Abstract
Adult-onset exposure is thought to result primarily in sensory and motor deficits but effects on learning are poorly understood. One mechanism by which chronic MeHg may exert its neurotoxicity is via sustained disruption of intracellular calcium homeostasis, with a consequent increase of intracellular Ca2+ ions in vulnerable neurons. A biochemically heterogeneous group of compounds, calcium channel blockers, have been shown in vitro to attenuate MeHg’s toxicity. To evaluate the role of calcium antagonism in MeHg toxicity in vivo, adult BALB/c mice were exposed chronically to 0 or 15 ppm of Hg (as MeHg) via drinking water and to nimodipine, a dihydropryidine, L-type Ca2+ channel blocker with action in the CNS. Nimodipine was administered orally in diets (0, 20, or 200 ppm, producing approximately 0, 2, or 20 mg/kg/day of nimodipine). An incremental repeated acquisition (IRA) of response chains procedure was used to detect MeHg-induced deficits in learning or motoric function and to evaluate possible neuroprotection by nimodipine. MeHg impaired performance on the IRA task, and this was partially or completely blocked by dietary nimodipine, depending on dose. Measures of learning co-varied with measures of motoric function as indicated by overall response rate. Nimodipine delayed or prevented the behavioral toxicity of MeHg exposure as evidenced by IRA performance; effects on learning seemed secondary to response rate decreases.
1. INTRODUCTION
A consistent finding from non-human models is that gestational exposure to the neurotoxicant methylmercury (MeHg) results in cognitive, motor and sensory impairment (Rice, 1983; 1992; 1996; Newland et al., 2004) while adult-onset exposure results in sensory and motor decline (Evans et al., 1975; Heath et al., 2010). Less is known, however, about the cognitive impact of chronic, adult-onset exposure and it is unclear whether apparent learning or memory deficits occur separate from sensory- and motor-toxicity.
In vitro, MeHg induces a biphasic elevation of intracellular calcium ([Ca2+]i) in the nerve terminal due to 1) rapid release of Ca2+ from intracellular stores (i.e. mitochondria, smooth endoplasmic reticulum (SER)), and 2) slower and longer-lasting increase of Ca2+ entry via voltage-gated calcium-channels (Hare and Atchison, 1995b). The elevation in [Ca2+]i disrupts Ca2+ signaling, alters neuronal excitability and neurotransmitter release, and can cause cell death (Atchison and Hare, 1994; Marty and Atchison, 1998; Atchison, 2003; Yuan and Atchison, 2007). A biochemically heterogeneous group of drugs referred to collectively as calcium-channel-blockers (CCBs) delays both the first- and second-phases of MeHg-induced Ca2+ entry (Marty and Atchison, 1997). The first phase, involving release from intracellular stores, might be buffered back to baseline levels but the second phase, which is slower and larger in magnitude, is hypothesized to be a major contributor to the long-term increase in [Ca2+]i (Marty and Atchison, 1997). The cumulative impact of elevated [Ca2+]i might be related to the well-known observation that MeHg’s neurotoxicity is significantly delayed (Reuhl, 1991; Davidson et al., 2006; Yoshida et al., 2008). If MeHg-induced increase in Ca2+ entry into nerve terminals is a major contributor to the neurotoxicity of chronic, adult-onset MeHg exposure then co-exposure to a Ca2+ channel blocker that penetrates the central nervous system should prevent or attenuate such toxicity.
In vivo, CCBs protect against the behavioral impairment associated with various Ca2+-induced CNS damage, like that caused by aging (Kowalska and Disterhoft, 1994; Thompson et al., 1990), brain trauma (Finger et al., 1990) and brain ischemia (Mršić et al., 1997; Yanpallewar et al., 2004). Sakamoto et al., (1996) reported that L- and T-type CCBs improved survival of cerebellar granule cells exposed to MeHg in culture and decreased mortality and prevented weight-loss in adult rats exposed orally to MeHg (Sakamoto et al., 1996). It is not known, however, whether CCBs prevent other, neurobehavioral, effects of MeHg exposure, including declining sensorimotor function or learning deficits.
Despite consistent benefits of CCBs following Ca2+-induced CNS damage, their behavioral effects in the absence of CNS insult are uncertain. Reported improvement (Quartermain et al., 2001), impairment and no effect (Maurice et al., 1995, Clements et al., 1995) on learning tasks following acute CCB treatment in uninjured animals renders conclusions about their effects tentative. Further, it is not clear whether CCBs are protective against certain types of neural insult or whether they are global cognitive enhancers.
Nimodipine is a 1,4-dihydropyridine compound used to treat vascular disorders. Nimodipine passes the blood-brain-barrier more readily than other CCBs (Van den Kerckhoff and Drewes, 1985) and binds with high-affinity and specificity to the dihydropyrine receptors on L-type calcium-channels in the brain (Peroutka and Allen, 1983; Belleman et al., 1983; Dompert and Traber, 1984) functionally blocking the calcium current through these voltage-dependent channels. Thus, nimodipine is often chosen to evaluate the neuroprotection offered by voltage-gated calcium antagonism in the injured CNS.
Using mice, we determined the behavioral relevance of CCB neuroprotection against MeHg’s neurotoxicity by providing nimodipine in the diet concurrently with drinking water containing MeHg. The mice were tracked longitudinally on an apical behavioral procedure that allowed us to examine effects on response-rate and learning. The incremental repeated acquisition of response chain (IRA) procedure generates a steady-state of acquisition by repeatedly requiring the acquisition of novel response chains. During a control condition, a previously-learned response chain is executed. This is unlike many learning tasks that use very few trials or measure discrimination performance meeting a pre-set criterion (e.g. 85% accuracy). These procedures are used in studies of drug action (Bailey et al., 2010; Paule and McMillan, 1984) and toxicant exposure (Cohn et al., 1993) as a sensitive indicator of subtle changes in learning following such exposure. IRA has also been helpful for generating an animal model of developmental disabilities (Wenger et al., 2004). In humans, IRA performance is correlated with IQ score (Paule et al., 1999).
Using IRA, and a 2 (MeHg) × 3 (nimodipine) full factorial design, we were able to examine 1) the impact of adult-onset, chronic MeHg exposure on behavioral endpoints, 2) neuroprotection by nimodipine and 3) nimodipine’s effects in the absence of CNS injury. Because this is a mechanistic study designed to investigate the potential role of L-type Ca++ channels, MeHg concentrations were used that generate neurotoxicity within several months (Heath et al., 2010) and the mice were tracked from the onset of MeHg exposure until overt toxicity appeared.
