Abstract
Hyphae of filamentous fungi maintain generally linear growth over long distances. In C. albicans, hyphae are able to reorient their growth in the direction of certain environmental cues. In previous work, the C. albicans bud-site selection proteins Rsr1 and Bud2 were identified as important for hyphae to maintain linear growth and were necessary for hyphal responses to directional cues in the environment (tropisms). To ask if hyphal directional responses are general functions of all yeast bud-site selection proteins, we studied the role of Rax2, ortholog of the S. cerevisiae bud-site selection protein Rax2, in C. albicans hyphal morphogenesis. Rax2-YFP localized to the hyphal cell surface in puncta and at the hyphal tip in a crescent. Strains lacking Rax2 had hyphal morphologies that did not differ from control strains. In non-cued growth conditions, rax2 mutant strains had defects in both yeast (bud) and hyphal (branch) site selection and mutant hyphae exhibited non-linear growth trajectories as compared to control hyphae. In contrast, when encountering a directional environmental cue, hyphae lacking Rax2 retained the ability to reorient growth in response to both topographical (thigmotropism) and electric-field (galvanotropism) stimuli but exhibited a reduced ability to establish hyphal growth in the direction of a cathodal stimulus. In conclusion, these results indicate that C. albicans Rax2 is important for establishing sites of emergence of yeast and hyphal daughters and for maintaining the linearity of hyphal growth. In contrast to Rsr1 and Bud2, Rax2 is not involved in responses that require a reorientation of the direction of already established hyphal growth (tropisms). Thus, it appears that some hyphal directionality responses are separable in that they are mediated by a different set of polarity proteins.
Keywords: Candida albicans, Fungal morphogenesis, Polarity establishment, Hyphal tropisms
1. INTRODUCTION
The hyphae of filamentous fungi maintain generally linear growth, sometimes over very long distances. In the opportunistic fungal pathogen Candida albicans, hyphae are able to reorient from a stable linear growth trajectory to a new direction in response to certain environmental cues. For example, hyphae reorient growth along topographical cues (thigmotropism) and align cathodally in an electric field (galvanotropism) (Gow, 1994; Gow et al., 1994; Sherwood et al., 1992). In addition, establishment of hyphal growth sites on mother yeast cells can be directed by cationic cues (Crombie et al., 1990). The directional growth responses of hyphae have been proposed to facilitate the invasion of host tissues during infection (Brand et al., 2008), although direct evidence for this remains to be shown. An understanding of the molecules involved in establishing and maintaining directional growth responses in C. albicans is important to gain because it could inform the development of therapies that prevent invasive disease in immunocompromised patients.
How directional growth is established has been extensively studied in the model yeast Saccharomyces cerevisiae. During yeast growth, bud sites are selected in one of two directions, based on the ploidy of the cell (Casamayor and Snyder, 2002). In haploid cells, the axial pattern predominates, where buds form adjacent to previous cell division sites. In diploid cells, buds form either adjacent to, or directly opposite of, the previous growth site, the so-called bipolar pattern of bud growth. C. albicans, a diploid fungus, exhibits both yeast-form and filamentous hyphal-form growth. During C. albicans yeast growth, both axial and bipolar budding patterns are exhibited. In contrast to S. cerevisiae, the predominance of one pattern over the other is dictated by the growth temperature; more axially budding cells are observed in cultures grown at lower temperatures (≤28°C) and an approximately equal mixture of axial and bipolar patterns are established as temperatures increase towards 37°C (Gale et al., 2001). In both S. cerevisiae and C. albicans yeast cells, axial and bipolar patterning is maintained by overlapping, yet distinct, sets of internal landmark proteins, the so-called bud-site selection proteins, which localize transiently at cell division sites and direct the polarity establishment machinery during the next cell cycle (Casamayor and Snyder, 2002; Gale et al., 2001; Hausauer et al., 2005).
In contrast to yeast bud growth where internal landmarks dictate the site of growth, C. albicans hyphal growth responds to external cues that override the internal landmarks to establish and reorient growth in the direction of the cue. To date, only two requirements for hyphal directional responses have been described. The first involves a calcium-regulated mechanism where calcium influx, via calcium channels, is required for both the establishment of hyphal growth in the direction of a cathodal stimulus and for the reorientation of growth during thigmotropic responses (Brand et al., 2007). The second involves a Ras-like GTPase, Rsr1, which is essential for all C. albicans hyphal tropism responses (Brand et al., 2008). Rsr1, a bud-site selection protein, is required for both axial and bipolar budding patterns in S. cerevisiae and C. albicans; yeast cells lacking Rsr1 initiate buds at random sites (Bender and Pringle, 1989; Hausauer et al., 2005). In addition, Rsr1 is important for hyphal growth maintenance by regulating the localization and distribution of Cdc42 Rho-GTPase activity at hyphal tips (Pulver et al., 2012).
To understand how other yeast bud-site selection proteins are involved in hyphal morphogenesis and directionality responses, we analyzed the role of Rax2, ortholog of the S. cerevisiae bud-site selection protein Rax2, during C. albicans hyphal morphogenesis. In S. cerevisiae, Rax2 is involved in maintenance of the bipolar budding pattern and is localized near incipient bud sites on the mother cell cortex, at the site of previous bud growth (Chen et al., 2000). Herein, we show that C. albicans Rax2, like the similar protein in S. cerevisiae, functions as an internal landmark for bud-site selection during yeast growth. In addition, we show that Rax2 is involved in the establishment of hyphal growth in the direction of an external cue. However, in contrast to the Rsr1 landmark, Rax2 does not appear to be important for re-orientation or tissue invasion responses of hyphae. Overall, our results support the idea that not all yeast bud-site selection proteins have a role in directional growth responses during hyphal morphogenesis.
2. MATERIALS AND METHODS
2.1. Strains and growth conditions
C. albicans strains used in this study are listed in Table 1. Strains were recovered from freezer stocks by culturing into synthetic dextrose complete (SDC) (Sherman, 1991) medium and incubated overnight at 30°C. For regulatable expression using the MET3 promoter, strains were grown either in SDC medium containing methionine (1500 μg/mL) and cysteine (1500 μg/mL) for repression of expression or in SDC lacking methionine and cysteine for induction of expression. For morphometric analyses of hyphae, following overnight incubation, 40 μl of yeast cells were subcultured into 10 ml of fresh media containing 10% serum, and grown on Poly-L-Lysine coated microscope slides at 37°C for 4h prior to imaging. For epithelial cell interaction assays, overnight yeast cultures were subcultured into fresh media at a dilution of 1:100 and grown at 30°C for 4h prior to inoculation into epithelial cell culture media and infection of epithelial cells.
