Study (Ovarian Cancer) Australian Cancer Study Group. Beck PA, Beesley J, Chanock S, Cramer DW, DiCioccio R, et al. Progesterone receptor variation and risk of ovarian cancer is limited to the invasive endometrioid subtype: results from the Ovarian Cancer Association Consortium pooled analysis. British Journal of Cancer. 2008;98:282–288. doi: 10.1038/sj.bjc.6604170. doi:10.1038/sj.bjc.6604170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce CL, Chung K, Pike MC, Wu AH. Increased ovarian cancer risk associated with menopausal estrogen therapy is reduced by adding a progestin. Cancer. 2009;115:531–539. doi: 10.1002/cncr.23956. doi:10.1002/cncr.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncology. 2012 doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso JJ. Non-genomic actions of progesterone in the normal and neoplastic mammalian ovary. Seminars in Reproductive Medicine. 2007;25:198–207. doi: 10.1055/s-2007-973432. doi:10.1055/s-2007-973432. [DOI] [PubMed] [Google Scholar]
- Peluso JJ. Progesterone signaling mediated through progesterone receptor membrane component-1 in ovarian cells with special emphasis on ovarian cancer. Steroids. 2011;76:903–909. doi: 10.1016/j.steroids.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso JJ, Liu X, Saunders MM, Claffey KP, Phoenix K. Regulation of ovarian cancer cell viability and sensitivity to cisplatin by progesterone receptor membrane component-1. Journal of Clinical Endocrinology and Metabolism. 2008;93:1592–1599. doi: 10.1210/jc.2007-2771. doi:10.1210/jc.2007-2771. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Gawkowska A, Liu X, Shioda T, Pru JK. Progesterone receptor membrane component-1 regulates the development and Cisplatin sensitivity of human ovarian tumors in athymic nude mice. Endocrinology. 2009;150:4846–4854. doi: 10.1210/en.2009-0730. doi:10.1210/en.2009-0730. [DOI] [PubMed] [Google Scholar]
- Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS. Mortality of women with polycystic ovary syndrome at long-term follow-up. Journal of Clinical Epidemiology. 1998;51:581–586. doi: 10.1016/s0895-4356(98)00035-3. doi:10.1016/S0895-4356(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertility and Sterility. 2004;82:186–195. doi: 10.1016/j.fertnstert.2004.03.013. doi:10.1016/j.fertnstert.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Poole R, Paridaens R. The use of third-generation aromatase inhibitors and tamoxifen in the adjuvant treatment of postmenopausal patients with hormone-dependent breast cancer: evidence based review. Current Opinion in Oncology. 2007;19:564–572. doi: 10.1097/CCO.0b013e3282f1c523. doi:10.1097/CCO.0b013e3282f1c523. [DOI] [PubMed] [Google Scholar]
- Prior JC. Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation. Endocrine. 2005;26:297–300. doi: 10.1385/ENDO:26:3:297. doi:10.1385/ENDO:26:3:297. [DOI] [PubMed] [Google Scholar]
- Ramirez PT, Schmeler KM, Milam MR, Slomovitz BM, Smith JA, Kavanagh JJ, Deavers M, Levenback C, Coleman RL, Gershenson DM. Efficacy of letrozole in the treatment of recurrent platinum- and taxane-resistant high-grade cancer of the ovary or peritoneum. Gynecologic Oncology. 2008;110:56–59. doi: 10.1016/j.ygyno.2008.03.014. doi:10.1016/j.ygyno.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Ramondetta LM, Johnson AJ, Sun CC, Atkinson N, Smith JA, Jung MS, Broaddus R, Iyer RB, Burke T. Phase 2 trial of mifepristone (RU-486) in advanced or recurrent endometrioid adenocarcinoma or low-grade endometrial stromal sarcoma. Cancer. 2009;115:1867–1874. doi: 10.1002/cncr.24197. doi:10.1002/cncr.24197. [DOI] [PubMed] [Google Scholar]