2. MATERIALS AND METHODS
2.1.Subjects
Adult male Balb/c mice purchased from Harlan laboratories (Indianapolis, IN; mice were from the Maryland facility) were housed (2 per cage) in clear polycarbonate cages with wire tops and woodchip bedding located in an AAALAC-accredited facility. A diagonal Plexiglas® barrier separated cage mates, who were always in the same exposure group, effectively creating single-housing. The vivarium was temperature- and humidity-controlled and maintained on a 12-h light-dark cycle (lights on at 06:00). All animals were maintained at approximately 24g by feeding a measured quantity of food daily (usually 2.5g). Thus the mice did acquire excess fat, which can accumulate MeHg, and the daily dose of nimodipine was held stable. Two MeHg water concentrations and three nimodipine diets produced a 2 (MeHg) × 3 (nimodipine) full factorial design with 12-14 animals in each of the six exposure groups. Both MeHg- and nimodipine-group assignments were designated so that groups were indistinguishable on pre-exposure evaluation of free-feeding body mass, performance on rotorod, and autoshaping of operant nose-poking.
2.2.Nimodipine and MeHg exposure
Mice were exposed chronically to 0 or 15 ppm of mercury as methyl mercuric chloride, dissolved in their only source of drinking water, corresponding to 0 and approximately 2.6 mg/kg/day based on calculations of water consumption undertaken during exposure (water consumption was measured daily for several weeks at various times during exposure). Within each MeHg exposure group, mice were exposed chronically to 0, 20 or 200 ppm of the dihyropryidine L-type calcium channel blocker, nimodipine, in their chow. These doses correspond to approximately 0, 2 and 20 mg/kg/day of nimodipine, based on daily consumption. Chow was manufactured by Harlan-Teklad custom research diets (Madison, WI) and based on the Global 18% protein rodent diet (TD.00588) formula. Methyl mercuric chloride was purchased from Alfa-Aesar (Ward Hill, MA) and nimodipine from Sigma-Aldrich (St. Louis, MO). Exposure to both methylmercury (MeHg) and nimodipine began three weeks after the mice arrived to the vivarium, when the mice were approximately 11 weeks of age, and continued for just over 17 weeks.
2.3.Apparatus
Experiments were conducted in 16 operant chambers manufactured by Med Associates (Med Associates Inc. St. Albans, VT, model # Med ENV-007, 12.0″ L × 9.5″ W × 11.5″ H) that were enclosed in sound-attenuating cabinets and modified to accommodate mice. Each chamber contained two nose-poke holes on the front panel (right “R” and left “L”), separated by a food tray (connected to a 20 mg pellet dispenser), and one nose-poke hole in the center of the back panel (back “B”). Inserting the nose (or paw, etc.) into the nose-hole interrupted an infrared beam located inside the hole, which registered as a response. A single 2.8-W house light was located near the ceiling of the chamber on the back panel. A Sonalert tone generator was located in the top left and a white-noise generator was located in the top right of the back panel.
2.4.Procedure
An IRA procedure was used in which a response chain incremented from a one- to a six-link chain within an experimental session. Nose-poke response-chains were built using backward chaining such that a new link was added before (or, in front of) the previously learned link(s). Criterion responses resulted in the delivery of a 20 mg sucrose pellet. For example, the 6-link response chain “LBLRBR” was trained as follows: R (right nose-poke) → sucrose, B – R (back then right nose-poke) → sucrose, R – B – R → sucrose, L – R – B – R → sucrose, etc. until a 6-link chain of nose-poking was formed. Chain-length was increased within a session using a pre-set mastery-based criterion: six consecutive correct chains (no errors) increased the chain from a one- to a two-link chain. All subsequent increases in chain length required three consecutive correct response-chains. Each chain-length was paired with a discrete auditory stimulus (e.g. first link paired with high tone). Nose-poking an incorrect nose-hole location at any point in the chain resulted in a 2” timeout period during which the house light was illuminated and nose-poking had no programmed consequences. Following this timeout period, the current chain length reset to the first link. Sessions ended after 1 hour passed or 50 consecutive correct 6-link response-chains occurred. All experimental sessions were conducted at approximately the same time each day, in the same testing room.
Animals were first exposed to the performance condition of IRA. Here, the same six-link sequence was required during every session. Learning chains, in which the animals were required to nose-poke in a changing (daily) sequence of locations for sucrose delivery, were introduced after 10 performance sessions, at which point the animals were reliably reaching a 4-link performance chain. A total of 17 learning chains were cycled through the course of the experiment with learning and performance sessions alternating daily. Learning chains were selected according to the following criteria: all three response locations (R,B,L) were used but response locations never consecutively repeated (e.g. RBBLCR would not qualify) and chains very similar to the performance chain or that had any obvious pattern (e.g. clockwise rotation) were excluded. The visual stimulus (a checkerboard pattern covering one wall of the experimental chamber) was provided during learning sessions, but not during performance sessions.
2.5.Criteria for euthanasia
Animals were inspected daily (body weight was measured 5 days per week) and if an animal appeared ill or moribund (e.g. weight loss, failure to eat, failure to explore or locomote when placed on an open surface, etc.) the attending veterinarian was consulted. Every effort was undertaken to keep an animal alive, providing it did not prolong distress. Animals meeting predefined criteria for euthanasia were euthanized using procedures approved by the Auburn University Institutional Animal Care and Use Committee. Over the course of the study, a total of 5 (6%) animals met criteria for euthanasia, all within the final months of exposure and all from the MeHg exposed groups.
2.6.Data analysis
Three main dependent measures are presented: response rate (total responses divided by time available to make a response, i.e., omitting timeouts), maximum chain length reached (MCL) and a type of accuracy score called progress quotient (PQ), previously described by Bailey et al. (2010):
| Equation 1 |
where i = weight (where weight = chain length), Ri = number of reinforcers earned on a chain of length i, and Rt = total reinforcers earned in the session. This index serves as a measure of progress and weights correct responses that comprise long chains more heavily than those comprising shorter chains. Characterized differently, the numerator is a count of all the responses comprising correct chains. The denominator normalizes PQ by the number of criterion chains that occur. A higher PQ score corresponds to better IRA performance (i.e. more reinforcers earned in longer chain lengths). This measure is favored over percent correct accuracy scores because high percent correct can occur even if the mastery criterion (6 or 3 consecutive criterion chains) keeps a mouse at a short chain. MCL, which provides some guide of progress, does not distinguish between a mouse reaching, say, a six link chain but producing it only once from another that produced that chain length reliably after reaching it (see Bailey et al. 2010 for details). Here, MCL is presented in addition to PQ because it is a more conventional measure of IRA performance.
