Abstract
Background
Inducible co-stimulator (ICOS), a member of the CD28 family of costimulatory molecules, is induced on CD4+ and CD8+ T-cells following their activation. ICOS functions as an essential immune regulator and ICOS blockade is a potential approach to immune modulation in allogeneic transplantation. Here, we describe the expression profile of ICOS in dogs and determine whether ICOS expression is up-regulated during chronic graft versus host disease (GVHD) and host versus graft (HVG) reactions in the canine hematopoietic cell transplantation model.
Methods
Monoclonal antibodies against cell surface-expressed ICOS were produced and tested in vitro for suppression of canine mixed leukocyte reactions (MLR). Expression of ICOS on CD3+ cells was evaluated by flow cytometry using peripheral blood, lymph nodes and splenocytes obtained from dogs undergoing GVH and HVG reactions.
Results
Canine ICOS was expressed in an inducible pattern on T-cells activated by Con A, anti-CD3 mAb in combination with anti-CD28 mAb, and alloantigen stimulation. Immunosuppressive effects of ICOS blockade were observed in MLR using peripheral blood mononuclear cells from dog-leukocyte-antigen-nonidentical dogs. Immunosuppressive effects of ICOS blockade were observed in MLR when anti-ICOS was combined with suboptimal concentrations of cytotoxic T-lymphocyte antigen 4-Ig (CTLA4-Ig) or cyclosporine. ICOS expression was significantly up-regulated on T-cells in dogs undergoing graft rejection or chronic GVHD after allogeneic hematopoietic cell transplantation.
Conclusion
These studies suggest that ICOS plays a role in graft rejection and GVHD in an out-bred animal model, and ICOS blockade may be an approach to prevention and treatment of chronic GVHD.
Keywords: dog, hematopoietic cell transplantation, ICOS, graft-versus-host disease, costimulatory molecule
INTRODUCTION
Inducible co-stimulator (ICOS), a member of the CD28 family of costimulatory molecules, is expressed on the surface of CD4+ and CD8+ T-cells following activation and is constitutively expressed on certain memory T-cell subsets (1). The nature of ICOS up-regulation on activated T-cells and the tissue expression of B7.h make ICOS a potential candidate for therapeutic approaches to two major issues in allogeneic hematopoietic cell transplantation (HCT): graft rejection and graft-vs.-host disease (GVHD).
Several studies indicate that ICOS plays a role in solid organ transplant rejection in mice and rats (2–4) and GVHD in mice (5–7). One report showed that ICOS blockade using either ICOS −/− mice or anti-ICOS monoclonal antibody (mAb) resulted in inhibition of GVHD and promotion of allogeneic hematopoietic engraftment (8). However, ICOS expression and blockade during hematopoietic graft rejection or GVHD have not been adequately characterized in an outbred large-animal model.
The canine model for allogeneic HCT is well-established and has played a fundamental role in numerous advances in the field, particularly in the prevention and treatment of acute GVHD (9–11). Despite these successes, identifying treatment strategies for chronic GVHD in any preclinical animal model has not been as successful. Murine studies have suggested that costimulatory molecule blockade using anti-ICOS mAb may be a promising approach to the treatment of chronic GVHD (12,13). Upon this foundation, we hypothesized that targeting ICOS may have therapeutic value in the canine GVHD model.
In a previous study, we cloned the full length of the open reading frame of canine ICOS cDNA and identified sequence similarities between the dog and other species (14). To further investigate the effect of ICOS blockade for the prevention of GVHD and graft rejection in dogs, we have produced and characterized the in vitro function of anti-canine (ca) ICOS monoclonal antibodies (mAb) and assessed ICOS expression in dogs undergoing hematopoietic graft rejection or chronic GVHD. Here we show that antagonistic anti-caICOS mAb can be produced and function synergistically with CD28/B7.1, B7.2 blockade or with the calcineurin inhibitor cyclosporine. ICOS expression is specifically increased on activated T-cells in dogs. Additionally, we show that ICOS expression is significantly up-regulated on T-cells in dogs experiencing graft rejection or chronic GVHD following dog leukocyte antigen (DLA)-haploidentical HCT.
RESULTS
Selection of Anti-caICOS mAb Clone 3F12 and ICOS Up-regulation on Activated T-cells
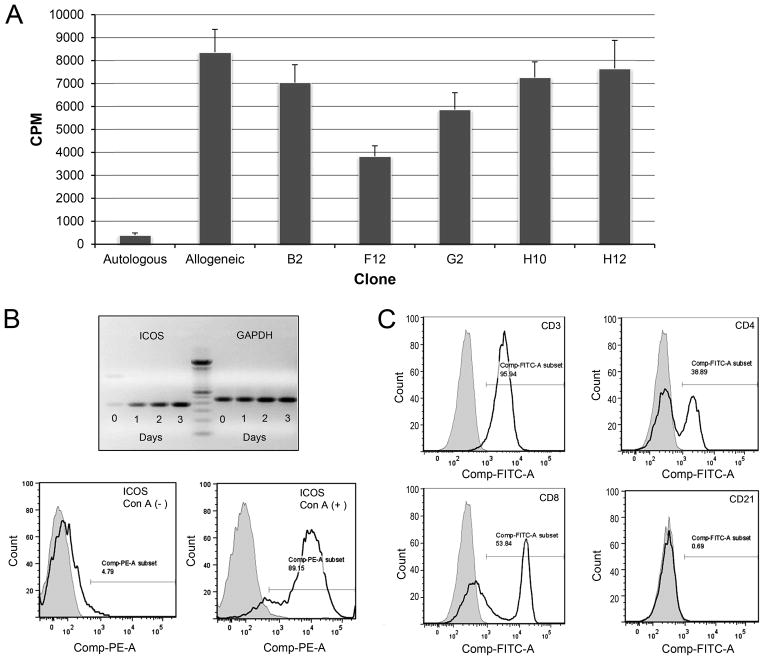
Clones of anti-caICOS mAb were screened for expression and the ability to suppress mixed leukocyte reactions (MLR). Of five clones identified, 3F12 was selected based on the acceptable levels of expression and antagonistic activity in MLR (Fig 1A).