- Rao BR, Slotman BJ. Endocrine factors in common epithelial ovarian cancer. Endocrine Reviews. 1991;12:14–26. doi: 10.1210/edrv-12-1-14. doi:10.1210/edrv-12-1-14. [DOI] [PubMed] [Google Scholar]
- Riman T, Persson I, Nilsson S. Hormonal aspects of epithelial ovarian cancer: review of epidemiological evidence. Clinical Endocrinology. 1998;49:695–707. doi: 10.1046/j.1365-2265.1998.00577.x. doi:10.1046/j.1365-2265.1998.00577.x. [DOI] [PubMed] [Google Scholar]
- Riman T, Nilsson S, Persson IR. Review of epidemiological evidence for reproductive and hormonal factors in relation to the risk of epithelial ovarian malignancies. Acta Obstetricia et Gynecologica Scandinavica. 2004;83:783–795. doi: 10.1111/j.0001-6349.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Dossus L, Lukanova A, Peeters PH, Allen NE, Key T, Bingham S, Khaw KT, Trichopoulos D, Trichopoulou A, et al. Endogenous androgens and risk of epithelial ovarian cancer: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Epidemiology, Biomarkers & Prevention. 2007;16:23–29. doi: 10.1158/1055-9965.EPI-06-0755. doi:10.1158/1055-9965.EPI-06-0755. [DOI] [PubMed] [Google Scholar]
- Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. Journal of the National Cancer Institute. 1998;90:1774–1786. doi: 10.1093/jnci/90.23.1774. doi:10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- Ritchie AA, Langdon SP. Estrogen-responsive ovarian cancer xenografts. Methods in Molecular Medicine. 2001;39:193–198. doi: 10.1385/1-59259-071-3:193. [DOI] [PubMed] [Google Scholar]
- Rocereto TF, Saul HM, Aikins JA, Jr, Paulson J. Phase II study of mifepristone (RU486) in refractory ovarian cancer. Gynecologic Oncology. 2000;77:429–432. doi: 10.1006/gyno.2000.5789. doi:10.1006/gyno.2000.5789. [DOI] [PubMed] [Google Scholar]
- Rocereto TF, Brady WE, Shahin MS, Hoffman JS, Small L, Rotmensch J, Mannel RS. A phase II evaluation of mifepristone in the treatment of recurrent or persistent epithelial ovarian, fallopian or primary peritoneal cancer: a gynecologic oncology group study. Gynecologic Oncology. 2010;116:332–334. doi: 10.1016/j.ygyno.2009.10.071. doi:10.1016/j.ygyno.2009.10.071. [DOI] [PubMed] [Google Scholar]
- Rochefort H, Chalbos D, Cunat S, Lucas A, Platet N, Garcia M. Estrogen regulated proteases and antiproteases in ovarian and breast cancer cells. Journal of Steroid Biochemistry and Molecular Biology. 2001;76:119–124. doi: 10.1016/s0960-0760(00)00142-4. doi:10.1016/S0960-0760(00)00142-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez GC, Walmer DK, Cline M, Krigman H, Lessey BA, Whitaker RS, Dodge R, Hughes CL. Effect of progestin on the ovarian epithelium of macaques: cancer prevention through apoptosis? Journal of the Society for Gynecologic Investigation. 1998;5:271–276. doi: 10.1016/s1071-5576(98)00017-3. doi:10.1016/S1071-5576(98)00017-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez GC, Nagarsheth NP, Lee KL, Bentley RC, Walmer DK, Cline M, Whitaker RS, Isner P, Berchuck A, Dodge RK, et al. Progestin-induced apoptosis in the Macaque ovarian epithelium: differential regulation of transforming growth factor-β. Journal of the National Cancer Institute. 2002;94:50–60. doi: 10.1093/jnci/94.1.50. doi:10.1093/jnci/94.1.50. [DOI] [PubMed] [Google Scholar]