In addition to the aforementioned dependent measures, reinforcer rate was also examined for the last 5 days. For graphical display, a LOESS smoothing algorithm was applied to the raw group PQ, MCL and response rate time-series data. Data from the performance and learning conditions are always presented separately. Tests for main effects and interactions between MeHg, nimodipine and session were carried out using a linear mixed-effects (hierarchical) model (LME) (Pinherio & Bates, 2004; Bates & DebRoy, 2004) using the statistical package SYStat 11®. LME was chosen, in part, because it is able to model unbalanced and incomplete repeated-measures data more effectively than repeated-measures ANOVAs.
The best LME model was arrived at via a series of model comparisons with the constraint that the final model must contain a priori hypotheses of interest: a term for Session or Session2 crossed with MeHg, Nimodipine, and MeHg × Nimodipine. A quadratic term (Session2) was required to describe the curvature evident when plotting most dependent measures across session. The first full model contained all terms and interactions. To identify the most parsimonious model that still captured the patterns present in the data sets, restricted models containing fewer terms and degrees of freedom were compared with fuller ones using likelihood ratios in which degrees of freedom for the test was the difference in degrees of freedom for the full and restricted models. Simplification proceeded iteratively; if there was no significant difference (p > 0.1) between a fuller and more restricted model then the restricted model served as the next base for comparison. Whether interactions of MeHg and nimodipine with session were best captured using Session, Session2, or both (as a linear combination) was determined using likelihood ratio tests. The session2 quadratic transformation was performed to accommodate the natural shape of the acquisition by time relationship (with rapid acquisition early on). For all dependent measures, the best fit was achieved with session, session2, MeHg dose × session2, nimodipine-dose × session2, and nimodipine dose × MeHg dose × session2 as fixed factors, and a fixed intercept. Analyses were conducted separately for performance and learning conditions. The highest order interactions (Nimodipine × MeHg × Session) are described first because they formed the hypotheses of greatest interest. Here, ß is the regression coefficient.
MeHg is known to cause motor impairment but cognitive effects are poorly understood, so in addition to the aforementioned analyses, we evaluated the extent to which PQ changes were independent of motor deficits as expressed in response rate. MeHg’s and nimodipine’s effects on response rate, reinforcer rate and PQ score during the last five sessions of exposure were analyzed using 2-way ANOVA’s, with nimodipine and MeHg dose as between-subject factors. Each of these dependent measures was affected similarly by MeHg and nimodipine, so we also examined the extent to which these measures co-vary using Pearson’s correlations and visually presenting scatterplots. All graphs were made in SigmaPlot 10® and statistical analyses were conducted using SYStat 11® (San Jose, CA).
2.7.Brain Hg Concentration
Whole-brain Hg concentrations (total Hg) from a control group (i.e. not exposed to MeHg or nimodipine) and from all three nimodipine (0, 20, 200 ppm) + MeHg (15 ppm) exposure groups were analyzed by Michigan State University Diagnostic Center for Population and Animal Health using inductively coupled plasma mass spectrometry (ICP/MS). The brains were taken when the animal was euthanized either due to MeHg neurotoxicity or at the end of the study. Group sizes for the 0, 20, & 200 ppm Nimodipine groups are N=11, N=11 and N=13, respectively.
3. RESULTS
3.1.PQ
Here, we describe the highest order interaction first, then 2-way interactions. The overall pattern of effects on PQ was captured by a three-way, nimodipine by MeHg by session2 interaction for the performance (β = 1.18×10−4, SE β = 1.1×10−5, z = 10.39, p<.001) and learning (β = 5.6×10−5, SE β = 1.2×10−5, z = 4.77, p < 0.001) conditions, indicating that MeHg’s effect on PQ across session depended upon nimodipine exposure. Note the quadratic term locates the peak value and indicates the degree of downward curvature.
In addition to significant three-way interactions, important two-way interactions and main effects were also observed. For performance sessions, there were significant session2 by MeHg (β = −2.89×10−4, SE β = 2.6×10−5, z = −11.22, p < 0.01) and session2 by nimodipine (β = −1.02×10−4, SE β = 1.7×10−5, z = −5.99, p < 0.01) interactions and main effects of session (β = 0.06, SE β = 0.002, z = 32.51, p < 0.01) and session2 (β = −1.55×10−4, SE β = 4.4×10−5 z = −3.49, p < 0.01). Similarly, for learning sessions MeHg by session2 (β = −1.23×10−4, SE β = 2.7×10−5, z = −4.64, p < 0.01) and nimodipine by session2 interactions (β = −3.6×10−5, SE β = 1.7×10−5, z = −2.04, p < 0.01) were identified as well as a main effect of session (β = 0.06, SE β = 0.003, z = 16.70, p < 0.01) and session2 (β = −3.04×10−4, SE β = 5.2×10−5 z = −5.81, p < 0.01).
The interpretation of these effects, specifically the three-way interaction, is clarified by examining Figure 1. Note that for MeHg-exposed mice, nimodipine conferred a dose-dependent protection: MeHg-exposed mice without dietary nimodipine had blunted improvement in PQ beginning about session 45 and declining PQ beginning at session 80 for the performance (Fig 1B, filled circles) and Learning (Fig 1D, filled circles) conditions. In contrast, no apparent effects of MeHg were seen at all in those mice consuming a diet containing 200 ppm nimodipine and MeHg’s toxicity was reduced considerably in those mice consuming a diet containing 20 ppm nimodipine (see Fig 1B & 1D). This clearly contrasts the effects of dietary nimodipine in the absence of MeHg exposure (see Fig 1A & C), in which no discernible main effect of nimodipine occurs.
Figure 1.
Top row is PQ score from the performance condition and the bottom row is from the learning condition of the IRA procedure. The left column contains data from the 0- ppm MeHg exposure group, for each of the three nimodipine condition (0, 20 and 200 ppm nimodipine). The right column contains data from the 15 ppm MeHg exposure group for each nimodipine condition. Data points represent the mean for each group as a function of MeHg exposure day. The curves represent a LOESS smoothing algorithm.
With respect to overall differences between performance and learning conditions in healthy mice, the control mice (no MeHg) showed an increase in PQ to an asymptote for performance sessions (Fig 1A) that was higher than the asymptote for learning sessions, indicating that final PQ was lower during the learning condition. Also, behavior during learning sessions was more variable than that during performance sessions, as would be expected for mice acquiring novel chains.
3.2. Maximum Chain Length Reached (MCL)
Similarly to PQ, a three-way interaction among nimodipine, MeHg and session2 captured the pattern of effects on MCL during performance (β = 1.25×10−4, SE β = 1.3×10−5, z =9.61, p <.001) and learning (β = 9.1×10−5, SE β = 1.5×10−5, z = 5.95, p <0.001) sessions, again indicating that MeHg’s effects on MCL across session depended upon nimodipine exposure (see Figure 2).