FIGURE 1. Anti-caICOS clone selection and ICOS expression on canine lymphocytes.
A) Five anti-caICOS mAbs were purified from hybridoma supernatants and added at 10 μg/ml to 7-day MLR comprised of PBMC from DLA-nonidentical dogs. Results are representative of two experiments and presented as counts per minute (CPM) of 3H-thymidine. B (upper) Real time PCR showing ICOS and GAPDH (control) mRNA expression in Con A activated PBMC over a three-day period. A 100 bp ladder (center) marks the expected size of the extracellular domain of canine ICOS at 363 bp. B (lower) Histogram showing ICOS-PE expression on naive and 3–day Con A activated canine CD3+ lymphocytes. (A and B representative of 4 dogs in 4 experiments) C) ICOS expression on PBMC from normal dogs cultured with Con A for 3 days. The histograms show ICOS+ on CD3+ gated cells (upper left panel); with this population stained with CD4-FITC and CD8-FITC antibodies (panels upper right and lower left respectively) or the B-cell marker, CD21-PE (lower right). Shaded regions represent isotype control fluorescence intensity. (Figure representative of 3 experiments using 3 different dogs).
The kinetics of up-regulation of ICOS expression on Con A-activated PBMC was determined using RT-PCR. ICOS mRNA expression increased time-dependently with significant levels of expression seen by day 3 (Figure 1B). ICOS expression was observed by flow cytometry on 89.2% of Con A-activated PBMC after 3 days of culture, but only 4.8% of resting PBMC expressed ICOS in the same period (Fig 1B). ICOS+ expression was observed on CD3+ lymphocytes, equally on both CD4+ and CD8+ cells, but not significantly on CD21+ cells (Fig 1C).
Specificity of Anti-caICOS mAb
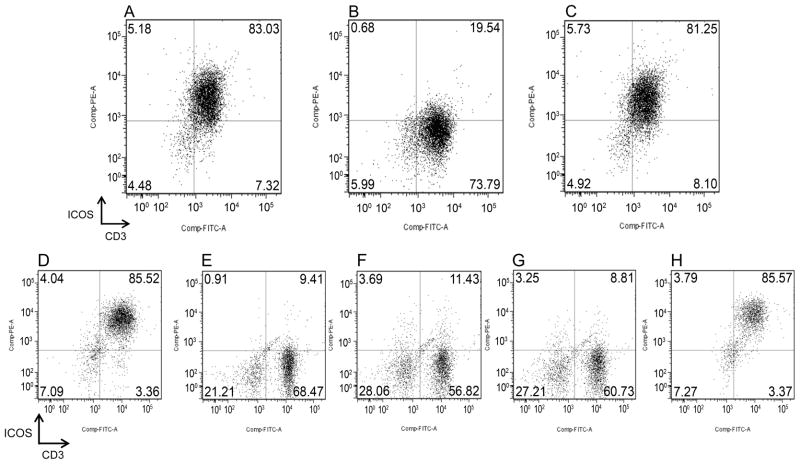
Specificity of anti-caICOS mAb was verified by binding studies of anti-caICOS mAb on activated T-cells in the presence or absence of caICOS-murine-Ig fusion protein. While anti-caICOS mAb bound to 83 % of Con A-activated CD3+ cells (Fig 2A), addition of 100-fold excess of ICOS-murine-Ig fusion protein effectively blocked anti-caICOS mAb binding to 19.5 % of CD3+ cells (Fig 2B). The same concentration of the negative control canine fusion protein, CTLA4-Ig, failed to prevent anti-caICOS mAb from binding to 81 % of Con A-activated T-cells (Fig 2C).
FIGURE 2. ICOS specificity and expression on T-cells mediated by anti-CD3/CD28 or alloantigen stimulation.
A) Gated CD3+ PBMC stained with anti-caICOS-PE. B) Gated CD3+ PBMC stained with a mixture of anti-caICOS-PE (5 μg/ml) and ICOSmurineIg fusion protein (500 μg/ml). C) Gated CD3+ PBMC stained with a mixture of anti-caICOS-PE (5 μg/ml) and the negative control fusion protein CTLA4-Ig (500 μg/ml). Flow cytometry analysis of ICOS expression on CD3+ cells gated from PBMC after culture for 7 days on tissue culture dishes coated with anti-CD3 mAb coated at 10 ug/ml (D), medium only (E), anti-CD3 mAb at 1 μg/ml (F), anti-CD28 mAb at 10 μg/ml (G), or the combination of anti-CD3 (1 μg/ml) and anti-CD28 (10 μg/ml) (H).