- Rodriguez GC, Turbov JM, Berchuck A, Stack MS, Hurteau JA, Thaete LG, Barry CP. Nonsteroidal antiinflammatory drugs and progestins synergistically enhance cell death in ovarian epithelial cells. American Journal of Obstetrics and Gynecology. 2012;253:e1–e9. doi: 10.1016/j.ajog.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Roger P, Pujol P, Lucas A, Baldet P, Rochefort H. Increased immunostaining of fibulin-1, an estrogen-regulated protein in the stroma of human ovarian epithelial tumors. American Journal of Pathology. 1998;153:1579–1588. doi: 10.1016/S0002-9440(10)65746-X. doi:10.1016/S0002-9440(10)65746-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez M, Peiper SC, Evans B, Wang Z, Catasús L, Ribe A, Prat J, Giri JG. Expression profile of heptahelical putative membrane progesterone receptors in epithelial ovarian tumors. Human Pathology. 2008;39:1026–1033. doi: 10.1016/j.humpath.2007.11.007. doi:10.1016/j.humpath.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Palmer JR, Zauber AG, Warshauer ME, Lewis JL, Jr, Strom BL, Harlap S, Shapiro S. A case-control study of oral contraceptive use and invasive epithelial ovarian cancer. American Journal of Epidemiology. 1994;139:654–661. doi: 10.1093/oxfordjournals.aje.a117055. [DOI] [PubMed] [Google Scholar]
- Rosenblatt KA, Thomas DB. Lactation and the risk of epithelial ovarian cancer. The WHO Collaborative Study of Neoplasia and Steroid Contraceptives. International Journal of Epidemiology. 1993;22:192–197. doi: 10.1093/ije/22.2.192. doi:10.1093/ije/22.2.192. [DOI] [PubMed] [Google Scholar]
- Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumors in a cohort of infertile women. New England Journal of Medicine. 1994;331:771–776. doi: 10.1056/NEJM199409223311204. doi:10.1056/NEJM199409223311204. [DOI] [PubMed] [Google Scholar]
- Santarosa M, Bidoli E, Gallo A, Steffan A, Boiocchi M, Viel A. Polymorphic CAG repeat length within the androgen receptor gene: identification of a subgroup of patients with increased risk of ovarian cancer. Oncology Reports. 2002;9:639–644. [PubMed] [Google Scholar]
- Scambia G, Benedetti-Panici P, Ferrandina G, Distefano M, Salerno G, Romanini ME, Fagotti A, Mancuso S. Epidermal growth factor, oestrogen and progesterone receptor expression in primary ovarian cancer: correlation with clinical outcome and response to chemotherapy. British Journal of Cancer. 1995;72:361–366. doi: 10.1038/bjc.1995.339. doi:10.1038/bjc.1995.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstetrics and Gynecology. 1996;88(4 Pt 1):554–559. doi: 10.1016/0029-7844(96)00226-8. doi:10.1016/0029-7844(96)00226-8. [DOI] [PubMed] [Google Scholar]
- Schildkraut JM, Calingaert B, Marchbanks PA, Moorman PG, Rodriguez GC. Impact of progestin and estrogen potency in oral contraceptives on ovarian cancer risk. Journal of the National Cancer Institute. 2002;94:32–38. doi: 10.1093/jnci/94.1.32. doi:10.1093/jnci/94.1.32. [DOI] [PubMed] [Google Scholar]
- Schildkraut JM, Murphy SK, Palmieri RT, Iversen E, Moorman PG, Huang Z, Halabi S, Calingaert B, Gusberg A, Marks JR, et al. Trinucleotide repeat polymorphisms in the androgen receptor gene and risk of ovarian cancer. Cancer Epidemiology, Biomarkers & Prevention. 2007;16:473–480. doi: 10.1158/1055-9965.EPI-06-0868. doi:10.1158/1055-9965.EPI-06-0868. [DOI] [PubMed] [Google Scholar]
- Schwartz PE, Keating G, MacLusky N, Naftolin F, Eisenfeld A. Tamoxifen therapy for advanced ovarian cancer. Obstetricia et Gynecologica. 1982;59:583–588. [PubMed] [Google Scholar]
- Shao R, Norström A, Weijdegård B, Egecioglu E, Fernandez-Rodriguez J, Feng Y, Stener-Victorin E, Brännström M, Billig H. Distinct expression pattern of Dicer1 correlates with ovarian-derived steroid hormone receptor expression in human Fallopian tubes during ovulation and the midsecretory phase. Journal of Clinical Endocrinology and Metabolism. 2011;96:E869–E877. doi: 10.1210/jc.2010-2353. doi:10.1210/jc.2010-2353. [DOI] [PubMed] [Google Scholar]