Figure 2.
Maximum chain length (MCL) reached across experimental days for the exposure groups, structured similarly as figure 1.
During performance sessions, there were main effects of session (β = 0.10, SE β = 0.002, z = 46.06, p < 0.04) and session2 (β = −4.97×10−4, SE β = 5.1×10−5, z = −9.76, p < 0.04) as well as significant nimodipine by session2 (β = −1.2×10−4, SE β = 2.0×10−5, z = −6.47, p < 0.04) and MeHg by session2 (β = −3.16×10−4, SE β =3.0×10−5, z = −10.71, p < 0.04) interactions. During learning sessions, main effects of session (β = 0.10, SE β = 0.004, z = 23.15, p < 0.001) and session2 (β = −6.18×10−4, SE β = 6.9×10−5, z = −8.95, p < 0.001) as well as nimodipine by session2 (β = −7.1×10−5, SE β = 2.3×10−5, z = −3.09, p < 0.001) and MeHg by session2 interactions (β = −2.01×10−4, SE β = 3.5×10−5, z = −5.79, p < 0.001) were significant. Figure 2 illustrates these results. As with PQ, there was no effect of nimodipine on MCL for the 0 MeHg mice but nimodipine prevented MeHg’s neurotoxicity (as expressed on this dependent measure).
3.3.Response Rate
For response rate (as for PQ and MCL) nimodipine offered dose-dependent protection from MeHg toxicity. A significant three-way interaction among nimodipine, MeHg and session2 was found for performance (β = 5.37×10−4, SE β = 1.12×10−4, z =4.77, p <.001) and learning sessions (β = 5.1×10−4, SE β = 1.2×10−4, z = 4.25, p<.001).
During performance sessions there were also main effects of session (β = 0.52, SE β = 0.02, z = 27.54, p < 0.0005) and session2 (β = −0.004, SE β = 4.39×10−4, z = −9.85, p < 0.0005) on response rate as well as a significant MeHg by session2 (β = −8.85×10−4, SE β = 2.55×10−4, z = −3.47, p < 0.0005) interaction term. During learning sessions main effects of session (β = .60, SE β = 0.04, z = 17.03, p < 0.009) and session2 (β = −5.2×10−3, SE β = 5.4×10−4, z = −9.62, p < 0.009) and a MeHg by session2 interaction (β = −7.10×10−4, SE β = 2.73×10−4, z = −2.59, p < 0.009) were significant. Figure 3 illustrates these results. Nimodipine did not affect response rate in the 0 MeHg mice but it prevented MeHg’s toxicity in the exposed mice.
Figure 3.
Response rate across experimental days for each exposure group, structured similarly as figure 1.
3.4.MeHg’s effects on response rate and PQ following prolonged exposure
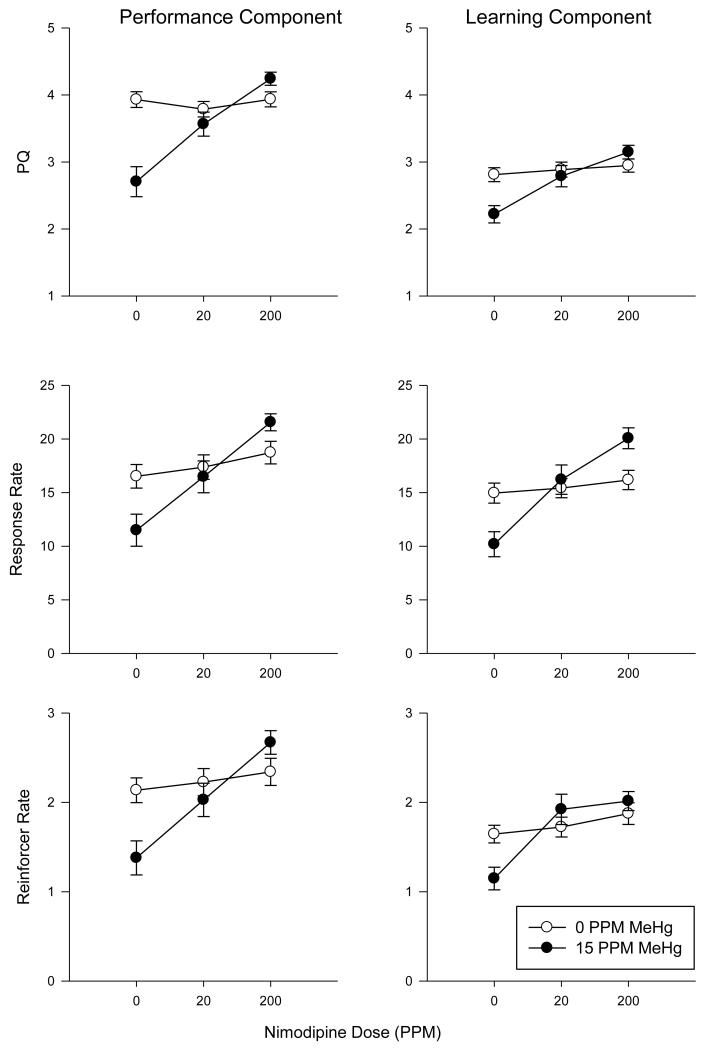
To examine the extent of nimodipine’s protection, and to better understand the independence from rate of learning effects, we examined the last five days of exposure to MeHg separately. Two-way ANOVA’s revealed a significant interaction between nimodipine and MeHg dose for PQ (F(2,284)= 14.19, p<0.0001), response rate (F(2,292)= 4.87, p<0.01) and reinforcer rate (F(2,292)= 5.10, p<0.01) during the performance condition. Similarly, during the learning condition, a nimodipine by MeHg interaction for all three measures PQ (F(2,344)= 4.56, p<0.001), response rate (F(2,352)= 7.48, p<0.001) and reinforcer rate (F(2,352)= 3.71, p<0.03) was significant. These data are plotted in Figure 4 and the relationship among these three measures is plotted in Figure 5. Pearson’s correlation revealed a significant and positive relationship among all dependent measures, which was true during both performance and learning conditions. All correlations p<0.001.
Figure 4.
PQ (top), response rate (middle) and reinforcer rate (bottom) data from the last 5 sessions of performance (left) and learning (right) for the control (open circle) and 15 ppm MeHg (filled circle) groups as a function of nimodipine dose (x-axis).
Figure 5.