Canine ICOS Expression is Regulated by T-cell Receptor/CD28 Costimulatory Pathway and Alloantigen Stimulation
Our previous studies showed that an agonist anti-caCD28 mAb in the presence of suboptimal concentrations of anti-CD3 mAb induced proliferation of canine lymphocytes in the absence of mitogens or other stimulation (15). Here we sought to determine whether the combination of the same concentrations of anti-CD28 and anti-CD3 could induce ICOS expression on canine PBMC. Anti-CD3 mAb at 10 μg/mL induced ICOS expression on T-cells (Fig 2D), while medium alone did not (Fig 2E). Anti-CD3 mAb at 1 μg/mL (Fig 2F) or anti-caCD28 mAb at 10 μg/mL (Fig 2G) alone failed to induce ICOS expression on T cells. However, the combination of anti-CD3 mAb and anti-CD28 mAb at these concentrations effectively induced ICOS expression (Fig 2H).
Anti-caICOS mAb Functions Synergistically with CD28 Blockade
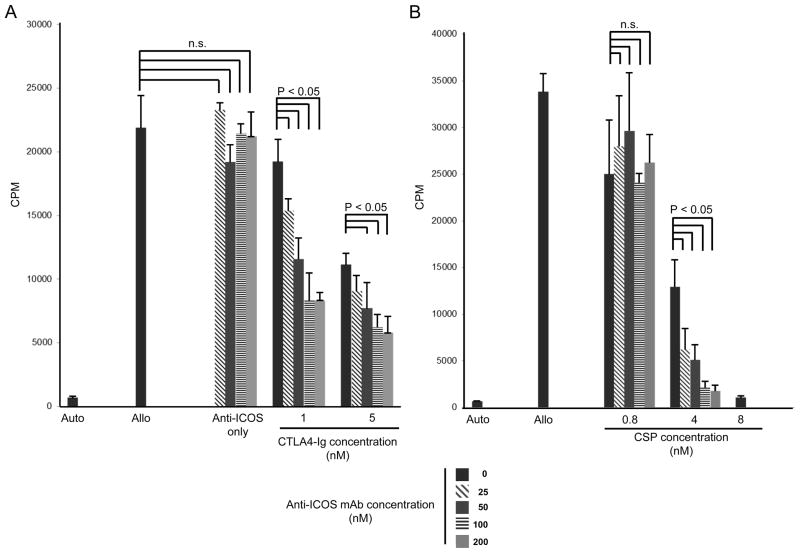
Initial studies showed that anti-caICOS alone was a weak and inconsistent antagonist for cell proliferation in a MLR (Figure 1). Here, we posed the question of whether blockade of the CD28/B7.1-B7.2 pathway with CTLA4-Ig (Abatacept) or immunosuppression with low doses of the calcineurin inhibitor, CSP, would improve the antagonistic activity of anti-caICOS in MLR. PBMC were collected from DLA-nonidentical pairs and were cultured with various concentrations of anti-caICOS mAb, with and without CTLA4-Ig. In one of four representative experiments, anti-caICOS mAb alone failed to suppress MLR, therefore not cytolytic at all concentrations tested (Fig 3A). However, consistent and significant immunosuppressive effects of anti-ICOS were observed in MLR when anti-caICOS was combined with suboptimal concentrations of CTLA4-Ig fusion protein (Fig 3A). Significant levels of immunosuppression were observed with 4 nM CSP only when combined with 25–200 nM anti-ICOS (Fig. 3B). Total MLR suppression was observed with 0.8nM CSP alone (Fig. 4B).
FIGURE 3. Synergy of anti-ICOS and CTLA4-Ig in suppressing MLR.
Anti-ICOS was added to 7-day MLR at 0–200 nM in the presence or absence of CTLA4-Ig at 1 or 5 nM (left panel), or presence or absence of CSP at 0.8–8 nM. The effect of CTLA4-Ig or CSP alone on the MLR is indicated by the black solid bars (positive control). Results are presented in CPM of 3H-thymidine incorporation and representative of 5 experiements).
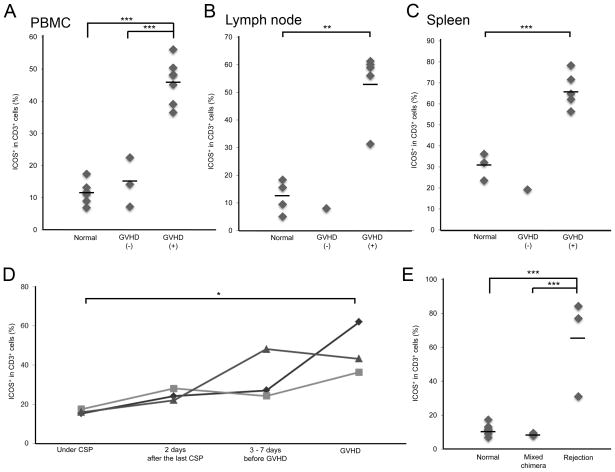
FIGURE 4. ICOS expression in dogs undergoing GVHD and HVG reactions.