Table 1.
C. albicans strains used in this study.
| Strain | Relevant Genotype | Source |
|---|---|---|
| BWP17 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | Wilson et al., 1999 |
| JB6284 | BWP17 HIS1::his1::hisG/his1::hisG ARG4-URA3::arg4::hisG/arg4::hisG | Bensen et al., 2002 |
| CA10476 | BWP17 rax2::HIS1/RAX2 | This study |
| CA10478 | BWP17 rax2::HIS1/rax2::ARG4 | This study |
| CA10500 | BWP17 rax2::HIS1/rax2::ARG4 arg4::hisG/ARG4-URA3::arg4::hisG | This study |
| CA12041 | CA10476 rax2::HIS1/URA3-PMET3::RAX2 | This study |
| CA10031 | BWP17 RAX2/RAX2::YFP-URA3 | This study |
| CA11891 | BWP17 rax2::HIS1/URA3-PMET3-YFP::RAX2 | This study |
| CA12531 | BWP17 RAX/RAX2::YFP-URA3 PIL1/PIL1::CFP-HIS1 | This study |
2.2. Strain constructions
Disruption of RAX2
The RAX2 open reading frames were sequentially deleted from C. albicans strain BWP17 (strains CA10476 and CA10478) by PCR-mediated gene disruption (Wilson et al., 1999). Deletion cassettes were amplified from plasmids pGEM-HIS1 and pGEM-ARG4 using primers 2564F (5’-tcctatatcttcaacttttaccaatcagtcattcgtttatattcctatatttctaatttacaattttacagTTTTCCCAGTCACGACGTT-3’ lowercase text refers to gene-specific sequence and uppercase text refers to plasmid-specific sequence throughout) and 2565R (5’-gcaaaaaccaatcttataataaacactaatcttctgtccaattttccaaaacaattttctctctatttgaTGTGGAATTGTGAGCGGATA-3’) and transformed into yeast. Transformants were selected on media lacking arginine and/or histidine as indicated and analyzed by PCR, using primers targeting regions outside of the area of integration, to confirm the deletions of RAX2. The rax2/rax2 strain was made prototrophic for URA3 by transformation with pRS-ARG-URA-BN (Davis et al., 2000) linearized with NotI to target integration to the ARG4 locus and generate strain CA10500.
Regulated expression of RAX2
The native RAX2 promoter was replaced with the regulatable MET3 promoter as previously described (Gerami-Nejad et al., 2004). A RAX2 locus specific URA3-PMET3 cassette was amplified from pURA3-PMET3 using primers 3889F (5’-attttttacattttttacatttttcaatttccttcatctataatatattttaatttattttattgttacaTCTAGAAGGACCACCTTTGATTG-3’) and 3890R (5’-cgttatcatctgcttgacatagtttaataattgatattagaagtaataataactgttgaaactgtactaaCATTTTAATAAACGCGGATCC-3’) or 3891R (5’-cgttatcatctgcttgacatagtttaataattgatattagaagtaataataactgttgaaactgtactaaTTTGTACAATTCATCCATAC-3’) and were transformed into rax2/RAX2 heterozygous strain CA10476 to generate strains allowing regulated expression of RAX2 or a RAX2-YFP fusion, respectively. Transformants were selected on media lacking uridine and analyzed by PCR using primers targeting regions outside of the area of integration to confirm insertion at the RAX2 locus. RAX2 expression/repression was evaluated in strain CA10476 by real-time PCR (data not shown) as follows. RNA was isolated from log phase yeast cells, grown as indicated in the legend to Figure 2, using the MasterPure RNA purification kit (EpiCentre Biotechnologies, Madison, WI). RNA quantities were normalized to 1μg (per 20 μl reaction) for subsequent cDNA synthesis using the High Capacity RNA to cDNA Kit (Applied Biosystems, Foster City, CA). 1μl of each cDNA synthesizing reaction was used in a PCR amplification reaction that targeted a sequence within the RAX2 open reading frame, using primers 4593F (5’-CACAATTTGATGAACAGTTGGTG-3’) and 4594R (5’-TCATAAACACATAATGAAGGAC-3’) to generate a product of 1258 bp.
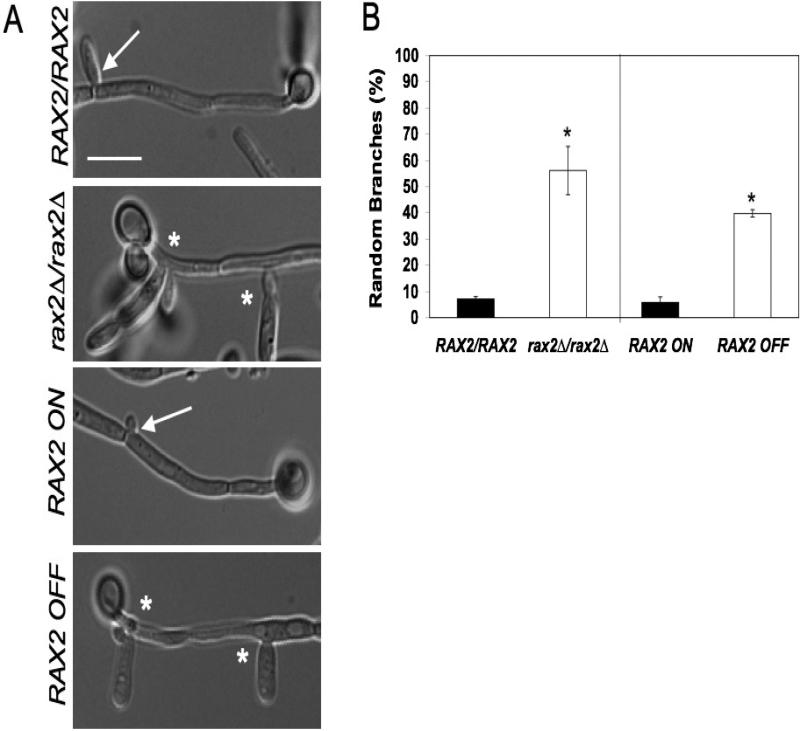
Fig. 2. Rax2 is needed for positioning daughter cell branch sites during hyphal morphogenesis.