- Shaw PA, Rittenberg PV, Brown TJ. Activation of androgen receptor-associated protein 70 (ARA70) mRNA expression in ovarian cancer. Gynecologic Oncology. 2001;80:132–138. doi: 10.1006/gyno.2000.6068. doi:10.1006/gyno.2000.6068. [DOI] [PubMed] [Google Scholar]
- Sheach LA, Adeney EM, Kucukmetin A, Wilkinson SJ, Fisher AD, Elattar A, Robson CN, Edmondson RJ. Androgen-related expression of G-proteins in ovarian cancer. British Journal of Cancer. 2009;101:498–503. doi: 10.1038/sj.bjc.6605153. doi:10.1038/sj.bjc.6605153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Zhang Y, Tong X, Yang Y, Shao Z. Dihydrotestosterone induces p27 degradation via direct binding with SKP2 in ovarian and breast cancer. International Journal of Molecular Medicine. 2011;28:109–114. doi: 10.3892/ijmm.2011.677. [DOI] [PubMed] [Google Scholar]
- Shirey DR, Kavanagh JJ, Jr, Gershenson DM, Freedman RS, Copeland LJ, Jones LA. Tamoxifen therapy of epithelial ovarian cancer. Obstetricia et Gynecologica. 1985;66:575–578. [PubMed] [Google Scholar]
- Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. Journal of the National Cancer Institute. 2004;96:926–935. doi: 10.1093/jnci/djh166. doi:10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- Sikic BI, Scudder SA, Ballon SC, Soriero OM, Christman JE, Suey L, Ehsan MN, Brandt AE, Evans TL. High-dose megestrol acetate therapy of ovarian carcinoma: a phase II study by the Northern California Oncology Group. Seminars in Oncology. 1986;13(4 Suppl):26–32. [PubMed] [Google Scholar]
- Simpson BJ, Langdon SP, Rabiasz GJ, Macleod KG, Hirst GL, Bartlett JM, Crew AJ, Hawkins RA, Macineira-Perez PP, Smyth JF, et al. Estrogen regulation of transforming growth factor-α in ovarian cancer. Journal of Steroid Biochemistry and Molecular Biology. 1998;64:137–145. doi: 10.1016/s0960-0760(97)00159-3. doi:10.1016/S0960-0760(97)00159-3. [DOI] [PubMed] [Google Scholar]
- Slayton RE, Pagano M, Creech RH. Progestin therapy for advanced ovarian cancer: a phase II Eastern Cooperative Oncology Group trial. Cancer Treatment Reports. 1981;65:895–896. [PubMed] [Google Scholar]
- Slevin ML, Harvey VJ, Osborne RJ, Shepherd JH, Williams CJ, Mead GM. A phase II study of tamoxifen in ovarian cancer. European Journal of Cancer and Clinical Oncology. 1986;22:309–312. doi: 10.1016/0277-5379(86)90396-2. doi:10.1016/0277-5379(86)90396-2. [DOI] [PubMed] [Google Scholar]
- Smyth JF, Gourley C, Walker G, MacKean MJ, Stevenson A, Williams AR, Nafussi AA, Rye T, Rye R, Stewart M, et al. Antiestrogen therapy is active in selected ovarian cancer cases: the use of letrozole in estrogen receptor-positive patients. Clinical Cancer Research. 2007;13:3617–3622. doi: 10.1158/1078-0432.CCR-06-2878. doi:10.1158/1078-0432.CCR-06-2878. [DOI] [PubMed] [Google Scholar]
- So WK, Cheng JC, Poon SL, Leung PC. Gonadotropin-releasing hormone and ovarian cancer: a functional and mechanistic overview. FEBS Journal. 2008;275:5496–5511. doi: 10.1111/j.1742-4658.2008.06679.x. doi:10.1111/j.1742-4658.2008.06679.x. [DOI] [PubMed] [Google Scholar]