PBMC were collected from normal dogs, transplanted dogs with or without GVHD and analyzed by flow cytometry for ICOS expression on CD3+ gated cells at time of necropsy for dogs with GVHD (A). Similarly, lymph nodes (B) and splenocytes (C) were collected and evaluated for ICOS expression from dogs also at time of necropsy. Kinetics of ICOS expression was done on CD3+ PBMC in 3 dogs before and at the onset of clinically diagnosed GVHD (significance * = p< 0.05, ** = p<0.01, *** = p<0.005) (D). ICOS expression on CD3+ PBMC isolated from normal dogs, stable mixed chimeric dogs (1–2 Gy TBI, DLA-identical marrow transplantation and postgrafting immunosuppression, CSP at 10 mg/kg BID for 37 days and MMF at 10 mg/kg BID for 28 days) and dogs undergoing graft rejection, HVG (4.5 Gy TBI, DLA-identical marrow transplantation, postgrafting immunosuppression of CSP at 10 mg/kg BID for 37 days and MMF at 10 mg/kg BID for 28 days) (E).
ICOS Up-regulation on T-cells in Dogs with chronic GVHD or Undergoing Marrow Graft Rejection
To determine whether ICOS could be a therapeutic target for chronic GVHD in dogs, we measured ICOS expression on CD3+ cells isolated from dogs with clinical symptoms of chronic GVHD. Table 1 lists the protocols used to establish the dogs with chronic GVHD, the time and type of GVHD diagnosed, and the samples collected. Six of the seven dogs that developed chronic GVHD were conditioned with 9.2 Gy TBI, transplanted with either DLA-nonidentical or -haploidentical marrow, followed by immunosuppression with methotrexate and CSP. An additional dog that developed chronic GVHD was given 4.5 Gy TBI and CSP + mycophenolate mofetil (MMF) immunosuppression. The median time to GVHD was 104 (range 34 to 213) days. All dogs with one exception were evaluated for ICOS expression by flow cytometry within two weeks of diagnosis of GVHD. Dog H236 was first diagnosed with GVHD on day 176 after transplantation and was then treated with CSP and MMF. GVHD resolved but rapidly progressed beginning 4 days after halting immunosuppression. Nine days later, the dog was tested for ICOS expression. The time to GVHD and the presence of lichenoid changes in the skin, seen in 6 out of 6 dogs listed in Table 1 with skin GVHD and exemplified in one dog (SDC, Supplemental Figure 1), suggested the GVHD was chronic and not acute.
TABLE 1.
Clinical outcomes of dogs following allogeneic hematopoietic transplantation
| ID | Transplantation Conditions | Samples | Onset of GVHD (Days) | ICOS Eval. (Days) | GVHD* |
|---|---|---|---|---|---|
| H236† | 4.5 Gy TBI + CSP (35 days)/MMF (28 days) DLA-haploidentical donor |
PB | 176/262 | 271 | Skin |
| H457 | 9.2 Gy TBI + CSP (120 days)/MMF (180 days) DLA-haploidentical donor |
PB, LN, SP | 213 | 220 | Skin |
| H316 | 9.2 Gy TBI + CSP (80days)/MTX (days 1, 3, 6) DLA-nonidentical donor |
PB, LN, SP | 93 | 103 | Skin/Liver |
| H432 | 9.2 Gy TBI + CSP (80 days)/MTX (days 1, 3, 6) DLA-nonidentical donor |
PB | 95 | 107 | Skin/Liver |
| H470 | 9.2 Gy TBI + CSP (100 days)/MTX (days 1, 3, 6, 11) DLA-nonidentical donor |
PB, LN, SP | 133 | 146 | Skin |
| H479 | 9.2 Gy TBI + CSP (80 days)/MTX (days 1, 3, 6) DLA-nonidentical donor |
PB, LN, SP | 34 | 36 | Liver |
| H481 | 9.2 Gy TBI + CSP (80 days)/MTX (days 1, 3, 6) DLA-nonidentical donor |
PB, LN, SP | 104 | 111 | Skin |
| H348 | 9.2 Gy TBI + CSP (100 days)/MTX (days 1, 3, 6, 11) DLA-nonidentical donor |
PB, LN, SP | ND | 181 | ND |
| H495 | 4.5 Gy TBI + CSP (35 days)/MMF (28 days) DLA-haploidentical donor |
PB | ND | 76 | ND |
| H371 | 4.5 Gy TBI + CSP (35 days)/MMF (28 days) DLA-haploidentical donor |
PB | ND | 252 | ND |
GVHD was confirmed by the clinical manifestation and histopathology evaluation
TBI, total body irradiation; CSP, cyclosporine; MMF, mycophenolate mofetil MTX, methotrexate; DLA, dog leukocyte antigen; PB, peripheral blood; LN, lymph node; SP, spleen; ND, not detected.
H236 presented cutaneous GVHD on day 176 after transplantation and was treated with MMF (5 mg/kg BID) and CSP (10 mg/kg, BID) to resolve the reaction. Immunosuppression ceased on day 258 and cutaneous GVHD verified on day 262 after transplantation.