Relationship between progress quotient (PQ) score and maximum chain length (MCL) (top left), PQ and response rate (responses/min) (top right) and reinforcer rate (reinforcers/min) and response rate (bottom right) during performance (filled circles) and learning (open circles) sessions from the last five days of the experiment. (Note: −0.1 has been applied to performance MCL and +0.1 has been applied to learning MCL).
Brain MeHg Concentrations
All three nimodipine groups of MeHg-treated animals had significantly higher brain concentrations of Hg than the non-MeHg treated animals (p’s < .0001). Within the nimodipine treated + MeHg exposed groups, brain Hg concentrations were statistically indistinguishable between the 15 ppm MeHg + 0 ppm nimodipine and the 15 ppm MeHg + 20 ppm nimodipine groups (p = .81) but the 200 ppm nimodipine group had significantly lower brain Hg concentrations than the 0 ppm nimodipine group (t(22)= 4.24, p < .0001). See Figure 6.
Figure 6.
MeHg brain concentration from the 0ppm MeHg 0 ppm nimodipine group (left-most position) and each chronic MeHg (15 ppm MeHg) exposure group across three nimodipine groups (0, 20 and 200 ppm nimodipine). Brain tissue was analyzed at the end on the experiment. “*” indicates p < .0001.
4. DISCUSSION
MeHg’s neurotoxicity has been linked to disrupted [Ca2+]i homeostasis in vitro (Komulainen and Bondy, 1987; Levesque and Atchison, 1991; Denny et al., 1993; Hare et al., 1993; Marty and Atchison, 1997; 1998; Bearrs et al., 2001; Limke et al., 2003) but the relevance of this to MeHg’s behavioral toxicity is not clear. We administered MeHg and the L-type CCB nimodipine chronically to mice as they engaged in an IRA task. Nimodipine or other L-type CCBs have has been shown to attenuate the behavioral deficits associated with other CNS insults (Deyo et al., 1989a; 1989b; Straube et al., 1990; Levere and Walker, 1992; Kabuto et al., 1995; Maxwell et al., 1999). Sakamoto et al., (1996) reported that several L-type CCBs, but not nimodipine, prevented body-weight loss, neurological signs, and mortality that was due to MeHg exposure and that flunarizine did so in a dose-related fashion. We used nimodipine because of its good penetration of the blood-brain barrier (Van den Kerckhoff and Drewes, 1985) and high specificity of binding to dihydropyridine receptors (Peroutka and Allen, 1983; Belleman et al., 1983; Dompert and Traber, 1984).
The present design permitted an evaluation of learning and performance deficits associated with chronic, adult-onset MeHg exposure as well as nimodipine’s effects in both exposed and un-exposed animals. Repeated acquisition procedures, like that used here, were designed to generate a steady state of acquisition and can capture even subtle changes in learning. IRA has the benefit of including a performance condition wherein a previously acquired response chain is performed, thus allowing for the assessment of memory (i.e. the performance of a previously learned sequence) and serving as control for motor function. In this way, IRA is capable of capturing and distinguishing novel acquisition (i.e. learning) from motoric ability, and from the performance of previously acquired behavior (Bailey et al., 2010).
While sensory and motor deficits are well-described following adult-onset MeHg exposure, learning deficits have been more difficult to capture and have often been assessed with procedures that make motor contributions difficult to determine. In the present study, mice exposed to MeHg in the absence of nimodipine, had markedly impaired acquisition (learning component) and performance (performance component) of the IRA response chain. MeHg-induced reductions of PQ, MCL, and response rate during both the learning and performance components were ameliorated by chronic nimodipine in a dose-related fashion. In fact, there was no evidence of MeHg neurotoxicity on any measure in mice concurrently consuming 200 ppm of nimodipine. This is consistent with reports from the molecular literature showing that CCBs delay MeHg-induced elevations in intracellular Ca2+, prevent cell death and diminish synaptic alterations following MeHg application (see Hare and Atchison, 1995a; Sakamoto et al., 1996). It also supports hypotheses that the delayed neurotoxicity of Methylmercury (e.g. Weiss et al., 2002; Landrigan et al., 2005) is linked to the cumulative impact of cellular dysfunction that results from disrupted intracellular Ca2+ homeostasis. This protection occurred, for the low-dose nimodipine group, in the absence of a reduction in total brain Hg concentration, therefore supporting the hypothesis that calcium regulation is a relevant mechanism of nimodipine’s neuroprotection. For the high-dose nimodipine group, there was modest reduction in brain mercury, suggesting that additional, perhaps non-neural, mechanisms may be invoked at high doses. It is unclear whether these mechanisms have a neural basis or instead involve some peripheral mechanism like reduced absorption of MeHg from the diet.
MeHg’s effect on PQ and MCL in both the learning and performance condition mirrored its effect on response rate. Such interdependence is not a necessary outcome of the IRA procedure since effects on measures of accuracy can occur independently of effects on response rate (Bailey et al., 2010; Cohn & Paule, 1995; Paule & McMillan, 1984). If, for instance, PQ had declined while response rate was unaffected or if deficits only appeared during the learning condition then that would be considered evidence of a purely cognitive deficit due to chronic MeHg exposure. Because, however, response rates were affected similarly as PQ and MCL and performance and learning conditions were similarly affected, it is concluded that the decreases in measures of learning were secondary to sensory-motor deficits. That is, “cognitive deficits” of adult-onset exposure were related to response-rate, and resulting reinforcer-rate, decreases and not actual cognitive deficits.
The present study is the first of our knowledge to track learning ability in healthy animals administered nimodipine chronically. Nimodipine alone did not affect measures of learning, performance, or response rate in mice (i.e. those that were not exposed to MeHg). This is true both of the time-series analyses and when the last five days were analyzed. The absence of an effect in the nimodipine + 0ppm MeHg groups is of interest because it shows that the nimodipine doses used were otherwise benign on all measures examined. Additionally, these data may help clarify some of the existing inconsistencies in the literature as disparate effects of acute CCBs have been demonstrated when administered in the absence of CNS insult. CCBs have been reported to produce benefits (Quartermain et al., 1993; McMonagle-Strucko and Fanelli, 1993; Deyo and Hittner, 1995), have no effect or result in impairment (Maurice et al., 1995a; 1995b; Clements et al., 1995) on learning endpoints in otherwise healthy animals. These divergent effects occurred despite the use of similar learning tasks, acute doses and routes of administration. Importantly however, these studies did not employ a chronic design, which is more akin to the human condition, and they used intra-peritoneal administration, which even at comparable doses likely resulted in higher peak blood concentrations of CCBs than in the present study. Interestingly, our own unpublished data has suggested acute high doses of some CCBs may affect performance on IRA, in healthy adults.