- Spurdle AB, Chen X, Abbazadegan M, Martin N, Khoo SK, Hurst T, Ward B, Webb PM, Chenevix-Trench G. CYP17 promotor polymorphism and ovarian cancer risk. International Journal of Cancer. 2000;86:436–439. doi: 10.1002/(sici)1097-0215(20000501)86:3<436::aid-ijc21>3.0.co;2-a. doi:10.1002/(SICI)1097-0215(20000501)86:3<436∷AID-IJC21>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Suzuki F, Akahira J, Miura I, Suzuki T, Ito K, Hayashi S, Sasano H, Yaegashi N. Loss of estrogen receptor β isoform expression and its correlation with aberrant DNA methylation of the 5′-untranslated region in human epithelial ovarian carcinoma. Cancer Science. 2008;99:2365–2372. doi: 10.1111/j.1349-7006.2008.00988.x. doi:10.1111/j.1349-7006.2008.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed V, Ho SM. Progesterone-induced apoptosis in immortalized normal and malignant human ovarian surface epithelial cells involves enhanced expression of FasL. Oncogene. 2003;22:6883–6890. doi: 10.1038/sj.onc.1206828. doi:10.1038/sj.onc.1206828. [DOI] [PubMed] [Google Scholar]
- Syed V, Ulinski G, Mok SC, Yiu GK, Ho SM. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Research. 2001;61:6768–6776. [PubMed] [Google Scholar]
- Syed V, Mukherjee K, Lyons-Weiler J, Lau KM, Mashima T, Tsuruo T, Ho SM. Identification of ATF-3, caveolin-1, DLC-1, and NM23-H2 as putative antitumorigenic, progesterone-regulated genes for ovarian cancer cells by gene profiling. Oncogene. 2005;24:1774–1787. doi: 10.1038/sj.onc.1207991. doi:10.1038/sj.onc.1207991. [DOI] [PubMed] [Google Scholar]
- Syed V, Mukherjee K, Godoy-Tundidor S, Ho SM. Progesterone induces apoptosis in TRAIL-resistant ovarian cancer cells by circumventing c-FLIPL overexpression. Journal of Cellular Biochemistry. 2007;102:442–452. doi: 10.1002/jcb.21304. doi:10.1002/jcb.21304. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Kato K, Kuboyama A, Inoue T, Tanaka Y, Kuhara A, Kinoshita K, Takeda S, Wake N. Induction of senescence by progesterone receptor-B activation in response to cAMP in ovarian cancer cells. Gynecologic Oncology. 2009;113:270–276. doi: 10.1016/j.ygyno.2008.12.032. doi:10.1016/j.ygyno.2008.12.032. [DOI] [PubMed] [Google Scholar]
- Tangjitgamol S, Manusirivithaya S, Khunnarong J, Jesadapatarakul S, Tanwanich S. Expressions of estrogen and progesterone receptors in epithelial ovarian cancer: a clinicopathologic study. International Journal of Gynecological Cancer. 2009;19:620–627. doi: 10.1111/IGC.0b013e3181a44b62. doi:10.1111/IGC.0b013e3181a44b62. [DOI] [PubMed] [Google Scholar]
- Tanner MM, Grenman S, Koul A, Johannsson O, Meltzer P, Pejovic T, Borg A, Isola JJ. Frequent amplification of chromosomal region 20q12-q13 in ovarian cancer. Clinical Cancer Research. 2000;6:1833–1839. [PubMed] [Google Scholar]
- Tansan S, Koç Y, Aydin H, Urbano G, McCaffrey R. Augmentation of vincristine cytotoxicity by megestrol acetate. Cancer Chemotherapy and Pharmacology. 1997;39:333–340. doi: 10.1007/s002800050580. doi:10.1007/s002800050580. [DOI] [PubMed] [Google Scholar]
- Treeck O, Pfeiler G, Mitter D, Lattrich C, Piendl G, Ortmann O. Estrogen receptor β1 exerts antitumoral effects on SK-OV-3 ovarian cancer cells. Journal of Endocrinology. 2007;193:421–433. doi: 10.1677/JOE-07-0087. doi:10.1677/JOE-07-0087. [DOI] [PubMed] [Google Scholar]
- Tropé C, Johnsson JE, Sigurdsson K, Simonsen E. High-dose medroxyprogesterone acetate for the treatment of advanced ovarian carcinoma. Cancer Treatment Reports. 1982;66:1441–1443. [PubMed] [Google Scholar]
- Tsilidis KK, Allen NE, Key TJ, Dossus L, Lukanova A, Bakken K, Lund E, Fournier A, Overvad K, Hansen L, et al. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. British Journal of Cancer. 2011;105:1436–1442. doi: 10.1038/bjc.2011.371. doi:10.1038/bjc.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumolo S, Rao BR, van der Burg ME, Guastalla JP, Renard J, Vermorken JB. Phase II trial of flutamide in advanced ovarian cancer: an EORTC Gynaecological Cancer Cooperative Group study. European Journal of Cancer. 1994;30A:911–914. doi: 10.1016/0959-8049(94)90112-0. doi:10.1016/0959-8049(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Lee IM, Buring JE, Hankinson SE. Plasma androgen concentrations and risk of incident ovarian cancer. American Journal of Epidemiology. 2008;167:211–218. doi: 10.1093/aje/kwm278. doi:10.1093/aje/kwm278. [DOI] [PubMed] [Google Scholar]