(A) Representative DIC microscopy images of hyphal branching in WT (RAX2/RAX2, BWP17), rax2Δ/rax2Δ (CA10500), and RAX2-regulatable expression (CA12041) strains. Scale bar, 10 μm. Asterisks indicate abnormal branch sites (non-adjacent to septa), while arrows show wild-type branch emergence site patterns. (B) The site of branch emergence was quantified in C. albicans strains described in (A) above. Data are presented as percentages of cells ± SEM with random branching patterns after 4h of growth on poly-L-lysine-coated slides at 37°C in SDC+10% serum. For regulatable expression of RAX2, hyphae were induced in SDC+10% serum either lacking (RAX2 ON) or containing (RAX2 OFF) Met and Cys). *, p ≤ 0.05 by Student's t-test with respect to the Rax2 expressing parent strain. Each assay was performed in triplicate, n~50 cells per strain for each assay.
Fluorescent protein fusions
YFP and cyan fluorescent protein sequences (CFP) were fused to the 3’-end of genes using PCR-mediated gene modification as previously described(Gerami-Nejad et al., 2001). A RAX2-specific YFP cassette was amplified using primers 2468F (5’-gaagatgaaatgatggatgcagttaacccagaagatttactacatgaaattgatttacaaagagagaaaGGTGGTGGTTCTAAAGGTGAAGAATTATT-3’) and 2469R (5’-taaaacatagcaaaaaccaatcttataataaacactaatcttctgtccaattttccaaaacaattttctTCTAGAAGGACCACCTTTGATTG-3’) and template plasmid pYFP-URA3. A PIL1-specific CFP cassette was amplified using primers 5102F (5’-cgaacacggtgaaattgatgatgctgctcaagaagaattcaacaaacacgatgaaaatgttgaacaccaaGGTGGTGGTTCTAAAGGTGAAGAATTATT-3’) and 5103R (5’-taaaagtactagcaaaaaccaattggtcaaaaattttttttaaaaaaacaatattattcaattaattgaaACTAGTATTGTAGTACAAGGTATC-3’) and template plasmid pCFP-HIS1. Transformants were selected on media lacking uridine or histidine as indicated and analyzed by PCR, using primers targeting regions outside of the area of integration, to confirm insertion at the appropriate gene locus.
2.3. Analysis of Rax2 expression by Western Blot
Proteins were isolated from 10 ml of fungal cells (grown as indicated in the legend to Figure 2) as described previously (Pulver et al., 2012), separated on a 15% SDS polyacrylamide gel, transferred to a PVDF blotting membrane, and subsequently incubated with mouse anti-GFP (Roche, Indianapolis, IN) followed by horseradish peroxidase-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies.
2.4. Cell morphometric and cell biological analyses
Budding pattern
To determine budding pattern, bud scar patterns were quantified as previously described (Hausauer et al., 2005). Yeast cells were grown in SDC at 28°C overnight, subcultured at a 1:10 dilution into fresh media, grown for 4 h at 28°C, and then stained with calcofluor white (Sigma, St. Louis, MO) to visualize chitin within bud scars. Bud scar patterns were determined for ~100 cells per strain. To determine the site of the first daughter cell, yeast cells were grown as described above with the exception of a 2 h subculture. Cells were counted if only a birth scar and a single bud scar were present and ~30 cells of each strain on each of three separate days were quantified. Statistical analysis was performed with Student's t-test and p values ≤ 0.05 were considered significant.
Hyphal branching pattern
Branch positions were determined from 50-100 cells of each strain on three separate days as previously described (Hausauer et al., 2005). Statistical analysis was performed utilizing Student's t-test and p values ≤ 0.05 were considered significant.
Hyphal Angles
Perturbations in linear hyphal growth were determined using Region Measurements in the Angle Measure feature of MetaMorph as described previously (Hausauer et al., 2005). 50 - 100 cells per strain were measured on each of three days and the results were analyzed statistically using a blocked analysis of variance (ANOVA) design (block on day) to control for day-to-day variations in growth conditions. p values of ≤ 0.05 were considered significant.
Morphological Index (MI)
MIs were determined as previously described (Merson-Davies and Odds, 1989). Measurements for cell width, length, and constriction/septation were obtained using MetaMorph imaging software (Molecular Devices LLC, Sunnyvale, CA). These data were used to calculate the MI of each cell using the equation 2 + 1.78 log10 (LS/D2), where L refers to cell length, S refers to constriction width, and D refers to cell width.
2.5. Microscopy
Differential interference contrast (DIC) and fluorescence microscopy were performed using a Nikon Eclipse E600 photomicroscope equipped with a 100W mercury lamp and epifluorescence illumination with standard YFP and CFP filter sets (both from Chroma Technology Corp., Bratteboro, VT) and standard UV filter sets (from Nikon). Digital images were collected using a photometrics CoolSNAP HQ camera (Tucson, AZ) and analyzed using Metamorph Software Series 6.3r7 (Universal Imaging Corporation, Downingtown, PA).
2.6 Hyphal tropism assays
Galvanotropism
Cells were cultured overnight at 30°C in yeast-peptone-dextrose (YPD) medium (Sherman, 1991). A volume of 7.5 μl was inoculated into 10 ml ddH2O and yeast cells allowed to adhere to poly-L-lysine-coated microscope slides. Hyphae were induced by incubation in YPD, pH 7.5 in a Biorad midi-sub cell electrophoresis tank at a temperature of 37°C ± 1°C (Brand et al., 2007). Emergence angle was determined after growth for 2 h at 10 V/cm and a current of 36 +/- 2 mA. Final angle was determined after incubation for 2 h with no electric field, followed by 3 h in an applied electric field, as above. Hyphal orientation at the site of germ tube emergence and at the germ tube tip relative to the cathode was measured using Improvision Openlab 2.0 software. The percentage cathodal orientation (%P) was calculated using %P = Σ (- sin θ / n) × 100, for n measurements, where n = > 100 per sample. The mean polarisation +/- SD was determined from 3 independent experiments.
Thigmotropism
Assays were carried as previously described (Brand et al., 2007). Briefly, cells were adhered to poly-L-lysine coated quartz slides featuring ridges of 0.79 μm +/- 40 nm and a pitch of 25 μm. Slides were incubated in 20 ml pre-warmed 20 % (v/v) newborn calf serum, 2 % (w/v) glucose at 37 °C for 6 h to induce hyphae. The number of hyphae re-orienting on contact with a ridge was expressed as a percentage of the total observed interactions. A minimum of 100 interactions was observed per sample and results reported as the mean value from 3 independent experiments +/- SD.