Three dogs (H348, H495, H371) that did not develop chronic GVHD underwent similar protocols of therapy, namely 4.5 or 9.2 Gy TBI, DLA-haploidentical HCT and postgrafting immunosuppression with CSP and MMF or methotrexate (MTX). Tissues from these dogs were also used to evaluate ICOS expression in the absence of chronic GVHD and considered as controls (Table 1).
ICOS expression was measured on CD3+ T-cells isolated from peripheral blood, lymph nodes, and spleens of normal dogs and dogs which underwent allogeneic HCT resulting in either stable mixed chimerism or GVHD. The means ± SD of percent ICOS+ CD3+ cells isolated from peripheral blood lymphocytes (n=6) (Fig 4A), lymph node cells (n=4) (Fig 4B) and splenocytes (n=3) (Fig 4C) from untreated normal dogs were 11.6 ± 3.6, 12.2 ± 6.0, 30.6 ± 6.5 percent, respectively. The means ± SD of percent ICOS+ CD3+ cells isolated from peripheral blood lymphocytes (n=3) (Fig 4A), lymph node (n=1) (Fig 4B) and splenocytes (n=1) (Fig 4C) from dogs which did not develop GVHD after allogenic HCT were 14.6 ± 7.7, 8.0, and 19.2 percent, respectively. The means + SD of percent ICOS+ CD3+ cells in peripheral blood (n=7) (Fig 4A), lymph node (n=5) (Fig 4B) and splenocytes (n=5) (Fig 4C) from dogs which developed chronic GVHD were 46.3 ± 6.7, 53.6 ± 12.6, and 66.7 ± 8.5 percent, respectively. The percentages of ICOS+ CD3+ cells were significantly higher in dogs with chronic GVHD than those in healthy and transplanted dogs without GVHD (Fig 4A–C).
ICOS expression on peripheral blood T-cells from postgrafting immunosuppression to the point of development of clinical sign of chronic GVHD was measured in three dogs (Fig. 4D). The mean ± SD percent CD3+ICOS+ cells obtained from dogs before the suspension of immunosuppression was 16.3 ± 1.1. After terminating CSP therapy, mean expression levels of ICOS on CD3+ peripheral blood cells increased to a mean of 24.8 ± 3.0 percent 3 to 7 days before the appearance of clinical signs of GVHD. In one of the dogs, ICOS expression levels on CD3+ cells increased significantly from 16.02 percent to 48.2 percent 3 days before clinical signs of GVHD were observed, whereas the remaining two dogs did not show significant ICOS up-regulation 6 and 7 days before clinical manifestation of chronic GVHD. The mean percent ± SD of ICOS+ cells in the dogs at the onset of GVHD was 47.2 ± 13.3. Significant differences of ICOS expression on T-cells were observed between the time of suspending CSP therapy and the onset of GVHD (Fig. 4D).
Next, we investigated whether ICOS expression is up-regulated in dogs undergoing host-versus-graft reactions. Three dogs were selected from a treatment arm consisting of 4.5 cGy TBI and a DLA-haploidentical HCT given concurrent with a vascularized composite allograft. ICOS expression on peripheral blood T-cells was assessed on days 2, 13, or 14 after marrow graft rejection. The mean ± SD percentage of ICOS+CD3+ T-cells in dogs with graft rejection was 64.2 ± 28.9, while the mean ± SD percentage of ICOS+ CD3+ cells in peripheral blood CD3+ cells in three dogs with stable mixed chimerism was 8.7 ± 0.7 (Fig. 4E).
DISCUSSION
Our results show that the anti-caICOS mAb 3F12 is specific for canine ICOS and functions as an antagonist. The anti-ICOS antibody, tested alone, produced inconsistent suppression of MLR. The addition of either CTLA4-Ig or CSP was synergistic with anti-caICOS and produced consistent suppression of MLR. The inconsistent inhibition by 3F12 alone is most likely due to superior co-stimulation through the CD28 pathway and the variable intensity of signaling by DLA mismatch in using PBMC from random pairs of dogs. Previous studies noted that combination therapy of anti-ICOS and CSP led to permanent cardiac allograft survival in MHC-mismatched mice (16). Similarly, our results suggest that effective suppression of chronic GVHD by the ICOS pathway will probably require additional immunosuppressive reagents or simultaneous blockade of other co-stimulatory pathways. One such strategy would be the combination of an antagonistic anti-CD28 and anti-caICOS mAb as has been applied in solid organ transplantation (17). A possible therapeutic alternative may be using radiolabeled conjugates such as 211At-anti-caICOS. This isotope, conjugated to an anti-CD45 mAb, has been successfully used in conditioning the dog for allogeneic HCT (18).
ICOS was expressed in an inducible pattern on activated T lymphocytes and expression was up-regulated by both TCR and CD28 signaling. These results are in accord with findings described in the murine model (19). Notably, up-regulated ICOS expression occurred in a high percentage of T-cells isolated from peripheral blood, lymph nodes and spleens of dogs with clinically confirmed chronic GVHD, but not in dogs without GVHD (the late onset of GVHD and the presence of lichenoid skin changes are consistent features of chronic GVHD (20). These findings suggest either a highly active polyclonal response or an enormous expansion of a limited number of alloreactive clones during chronic GVHD. Using PCR and spectrotyping analysis, Berrie et al. (21) showed restricted clonality of GVHD-associated T-cell clones isolated from patients with GVHD. Based on our current findings, ICOS expression on CD3+ canine lymphocytes provides a clue as to how extensive T cell expansion is in the case of GVHD.