- Van Der Velden J, Gitsch G, Wain GV, Friedlander ML, Hacker NF. Tamoxifen in patients with advanced epithelial ovarian cancer. International Journal of Gynecological Cancer. 1995;5:301–305. doi: 10.1046/j.1525-1438.1995.05040301.x. doi:10.1046/j.1525-1438.1995.05040301.x. [DOI] [PubMed] [Google Scholar]
- Vassilomanolakis M, Koumakis G, Barbounis V, Hajichristou H, Tsousis S, Efremidis A. A phase II study of flutamide in ovarian cancer. Oncology. 1997;54:199–202. doi: 10.1159/000227688. doi:10.1159/000227688. [DOI] [PubMed] [Google Scholar]
- Vaughan S, Coward JI, Bast RC, Jr, Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nature Reviews Cancer. 2011;11:719–725. doi: 10.1038/nrc3144. doi:10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhof CH, van der Burg ME, Nooy M, Aalders JG, Pecorelli S, Oliveira CF, Rotmensz N, Vermorken JB. Phase II study of high-dose megestrol acetate in patients with advanced ovarian carcinoma. European Journal of Cancer. 1994;30A:697–698. doi: 10.1016/0959-8049(94)90548-7. doi:10.1016/0959-8049(94)90548-7. [DOI] [PubMed] [Google Scholar]
- Verma S, Alhayki M, Le T, Baines K, Rambout L, Hopkins L, Fung Kee Fung M. Phase II study of exemestane (E) in refractory ovarian cancer (ROC) Journal of Clinical Oncology. 2006;24 [Google Scholar]
- Vlahos NF, Economopoulos KP, Creatsas G. Fertility drugs and ovarian cancer risk: a critical review of the literature. Annals of the New York Academy of Sciences. 2010;1205:214–219. doi: 10.1111/j.1749-6632.2010.05668.x. doi:10.1111/j.1749-6632.2010.05668.x. [DOI] [PubMed] [Google Scholar]
- Wada-Hiraike O, Imamov O, Hiraike H, Hultenby K, Schwend T, Omoto Y, Warner M, Gustafsson JA. Role of estrogen receptor β in colonic epithelium. PNAS. 2006;103:2959–2964. doi: 10.1073/pnas.0511271103. doi:10.1073/pnas.0511271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, du Bois A, Pfisterer J, Huober J, Loibl S, Lück HJ, Sehouli J, Gropp M, Staähle A, Schmalfeldt B, et al. Gefitinib in combination with tamoxifen in patients with ovarian cancer refractory or resistant to platinum-taxane based therapy–a phase II trial of the AGO Ovarian Cancer Study Group (AGO-OVAR 2.6) Gynecologic Oncology. 2007;105:132–137. doi: 10.1016/j.ygyno.2006.10.053. doi:10.1016/j.ygyno.2006.10.053. [DOI] [PubMed] [Google Scholar]
- Walker G, MacLeod K, Williams AR, Cameron DA, Smyth JF, Langdon SP. Insulin-like growth factor binding proteins IGFBP3, IGFBP4, and IGFBP5 predict endocrine responsiveness in patients with ovarian cancer. Clinical Cancer Research. 2007a;13:1438–1444. doi: 10.1158/1078-0432.CCR-06-2245. doi:10.1158/1078-0432.CCR-06-2245. [DOI] [PubMed] [Google Scholar]
- Walker G, MacLeod K, Williams AR, Cameron DA, Smyth JF, Langdon SP. Estrogen-regulated gene expression predicts response to endocrine therapy in patients with ovarian cancer. Gynecologic Oncology. 2007b;106:461–468. doi: 10.1016/j.ygyno.2007.05.009. doi:10.1016/j.ygyno.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Wang L, Yang CP, Horwitz SB, Trail PA, Casazza AM. Reversal of the human and murine multidrug-resistance phenotype with megestrol acetate. Cancer Chemotherapy and Pharmacology. 1994;34:96–102. doi: 10.1007/BF00685925. doi:10.1007/BF00685925. [DOI] [PubMed] [Google Scholar]