Brain concentrations of MeHg were in the range of 15 to 20 ppm in the present study after up to about four months of exposure. These concentrations are somewhat higher than reported in a previous study using rats exposed via a similar route, but lower doses. There, brain mercury concentration was about 8 and 10 ppm after six and eighteen months of exposure, respectively. By comparison, human brain mercury taken from autopsies is typically in the tens of ppb (Bjorkman, et al., 2007; Korbas et al., 2010, Nylander and Friberg, 1987, Matsuo et al., 1987).
Studies of the kinetics of intracellular Ca2+ have revealed that MeHg elevates calcium concentration in nerve terminals in two distinct temporal phases. The initial phase is due to the release of calcium from intracellular stores (i.e. mitochondria and smooth endoplasmic reticulum). In the second phase extracellular calcium enters the nerve terminal via voltage-gated calcium channels on the cell membrane (Marty and Atchison, 1997; Edwards et al., 2005). Marty and Atchison (1997) showed that CCB (L-, N- and T-type) application delayed the MeHg-induced onset of both phases of calcium flux and improved cell viability (Marty and Atchison, 1997). CCB’s block of voltage-gated calcium channels on the cell membrane is responsible for delaying the second phase of calcium flux, however the exact pathway by which the CCBs delay the first phase of increased intracellular calcium concentration is not yet understood.
The ability of nimodipine to delay or prevent MeHg’s behavioral toxicity, here, and to prevent weight-loss and mortality (Sakamoto et al. 1996), supports the results seen in the in vitro studies. By showing that drugs that reduce and delay the rise in intracellular Ca2+ in vitro associated with MeHg application also delay or block MeHg’s behavioral toxicity in vivo, we have provided strong experimental support for the hypothesis that Ca2+ dysregulation plays a crucial role in MeHg’s neurotoxicity. Because L-type Ca2+ blockers act by blocking the channels on the cell membrane, which 1) delays intracellular increases in calcium concentration and 2) prevents or delays behavioral signs of MeHg neurotoxicity, it might be concluded that the second phase of MeHg-induced Ca2+ dysregulation, reflecting the flow of Ca2+ across the cell membrane, is especially important in the behavioral manifestation of MeHg neurotoxicity. This conclusion should be tempered somewhat since the first phase of Ca2+ increase is also delayed somewhat by these drugs, but it is unclear the extent to which the first phase of Ca2+ increase contributes to the cellular dysfunction that is characteristic of MeHg exposure. Further, the magnitude of the first phase is similar to that seen with K+ depolarization and, as noted by Marty and Atchison (1997), would presumably be buffered back to baseline levels quickly.
In summary, the present behavioral data, in combination with data from in vitro designs, provide support for the hypothesis that a key mechanism by which MeHg exerts its cellular and behavioral effects is through disrupted [Ca2+]i homeostasis, particularly at lower nimodipine concentrations. These data extend the results of an earlier study (Sakamoto et al., 1996) to incorporate more subtle behavioral effects. Therapeutically, these data are important because they implicate oral CCB-administration for the prevention and possibly treatment of MeHg toxicity, although it is not known at present the extent to which CCBs may be useful if administered after exposure to MeHg occurs (a line of study that needs pursuing). The present data are also the first to utilize a sensitive learning task to evaluate adult-onset MeHg exposure. Because the effects on response rate were approximately coincident with PQ, it is concluded that MeHg effects that are likely sensorimotor can influence a cognitive endpoint (i.e. PQ and MCL) by reducing the opportunity to acquire new behavior. This is important because it suggests a sensorimotor route to deficits that might appear to be cognitive in nature. Finally, these data speak to the nature of CCB effects in the absence of CNS injury. Here, there was no impact, in terms of cognitive function, of any dose of nimodipine in groups not exposed to MeHg. This is inconsistent with some other reports in the literature that have suggested acute CCBs may have cognitive enhancing properties even in the absence of obvious CNS insult, but consistent with the conceptual framework of CCB protection.
Highlights.
MeHg’s neurotoxicity may be related to disrupted intracellular Ca++ homeostasis.
Cognitive effects of adult-onset MeHg exposure are poorly understood.
Chronic MeHg reduced accuracy and responding in a repeated acquisition procedure.
Cognitive effects may have been secondary to motor deficits.
A calcium channel blocker, nimodipine, conferred dose-dependent neuroprotection.
Acknowledgements
A. This research was supported by the National Institutes of Health [Grant ES R01 003299].
Non-standard abbreviation
- CCB
calcium channel blocker
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Atchison WD, Hare MF. Mechanisms of methylmercury-induced neurotoxicity. FASEB J. 1994;8:622–9. doi: 10.1096/fasebj.8.9.7516300. [DOI] [PubMed] [Google Scholar]
- Atchison WD. Effects of toxic environmental contaminants on voltage-gated calcium channel function: From past to present. J Bioenerg Biomembr. 2003;35:507–32. doi: 10.1023/b:jobb.0000008023.11211.13. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Johnson JE, Newland MC. Mechanisms and performance measures in mastery-based incremental repeated acquisition: behavioral and pharmacological analyses. Psychopharmacology. 2010;209:331–41. doi: 10.1007/s00213-010-1801-3. [DOI] [PubMed] [Google Scholar]
- Bates DM, DebRoy S. Linear mixed models and penalized least squares. J Multivariate Analysis. 2004;91:1–17. [Google Scholar]
- Bearrs JJ, Limke TL, Atchison WD. Methylmercury (MeHg) causes calcium release from smooth endoplasmic reticulum (SER) inositol-1,4,5-triphosphate receptors in rat cerebellar granule neurons. The Toxicologist. 2001;60:184. [Google Scholar]