Significant ICOS up-regulation was observed 3 days before clinical onset of chronic GVHD in one dog, whereas it was not detected 6 and 7 days before GVHD onset in two dogs, indicating that T-cell graft-vs.-host reaction occurred abruptly within a couple of days before GVHD could be clinically diagnosed by ICOS expression. Based on the few animals studied during this time frame, it is difficult to fully appreciate whether increases in ICOS expression may be used as a biomarker for alloreactive donor T-cells after allogeneic HCT. Future studies designed to better predict the onset of chronic GVHD in the dog will be used to verify this assertion.
Chronic GVHD is a major cause of morbidity and mortality in long-term survivors of allogeneic HCT (22). Immunosuppressive agents are currently the primary treatment for the disease, but decades of research have yielded disappointing results. Recently, a number of costimulatory molecules have been characterized, and antagonists and agonists directed against them tested in a variety of animal models. Mouse-specific ICOS-blocking reagents and inactivation of ICOS through gene knockout have been tested in a variety of allogeneic HCT mouse models (6–8,13). However, study results are discordant. One study showed that acute GVHD was reduced in recipients of ICOS−/− CD8+ T cells and wild type CD4+ T-cells (6), while a second study indicated that mice transplanted with ICOS−/− CD8+ T cells had enhanced GVHD morbidity and accelerated mortality (23). Taylor et al. (8) reported that both CD4+ and CD8+ cells were responsible for GVHD as either CD4 or CD8 knockout mice donors had increased survival. Studies using the non-irradiated parent-into-F1 murine model indicated that ICOS blockade inhibited chronic GVHD but exacerbated acute GVHD (12). However, later findings demonstrated that ICOS blockade also inhibited acute GVHD in irradiated transplanted mice (5,8). Given the contradictory results in murine models, we believe that further investigation of ICOS in large animal models is vital to clarify its role in alloreactivity. The canine model has been instrumental in the development and translation of numerous advances in HCT, particularly in GVHD prevention and treatment (24–26) and thus represents a logical platform to test hypotheses about ICOS and chronic GVHD.
ICOS blockade has not been studied in detail as a means of preventing allograft rejection. Only one study indicated that engraftment rates were significantly higher in ICOS−/− recipients of HCT compared to wild-type recipients, suggesting ICOS may be important in engraftment (8). Our data indicate that CD3+ T-cells up-regulate ICOS expression during hematopoietic graft rejection, suggesting that therapies directed towards ICOS may help abrogate host-vs.-graft reactions and facilitate engraftment. Future studies will also delineate whether up-regulation of ICOS can be used as a biomarker for the onset of graft rejection in canines.
In summary, we have successfully produced and characterized an anti-caICOS mAb and demonstrated that ICOS expression is up-regulated on activated T-cells in graft-vs.-host and host-vs.graft ractions. The present study supports the use of canine anti-caICOS mAb in the canine allogeneic HCT model both to enhance hematopoietic engraftment and to prevent or treat chronic GVHD.
MATERIAL AND METHODS
Experimental Animals
RBF/DnJ mice were purchased from The Jackson laboratory (Bar Harbor, ME). Beagles, mini-mongrel, and basenji crossbreeds were raised at the Fred Hutchinson Cancer Research Center, Seattle, WA, or purchased from commercial kennels. All were immunized against distemper, leptospirosis, hepatitis, papilloma virus and parvovirus. Animals were housed in facilities accredited by the American Association for Accreditation of Laboratory Animal Care, and study designs were approved by the Center’s Institutional Animal Care and Use Committee. DLA typing was done as previously described (27–30).
Production of anti-caICOS monoclonal antibodies
Methods for producing anti-ca ICOS mAb, cell culture, and protein production are described in the SDC, Supplemental Materials and Methods (online).
Functional Assays
The immunosuppressive activity of anti-ICOS mAbs was determined by MLR using previously reported methods (31). The kinetics of ICOS expression was determined using canine PBMC activated in vitro for 0, 24, 48 or 72 hours using Concanavalin A (Con A) (Sigma, St. Louis, MO) in complete dog medium containing 85% Waymouth’s, 10% heat inactivated dog serum, 1% non-essential amino acids, 1% Na Pyruvate, 1% PenStrep, 2% L-glutamine. After harvest, total RNA was extracted and reverse-transcribed using SuperScript III (Invitrogen) and Oligo dT. PCR was performed using primers based on the GenBank sequence (AY342349.1) of canis familiaris ICOS mRNA. Levels of extracellular ICOS were compared to those of the housekeeping gene GAPDH.