2.7. Epithelial cell penetration assay
H4 intestinal epithelial cell monolayers (Sanderson et al., 1996) were prepared, inoculated with Candida yeast cells (1 × 105) for 3h and then analyzed for fungal penetration by immunocytochemistry as previously described (Falgier et al., 2011). Candida cells were distinguished as either penetrating (unstained) or non-penetrating (stained) with respect to the epithelial cell layer. A total of ~15 fluorescent images along the z axis, in ~1 μm increments, were collected for each microscopic field to insure that any fluorescent signal within the fungal cell was captured. Data are presented as averages of at least three independent experiments (n ≥ 100 cells for each) ± SEM. Statistical analyses were performed using SPSS 17.0 for Windows (SPSS, Chicago, IL). A paired Student's t-test design was used to account for epithelial passage number variation among experiments. p values ≤ 0.05 were considered to be significant.
2.8. Epithelial cell cytotoxicity assay
H4 intestinal epithelial cell damage was measured using the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI) as previously described (Falgier et al., 2011). Results were reported as averages ±SEM and were analyzed statistically using SPSS 17.0 for Windows (IBM, Amronk, NY) with a paired Student's t-test design to account for epithelial passage number variation among experiments. p values ≤ 0.05 were considered to be significant.
3. RESULTS
3.1. RAX2 is needed for positioning daughter cell emergence sites during yeast and hyphal morphogenesis
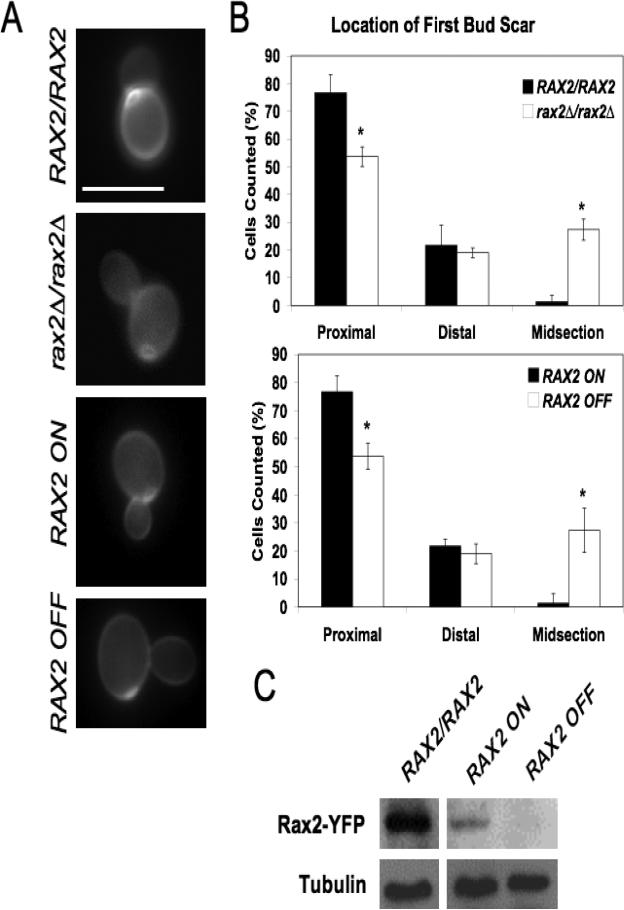
CaRAX2 was identified based on its similarity to ScRAX2, which encodes S. cerevisiae Rax2, a protein important for budding pattern during yeast morphogenesis. Similar to ScRax2, the CaRax2 sequence includes a predicted transmembrane domain at the carboxy-terminus and signal peptide at the amino-terminus (data not shown), consistent with the prediction that it is a membrane-spanning protein. CaRax2, like ScRax2, is involved in yeast bud-site selection. C. albicans yeast cells that lack Rax2 bud in a random pattern as compared to the wild-type parent strain for both first buds (Fig. 1A, B) and subsequent budding events (data not shown). Similarly, Rax2 repression in control strains containing the ability to regulate Rax2 expression (Fig. 1C) was associated with random selection of the first and subsequent bud sites (Fig. 1A, B). These data illustrate a role for CaRax2 in both the establishment and maintenance of bud-site selection patterns during yeast form growth.
Fig. 1. Rax2 is important for bud-site selection during C. albicans yeast-form growth.
(A) Representative fluorescence images of site of first budding event in wild-type (WT; BWP17), rax2Δ/rax2Δ (CA10500), and control strains allowing regulatable expression of RAX2 from the MET3 promoter (CA12041) stained with calcofluor white. Scale bar, 10 μm. (B) Quantification of position of first bud scars positions (after 2h of growth at 28°C in SDC) in the strains depicted in part (A). Data are presented as percentages of total cells counted ± SEM. (C) Western blot of whole protein lysates, reacted with anti-GFP antibody, from a WT strain expressing Rax2-YFP (CA10031) and a control strain allowing regulatable expression of Rax2-YFP from the MET3 promoter (CA11891). Tubulin levels for each condition are included as an indication of total protein load.
WT C. albicans hyphal cells branch at sites adjacent to septation sites, on the side proximal to the mother cell. In contrast, in C. albicans hyphae lacking Rax2, branches emanate from random sites along the hyphal compartment (Fig. 2). In addition, it has been proposed that positioning of secondary hyphal emergence sites is regulated by yeast bud-site selection proteins (Li et al., 2012). In WT strains, secondary hyphae predominantly emerge at sites distal (bipolar) to the initial hyphal site. In contrast, when we analyzed hyphae lacking Rax2, secondary hyphae had a reduced frequency of distal patterning (45% for strain CA10500 vs. 76% for strain BWP17, p < 0.05). Results using regulatable Rax2 expression showed a similar relationship; Rax2 repression was associated with randomly positioned branch sites (Fig. 2) and reduced bipolar positioning of secondary hyphae as compared to the Rax2-expressing strain (53% for strain 12041-OFF vs. 73% for strain 12041-ON, p < 0.01). Altogether, the results indicate that Rax2 functions in positioning daughter cell sites during both yeast and hyphal morphogenesis.