- Weiner SA, Alberts DS, Surwit EA, Davis J, Grosso D. Tamoxifen therapy in recurrent epithelial ovarian carcinoma. Gynecologic Oncology. 1987;27:208–213. doi: 10.1016/0090-8258(87)90294-0. doi:10.1016/0090-8258(87)90294-0. [DOI] [PubMed] [Google Scholar]
- Whiteman DC, Murphy MF, Cook LS, Cramer DW, Hartge P, Marchbanks PA, Nasca PC, Ness RB, Purdie DM, Risch HA, et al. Multiple births and risk of epithelial ovarian cancer. Journal of the National Cancer Institute. 2000;92:1172–1177. doi: 10.1093/jnci/92.14.1172. doi:10.1093/jnci/92.14.1172. [DOI] [PubMed] [Google Scholar]
- Wiernik PH, Greenwald ES, Ball H, Young JA, Vogl S. High-dose megestrol acetate in the treatment of patients with ovarian cancer who have undergone previous treatment: Eastern Cooperative Oncology Group Study PD884. American Journal of Clinical Oncology. 1998;21:565–567. doi: 10.1097/00000421-199812000-00007. doi:10.1097/00000421-199812000-00007. [DOI] [PubMed] [Google Scholar]
- Wilailak S, Linasmita V, Srisupundit S. Phase II study of high-dose megestrol acetate in platinum-refractory epithelial ovarian cancer. Anticancer Drugs. 2001;12:719–724. doi: 10.1097/00001813-200110000-00002. doi:10.1097/00001813-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Williams CJ. Tamoxifen for relapse of ovarian cancer. Cochrane Database of Systematic Reviews. 2001:CD001034. doi: 10.1002/14651858.CD001034. [DOI] [PubMed] [Google Scholar]
- Williams C, Simera I, Bryant A. Tamoxifen for relapse of ovarian cancer. Cochrane Database of Systematic Reviews. 2010:CD001034. doi: 10.1002/14651858.CD001034.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsberg TC. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor α for degradation in breast cancer cells. Cancer Research. 2009;69:1722–1727. doi: 10.1158/0008-5472.CAN-08-3933. doi:10.1158/0008-5472.CAN-08-3933. [DOI] [PubMed] [Google Scholar]
- Yang CP, DePinho SG, Greenberger LM, Arceci RJ, Horwitz SB. Progesterone interacts with P-glycoprotein in multidrug-resistant cells and in the endometrium of gravid uterus. Journal of Biological Chemistry. 1989;264:782–788. [PubMed] [Google Scholar]
- Yap OW, Bhat G, Liu L, Tollefsbol TO. Epigenetic modifications of the Estrogen receptor β gene in epithelial ovarian cancer cells. Anticancer Research. 2009;29:139–144. [PMC free article] [PubMed] [Google Scholar]
- Zaino RJ, Brady MF, Lele SM, Michael H, Greer B, Bookman MA. Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal: a Gynecologic Oncology Group study. Cancer. 2011;117:554–562. doi: 10.1002/cncr.25460. doi:10.1002/cncr.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Kavanagh JJ, Hu W, Liao Q, Fu S. Hormonal therapy in ovarian cancer. International Journal of Gynecological Cancer. 2007;17:325–338. doi: 10.1111/j.1525-1438.2006.00749.x. doi:10.1111/j.1525-1438.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Hua K, Sun H, Yu Y, Jin H, Feng Y. Re-expression of estrogen receptor β inhibits the proliferation and migration of ovarian clear cell adenocarcinoma cells. Oncology Reports. 2011;26:1497–1503. doi: 10.3892/or.2011.1430. [DOI] [PubMed] [Google Scholar]
- Zhu J, Lu X, Hua KQ, Sun H, Yu YH, Feng YJ. Oestrogen receptor α mediates 17β-estradiol enhancement of ovarian cancer cell motility through up-regulation of survivin expression. Archives of Gynecology and Obstetrics. 2012;286:729–737. doi: 10.1007/s00404-012-2368-5. doi:10.1007/s00404-012-2368-5. [DOI] [PubMed] [Google Scholar]