Ex Vivo Analysis of ICOS Expression
PBMC, splenocytes and lymph node cells were obtained from dogs on various protocols under the direction of other investigators. The common feature of these studies was that transplantation was between haploidentical canine littermates with the occurrence of GVHD. This has been a routinely productive method of producing GVHD in a variety of canine HCT studies (32–35). Cells were obtained from peripheral blood during the course of GVHD or from lymph nodes and spleen at time of necropsy. Samples were also obtained from control healthy dogs. The treatment protocols of dogs enrolled in GVHD studies were summarized in Table 1. All tissue samples were collected from dogs that showed clinical signs of GVHD at a median of 104 days after transplant which was verified histopathologically by lichenoid changes of the skin. The time of onset and lichenoid changes are clearly defined as pathognomonics for chronic GVHD in humans (20). To evaluate ICOS expression on peripheral blood T-cells in dogs with graft rejection, three dogs (H266, H451, H476) received 4.5 Gy TBI followed by infusion of DLA-haploidentical hematopoietic stem cells. After chimerism was established the dogs received vascularized composite allograft transplantation from the marrow donors. To evaluate ICOS expression in peripheral blood T-cells in dogs with stable mixed chimerism, three dogs (H118, H304, H382) underwent reduced-intensity conditioning (1–2 Gy TBI) followed by DLA-identical stem cell transplantation.
Statistical Analysis
Statistical significance was determined by a Student t test (between two groups) or ANOVA with a post-hoc test (three or more groups). P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by grants P01CA078902 and P30CA015704 from the National Institutes of Health, Bethesda MD and by awards from the Joseph Steiner Krebsstifung, Bern, Switzerland, and Lupin Foundation, Metairie, Louisiana (R.S.).
ABBREVIATIONS
- caICIOS
canine inducible costimulatory
- CTLA4-Ig
(recombinant) cytotoxic T cell associated antigen 4-immunoglobulin fusion protein
- CSP
cyclosporine A
- DLA
dog leukocyte antigen
- ELISA
enzyme linked immonoabsorbant assay
- FACS
fluorescent activated cell sorter
- MLR
mixed leukocyte reaction
- PBMC
peripheral blood mononuclear cells
- RT-PCR
real time polymerase chain reaction
Footnotes
Authorship:
M.S. performed the flow cytometry studies and co-authored the manuscript
R.S. participated in the study design and edited the manuscript.
C.L. participated in the cloning of canine ICOS, hybridoma culture and MLR.
D.S. participated in cloning of ICOS and flow cytometry
M.M. conducted canine studies and participated in manuscript review
G.E.S performed histological evaluations of dogs with GVHD
A.R. researched background on chronic GVHD and edited the manuscript
S.S.G. directed the experiments and coauthored the manuscript.
The authors have no relevant conflicts of interest to report.
References
- 1.Rothstein DM, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance (Review) [erratum appears in Immunol Rev 2004 Feb;197:243] Immunol Rev. 2003;196:85. doi: 10.1046/j.1600-065x.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhang QW, Rabant M, Schenk A, Valujskikh A. ICOS-Dependent and -independent functions of memory CD4 T cells in allograft rejection. Am J Transplant. 2008;8:497. doi: 10.1111/j.1600-6143.2007.02096.x. [DOI] [PubMed] [Google Scholar]
- 3.Pan XC, Guo L, Deng YB, et al. Further study of anti-ICOS immunotherapy for rat cardiac allograft rejection. Surgery Today. 2008;38:815. doi: 10.1007/s00595-007-3734-y. [DOI] [PubMed] [Google Scholar]
- 4.Guillonneau C, Aubry V, Renaudin K, et al. Inhibition of chronic rejection and development of tolerogenic T cells after ICOS-ICOSL and CD40-CD40L co-stimulation blockade [Republished from Transplantation. 2005 Jul 27;80(2):255–63] Transplantation. 2005;80:546. [PubMed] [Google Scholar]
- 5.Li J, Semple K, Suh WK, et al. Roles of CD28, CTLA4, and inducible costimulator in acute graft-versus-host disease in mice. Biol Blood Marrow Transplant. 2011;17:962. doi: 10.1016/j.bbmt.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujimura J, Takeda K, Kaduka Y, et al. Contribution of B7RP-1/ICOS co-stimulation to lethal acute GVHD. Pediatr Transplant. 2010;14:540. doi: 10.1111/j.1399-3046.2009.01279.x. [DOI] [PubMed] [Google Scholar]
- 7.Mollweide A, Staege MS, Hoeschen C, Hideo Y, Burdach S, Richter GH. Only therapeutic ICOS:ICOSL blockade alleviates acute graft versus host disease. Klin Padiatr. 2009;221:344. doi: 10.1055/s-0029-1239532. [DOI] [PubMed] [Google Scholar]
- 8.Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, et al. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM) Blood. 2005;105:3372. doi: 10.1182/blood-2004-10-3869. [DOI] [PubMed] [Google Scholar]
- 9.Storb R, Graham TC, Shiurba R, Thomas ED. Treatment of canine graft-versus-host disease with methotrexate and cyclophosphamide following bone marrow transplantation from histoincompatible donors. Transplantation. 1970;10:165. doi: 10.1097/00007890-197008000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Deeg HJ, Storb R, Weiden PL, et al. Cyclosporin A and methotrexate in canine marrow transplantation: engraftment, graft-versus-host disease, and induction of tolerance. Transplantation. 1982;34:30. doi: 10.1097/00007890-198207000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Yu C, Seidel K, Nash RA, et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood. 1998;91:2581. [PubMed] [Google Scholar]