3.2. RAX2 is important for maintaining linear hyphal growth but not overall hyphal shape
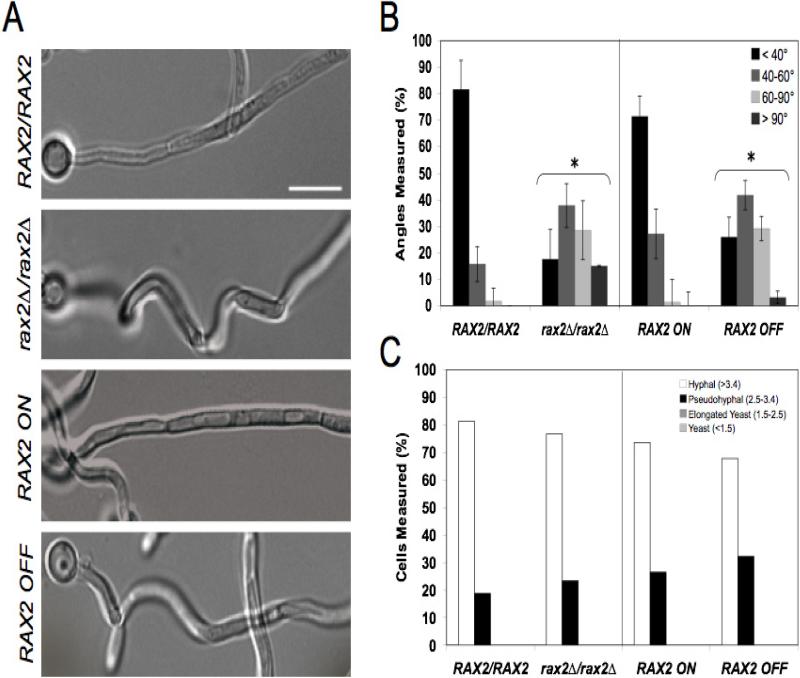
Typically, wild-type hyphae grow in a linear manner over long distances. In strains lacking Rax2, we qualitatively observed that hyphae were curvier (Fig. 3A). To quantitatively compare wild-type and mutant strains, we measured hyphal angles of the strains during hyphal growth on serum-containing agar. The vast majority (~80%) of wild-type hyphae did not veer more than 40° from a linear trajectory (Fig. 3B). In strains lacking Rax2, ~80% of hyphae deviated more than 40°, with half of these exhibiting more than 60° of deviation (Fig. 3B). The same significant trend was observed for the control strain in which Rax2 expression was regulated (Fig. 3B). Thus, Rax2 is needed to maintain the linear directionality of growth during hyphal morphogenesis.
Fig. 3. RAX2 is important for maintaining linear hyphal growth, but not for overall hyphal morphology.
(A) Representative DIC microscopy images of cell growth trajectories in strains listed and grown as described in Fig. 2A. In the absence of RAX2 hyphae are visibly curvier. Scale bar, 10 μm. (B) Quantification of hyphal angles. The first angle of each hypha was measured using the MetaMorph® Angle Measurements tool and the mean angle ± SEM for each strain/condition is shown. n~30 hyphae for each strain and each assay was performed in triplicate. *, comparisons between strains lacking Rax2 and their isogenic Rax2-expressing parent strains were statistically different at the p<0.001 level. (C) Morphology Index (MI) values of WT the strains depicted in A. The classic morphology definitions with respect to morphology index (Merson-Davies and Odds, 1989) are indicated on the graph. n~30 hyphae for each strain.
To evaluate the role of Rax2 in cell shape and its impact on the hyphal morphogenesis program, we compared the Morphology Index (MI) of strains after growth in hyphal induction media. Strains lacking Rax2 had similar MI's to the Rax2-expressing control strains, with the majority of the cells (> 70%) in all strains having “hyphal” MIs (> 3.4) (Fig. 3C). The remaining minority of cells, for all strains, scored in the pseudohyphal MI category. Altogether, the results are consistent with the idea that Rax2 is important for hyphal linearity but not for cell shape or the ability to undergo true hyphal morphogenesis.
3.3. Rax2 localizes to the hyphal cell surface with enrichment at the hyphal tip
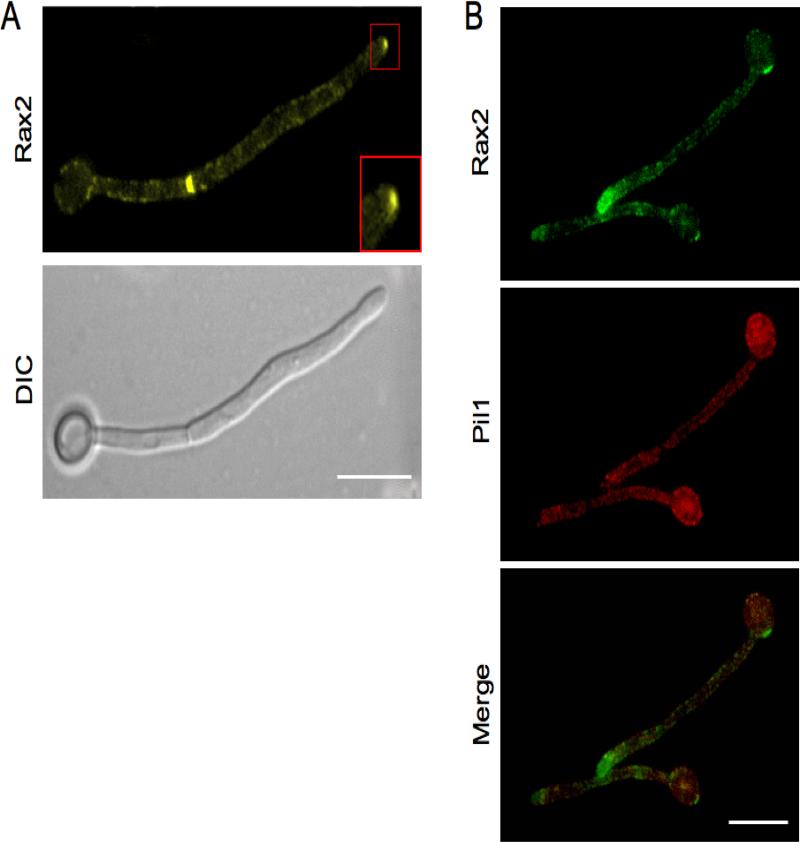
To understand the relationship between the physical localization of Rax2 and its function in C. albicans morphogenesis, we analyzed the localization of Rax2 fused to YFP. In yeast, Rax2-YFP localized at mother-daughter bud necks and to bud scars (data not shown), similar to the orthologous protein in S. cerevisiae (Kang et al., 2004). In hyphae, Rax2-YFP localized in a punctate pattern along the cell surface (both mother cells and hyphal daughters) and at septation sites (Fig. 4A). In addition, Rax2-YFP was enriched at the hyphal tip (Fig. 4A, inset), localizing in a crescent-shape that was reminiscent of the localization of polarisome proteins such as Spa2 and Bud6 (Crampin et al., 2005). The punctate pattern of Rax2-YFP raised the possibility that it was localized within eisosomes, which are specialized, static membrane structures thought to have a role in plasma membrane organization, cell wall synthesis and endocytosis in C. albicans (Alvarez et al., 2008; Alvarez and Konopka, 2007). C. albicans mutant strains lacking eisosome-associated proteins have abnormal hyphal morphologies (Alvarez et al., 2008). To evaluate this, we analyzed the extent to which Rax2-YFP co-localized with a CFP tagged version of the eisosome-associated protein, Pil1 (Reijnst et al., 2011). Rax2-YFP did not significantly co-localize with Pil1-CFP (Fig. 4B). Therefore, the curved shapes of rax2 null hyphae are not likely to be due to a reduced function of eisosomes. The localization of Rax2 at hyphal tips, however, poises it for a function in hyphal growth directionality.