- Belleman P, Sehade A, Towart R. Dihydropyridine receptor in rat brain labeled with [3-H]nimodipine. Proc Natl Acad Sci. 1983;80:2356–60. doi: 10.1073/pnas.80.8.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman L, Lundekvam B, Laegreid T, Bertelsen B, Morild I, Lilleng P, et al. Mercury in human brain, blood, muscle and toenails in relation to exposure: an autopsy study. Environ Health. 2007;6:30. doi: 10.1186/1476-069X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements MP, Rose SPR, Tiunova A. ω-Conotoxin GIVA disrupts memory formation in the day old chick. Neurobiol Learn Mem. 1995;64:276–84. doi: 10.1006/nlme.1995.0010. [DOI] [PubMed] [Google Scholar]
- Cohn J, Cox C, Cory-Slechta DA. The effects of lead exposure on learning in a multiple repeated acquisition and performance schedule. Neurotoxicology. 1993;14:329–46. [PubMed] [Google Scholar]
- Davidson PW, Myeres GJ, Weiss B, Shamlaye CF, Cox C. Prenatal methyl mercury exposure from fish consumption and child development: A review of evidence and perspectives from the Seychelles Child Development Study. Neurotoxicology. 2006;27:1106–9. doi: 10.1016/j.neuro.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Denny MF, Hare MF, Atchison WD. Methylmercury altars intrasynaptosomal concentrations of endogenous polyvalent cations. Toxicol Appl Pharmacol. 1993;122:222–32. doi: 10.1006/taap.1993.1191. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Hittner JM. Effects of the Ca2+ channel antagonist flunarizine on visual discrimination learning. Neurobiol Learn Mem. 1995;64:10–6. doi: 10.1006/nlme.1995.1039. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Straube KT, Disterhoft JF. Nimodipine facilitates associative learning in aging rabbits. Science. 1989;243:809–11. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Straube KT, Moyer JR, Disterhoft JF. Nimodipine ameliorates aging-related changes in open-field behaviors of the rabbit. Exp Aging Resch. 1989;15:169–75. doi: 10.1080/03610738908259771. [DOI] [PubMed] [Google Scholar]
- Dompert WU, Traber J. Binding sites for dihydropyridine calcium antagonists. In: Opie LH, editor. Calcium Antagonists and Cardiovascular Disease. Raven Press; New York: 1989. pp. 175–179. [Google Scholar]
- Edwards JR, Marty MS, Atchison WD. Comparative sensitivity of rat cerebellar neurons to dysregulation of divalent cation homeostasis and cytotoxicity caused by methylmercury. Toxicol. Appl. Pharmacol. 2005;208:222–32. doi: 10.1016/j.taap.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Evans HL, Laties VG, Weiss B. Behavioral effects of mercury and methylmercury. Fed Proc. 1975;34:1858–67. [PubMed] [Google Scholar]
- Finger S, Green L, Tarnoff ME, Mortman KD, Andersen A. Nimodipine enhances new learning after hippocampal damage. Exp Neurol. 1990;109:279–85. doi: 10.1016/s0014-4886(05)80018-4. [DOI] [PubMed] [Google Scholar]
- Hare MF, Atchison WD. Methylmercury mobilizes Ca2+ from intracellular stores sensitive to inositol 1,4,5-trophosphate in NG108-15 cells. J Pharmacol Exp Ther. 1995b;272:1016–23. [PubMed] [Google Scholar]
- Hare MF, Atchison WD. Nifedipine and tetrodotoxin delay the onset of methylmercury-induced increases in [Ca2+]i. Toxicol Appl Pharmacol. 1995a;135:299–307. doi: 10.1006/taap.1995.1236. [DOI] [PubMed] [Google Scholar]
- Hare MF, McGinnis KM, Atchison WD. Methylmercury increases intracellular concentrations of Ca2+ and heavy metals in NG108-15 cells. J Pharmacol Exp Ther. 1993;266:1626–35. [PubMed] [Google Scholar]
- Heath JC, Banna KM, Reed MN, Pesek EF, Cole N, Li J, Newland MC. Dietary selenium protects against selected signs of methylmercury exposure and aging. Neurotoxicology. 2010;31:169–79. doi: 10.1016/j.neuro.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabuto H, Yokoia I, Moria A, Murakamib M, Sawadab S. Neurochemical changes related to ageing in the senescence-accelerated mouse brain and the effect of chronic administration of nimodipine. Mech Ageing Dev. 1995;80:1–9. doi: 10.1016/0047-6374(94)01542-t. [DOI] [PubMed] [Google Scholar]
- Komulainen H, Bondy SC. Increased free intrasynaptosomal Ca2+ by neurotoxic organometals: distinctive mechanisms. Toxicol Appl Pharmacol. 1987;88:77–86. doi: 10.1016/0041-008x(87)90271-7. [DOI] [PubMed] [Google Scholar]
- Korbas M, O’Donoghue JL, Watson GE, Pickering IJ, Singh SP, Myers GJ, et al. The Chemical Nature of Mercury in Human Brain Following Poisoning or Environmental Exposure. ACS Chemical Neuroscience. 2010;1:810–8. doi: 10.1021/cn1000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska M, Disterhoft JF. Relation of nimodipine dose and serum concentration to learning enhancement in aging rabbits. Exp Neurol. 1994;127:159–66. doi: 10.1006/exnr.1994.1090. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D. Early Environmental Origins of Neurodegenerative Disease in Later Life. Environ Health Perspect. 2005;113:1230–3. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levere TE, Walker A. Old age and cognition: Enhancement of recent memory in aged rats by the calcium channel blocker nimodipine. Neurobiol Aging. 1992;13:63–6. doi: 10.1016/0197-4580(92)90010-u. [DOI] [PubMed] [Google Scholar]
- Levesque PC, Atchison WD. Disruption of brain mitochondrial calcium sequestration by methylmercury. J Pharmacol Exp Ther. 1991;256:236–42. [PubMed] [Google Scholar]
- Limke TL, Otero-Montanez JK, Atchison WD. Evidence for interactions between intracellular calcium stores during methylmercury-induced intracellular calcium dysregulation in rat cerebellar granule neurons. J Pharmacol Exp Ther. 2003;304:949–58. doi: 10.1124/jpet.102.042457. [DOI] [PubMed] [Google Scholar]
- Marty MS, Atchison WD. Elevations in intracellular Ca2+ as a probable contributor to decreased viability in cerebellar granule cells following acute exposure to methylmercury. Toxicol Appl Pharmacol. 1998;150:98–105. doi: 10.1006/taap.1998.8383. [DOI] [PubMed] [Google Scholar]
- Marty MS, Atchison WD. Pathways mediating Ca2+ entry in rat cerebellar granule cells following in vitro exposure to methylmercury. Toxicol Appl Pharmacol. 1997;147:319–30. doi: 10.1006/taap.1997.8262. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Suzuki T, Akagi H. Mercury Concentration in Organs of Contemporary Japanese. Archives of Environmental Health: An International Journal. 1989;44:298–303. doi: 10.1080/00039896.1989.9935897. [DOI] [PubMed] [Google Scholar]