- 12.Ogawa S, Nagamatsu G, Watanabe M, et al. Opposing effects of anti-activation-inducible lymphocyte-immunomodulatory molecule/inducible costimulator antibody on the development of acute versus chronic graft-versus-host disease. J Immunol. 2001;167:5741. doi: 10.4049/jimmunol.167.10.5741. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe S, Ogawa S, Hara Y, Tanabe K, Toma H, Abe R. Expression level of costimulatory receptor ICOS is critical for determining the polarization of helper T cell function. Transpl Immunol. 2006;15:255. doi: 10.1016/j.trim.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Lee J-H, Joo Y-D, Yim D, et al. Molecular cloning and characterization of canine ICOS. Genomics. 2004;84:730. doi: 10.1016/j.ygeno.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Graves SS, Stone DM, Loretz C, et al. Antagonistic and agonistic anti-canine CD28 monoclonal antibodies: tools for allogeneic transplantation. Transplantation. 2011;91:833. doi: 10.1097/TP.0b013e31820f07ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nanji SA, Hancock WW, Anderson CC, Zhu LF, Kneteman NM, Shapiro AM. Combination therapy with anti-ICOS and cyclosporine enhances cardiac but not islet allograft survival. Transplant Proc. 2003;35:2477. doi: 10.1016/j.transproceed.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 17.Hara Y, Kitazawa Y, Funeshima N, et al. Anergic lymphocytes generated by blocking CD28 and ICOS pathways in vitro prolong rat cardiac graft survival. International Immunopharmacology. 2006;6:1143. doi: 10.1016/j.intimp.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Kornblit B, Hamlin DK, et al. Durable donor engraftment after radioimmunotherapy using a-emitter astatine-211-labeled anti-CD45 antibody for conditioning in allogeneic hematopoietic cell transplantation. Blood. 2012;119:1130. doi: 10.1182/blood-2011-09-380436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAdam AJ, Chang TT, Lumelsky AE, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 20.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Berrie JL, Kmieciak M, Sabo RT, et al. Distinct oligoclonal T cells are associated with graft versus host disease after stem-cell transplantation. Transplantation. 2012;93:949. doi: 10.1097/TP.0b013e3182497561. [DOI] [PubMed] [Google Scholar]
- 22.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease (Review) Lancet. 2009;373:1550. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu XZ, Liang Y, Nurieva RI, Guo F, Anasetti C, Dong C. Opposing effects of ICOS on graft-versus-host disease mediated by CD4 and CD8 T cells. J Immunol. 2006;176:7394. doi: 10.4049/jimmunol.176.12.7394. [DOI] [PubMed] [Google Scholar]
- 24.Storb R, Weiden PL, Schroeder M-L, Graham TC, Lerner KG, Thomas ED. Marrow grafts between canine littermates homozygous or heterozygous for lymphocyte-defined histocompatibility antigens. Transplantation. 1976;21:299. doi: 10.1097/00007890-197604000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Storb R, Yu C, Zaucha JM, et al. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant. Blood. 1999;94:2523. [PubMed] [Google Scholar]
- 26.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048. [PubMed] [Google Scholar]
- 27.Wagner JL, Burnett RC, Storb R. Molecular analysis of the DLA DR region. Tissue Antigens. 1996;48:549. doi: 10.1111/j.1399-0039.1996.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 28.Wagner JL, Burnett RC, Works JD, Storb R. Molecular analysis of DLA-DRBB1 polymorphism. Tissue Antigens. 1996;48:554. doi: 10.1111/j.1399-0039.1996.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 29.Burnett RC, Francisco LV, DeRose SA, Storb R, Ostrander EA. Identification and characterization of a highly polymorphic microsatellite marker within the canine MHC class I region. Mamm Genome. 1995;6:684. doi: 10.1007/BF00352386. [DOI] [PubMed] [Google Scholar]
- 30.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee WS, Suzuki Y, Graves SS, et al. Canine bone marrow-derived mesenchymal stromal cells suppress alloreactive lymphocyte proliferation in vitro but fail to enhance engraftment in canine bone marrow transplantation. Biol Blood Marrow Transplant. 2011;17:465. doi: 10.1016/j.bbmt.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deeg HJ, Storb R, Shulman HM, Weiden PL, Graham TC, Thomas ED. Engraftment of DLA-nonidentical unrelated canine marrow after high-dose fractionated total body irradiation. Transplantation. 1982;33:443. doi: 10.1097/00007890-198204000-00021. [DOI] [PubMed] [Google Scholar]
- 33.Georges GE, Storb R, Bruno B, et al. Engraftment of DLA-haploidentical marrow with ex vivo expanded, retrovirally transduced cytotoxic T lymphocytes. Blood. 2001;98:3447. doi: 10.1182/blood.v98.12.3447. [DOI] [PubMed] [Google Scholar]
- 34.Ding Y, Rotta M, Graves SS, et al. Delaying DLA-haploidentical hematopoietic cell transplantation after total body irradiation. Biol Blood Marrow Transplant. 2009;15:1244. doi: 10.1016/j.bbmt.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mielcarek M, Storb R, Georges GE, et al. Mesenchymal stromal cells fail to prevent acute graft-versus-host disease and graft rejection after dog leukocyte antigen-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2011;17:214. doi: 10.1016/j.bbmt.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.