Fig. 4. Rax2 localizes to the cell surface with enrichment at the hyphal tip and does not colocalize with eisosomes.
(A) Representative fluorescence (top) and DIC (bottom) photomicrographs of a C. albicans hypha expressing Rax2-YFP (CA10031) after growth for 3h in SDC + 10% serum at 37°C. Fluorescent image was taken with a 3000 msec exposure. Scale bar, 10 μm. Inset, localization of Rax2-YFP at hyphal tips. (B) Colocalization analysis of Rax2-YFP (top) and the eisosomal protein Pil1 tagged with CFP (middle) in hyphae expressing the two proteins simultaneously (CA12531). Merged image, bottom panel.
3.4. Rax2 is needed for the establishment of growth direction, but not for the repositioning of growth, in response to environmental cues
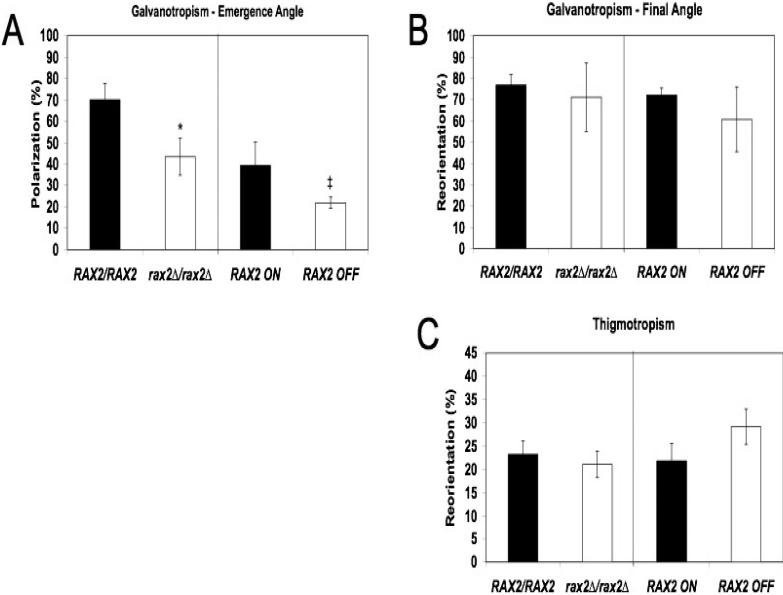
Because Rax2 is important for positioning daughter cell emergence sites and in maintaining hyphal linearity during growth in a non-cued environment, we asked if Rax2 also has a role in positioning growth direction in response to environmental cues (hyphal tropisms). We analyzed three tropism responses: emergence angle galvanotropism, the ability to establish hyphal growth in a direction dictated by an electrical field, final angle galvanotropism, the ability to re-orient hyphal growth according to electric field cues, and thigmotropism, the ability to re-orient hyphal growth in response to a topographical stimulus. Strains lacking Rax2 had reduced ability to establish growth in the direction of the cathode (emergence angle galvanotropism, Fig. 5A). Interestingly, strains lacking Rax2 retained intact galvanotropic and thigmotropic reorientation responses (Fig. 5B,C). Altogether, the tropism response results are consistent with the idea that Rax2 is important for establishing sites of growth in response to environmental cues but not in reorienting already established hyphal growth directions. These results contrast with those seen previously for strains lacking Rsr1, which was required for all three tropism responses. The differing tropism phenotypes of rax2 compared to rsr1 null cells suggest that there are distinguishing molecular requirements among these directionality responses in C. albicans.
Fig. 5. Rax2 is needed for cathodal polarization, but not for the reorientation of hyphal growth in response to environmental cues.
Yeast strains (listed in the legend to Fig. 2A) were adhered to poly-L-lysine-coated slides and hyphae were induced as described in Materials and Methods. (A) Hyphal emergence angles relative to a cathodal stimulus (emergence angle galvanotropism) are shown and reported as means ± SD (n ≥ 100 cells for each strain, each assay performed in triplicate) where +100% cathodal polarization indicates perfect cathodal orientation, -100% indicates anodal orientation, and 0% is obtained for a randomly oriented population. *p<0.05 and ‡p=0.055, based on comparisons with the parent strains. (B) Final hyphal orientation angles relative to a cathodal stimulus applied after hyphal elongation was already established (final angle galvanotropism) are shown with values as described in (A) above. (n ≥ 100 cells for each strain, each assay performed in triplicate). No significant differences were observed between mutant and parent strains. (C) The number of hyphal contacts with ridges that resulted in reorientation of growth direction (thigmotropism reorientation response) is expressed as a percentage of total hypha-ridge contacts ±SD (n ≥ 100 contact events for each strain per experiment, each experiment performed in triplicate). No significant differences were observed between mutant and parent strains. For A-C, statistical analyses were by ANOVA followed by post-hoc Dunnett's t-test.