- Maurice T, Bayle J, Privat A. Learning impairment following acute administration of the calcium channel antagonist nimodipine in mice. Behav Pharmacol. 1995;6:167–75. [PubMed] [Google Scholar]
- Maurice T, Su T-P, Parish DW, Privat A. Prevention of nimodipine-induced impairment of learning by the selective σ ligand PRE-084. J Neural Transm. 1995;102:1–18. doi: 10.1007/BF01276561. [DOI] [PubMed] [Google Scholar]
- Maxwell CJ, Hogan DB, Ebly EM. Calcium-channel blockers and cognitive function in elderlypeople: results from the Canadian Study of Health and Aging. CMAJ. 1999;161:501–6. [PMC free article] [PubMed] [Google Scholar]
- McMonagle-Strucko K, Fanelli RJ. Enhanced acquisition of reversal training in a spatial learning task in rats treated with chronic nimodipine. Pharmacol Biochem Behav. 1993;44:827–35. doi: 10.1016/0091-3057(93)90013-j. [DOI] [PubMed] [Google Scholar]
- Mršić J, Zupan G, Eraković V, Simonić A, Varljen A. The influence of nimodipine and MK-801 on the brain free arachidonic acid level and the learning ability in hypoxia-exposed rats. Progr Neuro Psychopharmacol Biol Psychiatr. 1997;21:345–58. doi: 10.1016/s0278-5846(97)00005-5. [DOI] [PubMed] [Google Scholar]
- Newland MC, Paletz EM, Reed MN. Methylmercury and nutrition: Adult effects of fetal exposure in experimental models. Neurotoxicology. 2008;29:783–801. doi: 10.1016/j.neuro.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Rasmussen EB. Aging unmasks adverse effects of gestational exposure to methylmercury in rats. Neurotoxicol Teratol. 2000;22:819–28. doi: 10.1016/s0892-0362(00)00107-0. [DOI] [PubMed] [Google Scholar]
- Newland MC, Reile PA, Langston JL. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicol Teratol. 2004;26:179–94. doi: 10.1016/j.ntt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Nylander M, Friberg L, Lind B. Mercury concentrations in the human brain and kidneys in relation to exposure from dental amalgam fillings. Swed Dent J. 1987;11:179–87. [PubMed] [Google Scholar]
- Paule MG, Chelonis JJ, Buffalo EA. Operant Test Battery Performance in Children: Correlation with IQ. Neurotoxicol Teratol. 1999;21:223–230. doi: 10.1016/s0892-0362(98)00045-2. [DOI] [PubMed] [Google Scholar]
- Paule MG, McMillan DE. Incremental repeated acquisition in the rat: Acute effects of drugs. Pharmacol Biochem Behav. 1984;21:431–39. doi: 10.1016/s0091-3057(84)80106-9. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Allen GS. Calcium channel antagonist binding sites labeled by 3H-nimodipine in human brain. J Neurosurg. 1983;59:933–7. doi: 10.3171/jns.1983.59.6.0933. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects Models in S and S-Plus. Springer; New York: 2004. [Google Scholar]
- Quartermain D, Garcia deSoria V, Kwan A. Calcium channel antagonists enhance retention of passive avoidance and maze learning in mice. Neurobiol Learn Mem. 2001;75:77–90. doi: 10.1006/nlme.1999.3958. [DOI] [PubMed] [Google Scholar]
- Quartermain D, Hawxhurst A, Ermita B, Puente J. Effect of the calcium channel blocker amlodipine on memory in mice. Behav Neural Biol. 1993;60:211–9. doi: 10.1016/0163-1047(93)90390-4. [DOI] [PubMed] [Google Scholar]
- Reed MN, Paletz EM, Newland MC. Gestational exposure to methylmercury and selenium: Effects on a spatial discrimination reversal in adulthood. Neurotoxicology. 2006;27:721–32. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuhl KR. Delayed Expression of Neurotoxicity: The Problem of Silent Damage. Neurotoxicology. 1991;12:341–6. [PubMed] [Google Scholar]
- Rice DC. Effects of pre- plus postnatal exposure to methylmercury in the monkey on fixed interval and discrimination reversal performance. Neurotoxicology. 1992;13:443–52. [PubMed] [Google Scholar]
- Rice DC. Evidence for delayed neurotoxicity produced by methylmercury. Neurotoxicology. 1996;17:583–96. [PubMed] [Google Scholar]
- Rice DC. Nervous system effects of perinatal exposure to lead or methylmercury in monkeys. In: Clarkson TW, Nordberg G, Sager P, editors. Reproductive and Developmental Toxicity of Metals. Plenum Press; New York: 1983. pp. 517–40. [Google Scholar]
- Sakamoto M, Ikegami N, Nakano A. Protective Effects of Ca2+ Channel Blockers against Methyl Mercury Toxicity. Basic Clin Pharmacol Toxicol. 1996;78:193–9. doi: 10.1111/j.1600-0773.1996.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Straube KT, Deyo RA, Moyer JR, Jr., Disterhoft JF. Dietary nimodipine improves associative learning in aging rabbits. Neurobiol Aging. 1990;11:659–61. doi: 10.1016/0197-4580(90)90033-v. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Disterhoft JF, Deyo RA. Nimodipine enhances spontaneous activity of hippocampal pyramidal cells in aging rabbits at a dose that facilitates learning. Brain Res. 1990;535:119–30. doi: 10.1016/0006-8993(90)91830-a. [DOI] [PubMed] [Google Scholar]
- Van den Kerckhoff W, Drewes LR. Transfer of the Ca-antagonists nifedipine and nimodipine across the blood-brain barrier and their regional distribution in vivo. J Cereb Blood Flow Metab. 1985;5:459–60. [Google Scholar]
- Weiss B, Clarkson TW, Simon W. Silent Latency Periods in Methylmercury Poisoning and in Neurodegenerative Disease. Environ Health Perspect. 2002;110:851–4. doi: 10.1289/ehp.02110s5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger G, Schmidt C, Davisson M. Operant Conditioning in the Ts65Dn Mouse: Learning. Behav Genet. 2004;34:105–19. doi: 10.1023/B:BEGE.0000009480.79586.ee. [DOI] [PubMed] [Google Scholar]
- Yanpallewar SU, Hotab D, Raic S, Kumarc M, Acharyaa SB. Nimodipine attenuates biochemical, behavioral and histopathological alterations induced by acute transient and long-term bilateral common carotid occlusion in rats. Pharmacol Res. 2004;49:143–50. doi: 10.1016/j.phrs.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Shimizu N, Suzuki M, Watanabe C, Satoh M, Mori K, Yasutake A. Emergence of Delayed Methylmercury Toxicity after Perinatal Exposure in Metallothionein-Null and Wild-Type C57BL Mice. Environ Health Perspect. 2008;116:746–51. doi: 10.1289/ehp.10906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Methylmercury-induced increase of intracellular Ca2+ increases spontaneous synaptic current frequency in rat cerebellar slices. Mol Pharmacol. 2007;71:1109–21. doi: 10.1124/mol.106.031286. [DOI] [PubMed] [Google Scholar]