3.5. Rax2 is not required for penetration and damage of immature intestinal epithelial cells by C. albicans hyphae
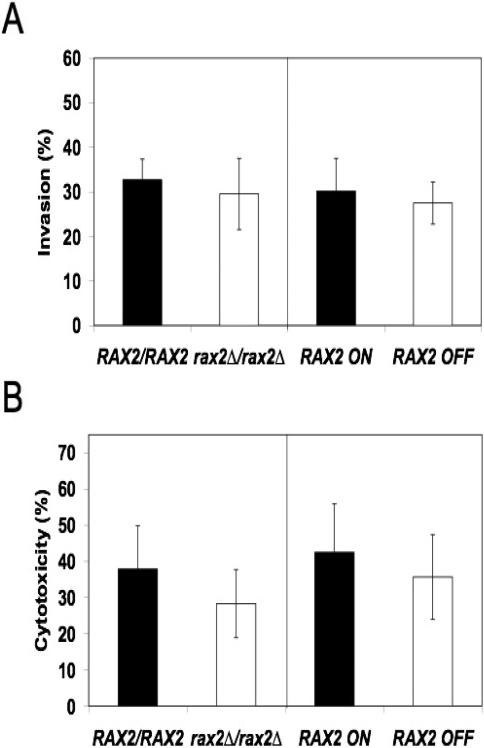
Tropism responses of C. albicans hyphae have been hypothesized to contribute to the pathogenesis of invasive candidiasis by guiding hyphal growth into “infectable” sites on host tissue surfaces (Brand et al., 2008; Davies et al., 1999; Gow, 1997; Jayatilake et al., 2005; Odds, 1994; Reichart et al., 1995; Sherwood et al., 1992). In C. albicans, Rsr1, a bud-site selection protein that mediates tropism responses of hyphae, is important for penetration and injury of human epithelial cells, although it remains unclear if the tropism defects are directly linked to decreased tissue invasiveness (Brand et al., 2008). To ask if Rax2 is involved in invasive interactions between C. albicans hyphae and human epithelial cells, we first analyzed rax2 mutant strains for their ability to penetrate H4 intestinal epithelial cells. H4 cells are an immature human intestinal cell line that models the gut epithelia of premature infants, the primary site for invasion of Candida species that leads to disseminated candidiasis in this patient population (Falgier et al., 2011; Saiman et al., 2000). Strains lacking Rax2 had no significant defects in their ability to penetrate H4 cells (Fig. 6A) as compared to isogenic control strains. We then evaluated rax2 mutant strains for their ability to damage H4 cells and found that they did not differ from control strains (Fig. 6B). C. albicans penetration of host tissue is thought to differ depending on the specific host cell type (Dalle et al., 2010). Thus, we also analyzed the ability of the rax2 mutant strains to penetrate oral epithelial (TERT2) cells. Similar to the results with H4 cells, strains lacking Rax2 had no reduction in their ability to invade TERT2 cells as compared to the wild-type control strain (data not shown). Thus, in contrast to Rsr1, Rax2 does not appear to be important for penetration or injury of two different human epithelial cell types.
Fig. 6. Rax2 is not required for penetration (A) and damage (B) of immature intestinal epithelial cells by C. albicans hyphae (strains as listed in the legend to Fig. 1A).
(A) Penetration of intestinal epithelial cells (invasion %, mean ± SEM) by C. albicans strains after 3h of incubation. n≈100 cells for each strain per experiment, each experiment performed in triplicate. No significant differences were observed between mutant and parent strains. (B) Intestinal epithelial cell damage (cytotoxicity %, mean ± SEM) after incubation with C. albicans strains. n=3 independent experiments, each performed in triplicate. For both assays, statistical analyses were performed using Student's t-test.
4. DISCUSSION
In C. albicans hyphae, polarity establishment and reorientation of growth in the direction of an external stimulus occurs in response to electrical field and topographical cues (Gow 1982 and 1994). To date, only two requirements for hyphal growth directionality mechanisms have been described. The first, calcium influx, is mediated by calcium channels and is required for the establishment of germ tube growth in the direction of a cathodal stimulus as well as for re-orientation of growth along a topographical cue (Brand et al., 2007). The second, the Ras-like GTPase, Rsr1, is needed for all C. albicans hyphal tropism responses (Brand et al., 2008). A role for Rsr1 in hyphal growth directionality was predicted by its role as a landmark protein in the selection of bud emergence sites during yeast growth in both S. cerevisiae and C. albicans (Bender and Pringle, 1989; Hausauer et al., 2005). Thus, in this study, we sought to test the hypothesis that other yeast bud-site selection proteins are required for hyphal tropisms.
Our studies found that CaRAX2, ortholog of ScRAX2, is needed for yeast bud-site selection. In S. cerevisiae, Rax2 is important for both establishing and maintaining the bipolar pattern of yeast growth (Chen et al., 2000; Kang et al., 2004). In wild-type S. cerevisiae diploid cells, the first bud is placed almost exclusively at the pole distal to the birth scar and after several cell cycles, cells exhibit bud scars at both poles (bipolar pattern). In S. cerevisiae cells lacking Rax2, the first bud tends to emerge at sites proximal to the birth scar and subsequent buds emerge predominantly at non-patterned, random sites. In contrast to S. cerevisiae, we observed that wild-type C. albicans yeast cells place the first bud at sites proximal to the birth scar and, over time, the budding pattern becomes more axial or bipolar, depending upon the temperature of growth (Gale et al., 2001). Despite the differences in basic budding patterns exhibited by S. cerevisiae and C. albicans yeast cells, we nevertheless observed that Rax2 was important for bud-site selection in C. albicans. For both first buds and subsequent budding events, C. albicans strains lacking Rax2 exhibited an increase in bud sites occuring at random, or non-polar locations. Thus, CaRax2 is important for both the establishment and maintenance of bud-site selection patterns during yeast-form growth.
Similar to its role in yeast bud-site selection, we found that CaRax2 was also important for the selection of hyphal branch and secondary hyphal sites. In addition, rax2 mutant strains were unable to maintain linear hyphal growth in non-cued growth conditions. Similar hyphal linearity defects have been reported for C. albicans and Ashbya gossypii strains lacking the Ras-like GTPase Rsr1 (Hausauer et al., 2005; Bauer et al., 2004), providing additional support for the idea that landmark proteins direct the establishment of daughter cell emergence sites and help to maintain growth axes once established during fungal morphogenesis. Of note, deletion of Rax2 did not affect overall hyphal shape. This finding is in contrast to that observed for deletion of the bud-site selection protein Rsr1, which causes wider hyphal shapes with constricted septations. Thus, bud-site selection proteins are not completely generalizable with respect to their cellular functions.
The punctate membrane localization pattern of Rax2 in hyphae raised the possibility that Rax2 is a component of eisosomes, static protein complexes on fungal cell surfaces (Walther, et al., 2006; Alvarez et al., 2008; Alvarez and Konopka, 2007; Vangelatos, et al., 2010; Seger, et al., 2011; Reijnst et al., 2011). Eisosomes have been proposed to mark endocytic sites in S. cerevisiae and C. albicans (Walther, et al., 2006; Alvarez et al., 2008; Alvarez and Konopka, 2007) but are not involved in endocytosis in A. gossypii or Aspergillus nidulans (Seger, et al., 2011; Vangelatos, et al., 2010). We found that, in C. albicans, Rax2 did not significantly colocalize with the eisosome-associated protein Pil1. Importantly, however, Rax2 localization was enriched at hyphal tips, poising it for a role in the directional growth of the hyphal apex. This prediction was supported by the findings that Rax2 was needed for the maintenance of hyphal linearity in non-cued conditions and in cathodal hyphal emergence responses. However, Rax2 was not required for final angle galvanotropism or thigmotropism responses of hyphae. Thus, Rax2 has a role in directing and maintaining the initial site of polarized growth, but not in the re-orientation of the polarity axis once it has been established. In strains with mutations of genes encoding the calcineurin catalytic subunits CNA1 and CNB1, a similar phenotype is seen in that cathodal emergence responses are reduced but the ability to reorient already established hyphae in the direction of a stimulus is maintained (Brand et al., 2007). In contrast, mutation of CaMID1, ortholog of the stretch-activated channel for the high-affinity calcium uptake system in S. cerevisiae, resulted in the opposite response; hyphae lacking Mid1 were able to initiate germ tube growth in the direction of the cathode, but were unable to reorient when an external stimulus was presented (Brand et al., 2007). Altogether, these results indicate that distinct mechanisms exist for the establishment/maintenance versus the re-orientation of hyphal growth direction.
Previous studies have suggested that intact tropism responses are needed for C. albicans hyphae to efficiently penetrate and injure host tissue (Brand et al., 2008), however direct evidence of this remains to be shown. Herein, we found that hyphae lacking Rax2 retain the ability to invade and damage human epithelial cell monolayers, correlating with intact re-orientation responses and normal, polarized hyphal cell shapes. Although the rax2 strain had defective cathodal emergence/establishment responses and exhibited curvier hyphae in non-cued growth conditions, their penetration and damage responses remained intact. Thus, the mechanisms that dictate daughter cell emergence sites and that maintain hyphal linearity do not appear to play a role in epithelial cell invasion and injury. Hyphal re-orientation responses, in contrast, remain potentially important in the pathogenesis process. Alternatively, intact re-orientation mechanisms may be a general indicator of a functional, tip-localized polarity complex that is required for hyphal-mediated invasive disease.
Highlights.
RAX2 is required for proper bud and branch site selection in Candida albicans.
Candida albicans Rax2 is important for maintaining the linearity of hyphal growth.
Rax2 establishes polarity during galvanotropic responses of Candida albicans hyphae.
Distinct mechanisms exist for maintenance vs. re-orientation of hyphal growth direction.
Acknowledgements
We thank W. Allan Walker for providing the H4 intestinal epithelial cell line, Karen Ross for providing TERT2 epithelial cells and assistance with epithelial cell assays, and Abigail DiPasquale for technical assistance.
This study was funded by NIH R01AI057440 and University of Minnesota Pediatrics Foundation awards to CAG.
ABBREVIATIONS
- CFP
cyan fluorescent protein
- DIC
differential interference contrast
- MI
morphological index
- YFP
yellow fluorescent protein
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Jennifer Norton, current address: University of Arizona College of Medicine, Department of Cellular and Molecular Biology, Life Sciences South Bldg., 1007 E. Lowell St., P.O. Box 210016, Tucson, AZ 85721, jnorton@email.arizona.edu
Lindy Watanaskul, current address: University of Maryland Medical Center, 201 East University Parkway, Baltimore, MD 21218
REFERENCES
- Alvarez FJ, et al. The Sur7 protein regulates plasma membrane organization and prevents intracellular cell wall growth in Candida albicans. Mol Biol Cell. 2008;19:5214–25. doi: 10.1091/mbc.E08-05-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Konopka JB. Identification of an N-acetylglucosamine transporter that mediates hyphal induction in Candida albicans. Mol Biol Cell. 2007;18:965–75. doi: 10.1091/mbc.E06-10-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer Y, et al. A Ras-like GTPase is involved in hyphal growth guidance in the filarmentous fungus Ashbya gossypii. Mol Biol Cell. 2004;15:4622–32. doi: 10.1091/mbc.E04-02-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Pringle JR. Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc Nal Acad Sci U S A. 1989;86:9976–80. doi: 10.1073/pnas.86.24.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensen ES, et al. A forkhead transcription factor is important for true hyphal as well as yeast morphogenesis in Candida albicans. Eukaryot Cell. 2002;1:787–98. doi: 10.1128/EC.1.5.787-798.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, et al. Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr Biol. 2007;17:347–52. doi: 10.1016/j.cub.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, et al. An internal polarity landmark is important for externally induced hyphal behaviors in Candida albicans. Eukaryot Cell. 2008;7:712–20. doi: 10.1128/EC.00453-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamayor A, Snyder M. Bud-site selection and cell polarity in budding yeast. Curr Opin Microbiol. 2002;5:179–86. doi: 10.1016/s1369-5274(02)00300-4. [DOI] [PubMed] [Google Scholar]
- Chen T, et al. Multigenerational cortical inheritance of the Rax2 protein in orienting polarity and division in yeast. Science. 2000;290:1975–8. doi: 10.1126/science.290.5498.1975. [DOI] [PubMed] [Google Scholar]
- Crampin H, et al. Candida albicans hyphae have a Spitzenkorper that is distinct from the polarisome found in yeast and pseudohyphae. Journal of Cell Science. 2005;118:2935–47. doi: 10.1242/jcs.02414. [DOI] [PubMed] [Google Scholar]
- Crombie T, et al. Influence of applied electrical fields on yeast and hyphal growth of Candida albicans. J Gen Microbiol. 1990;136:311–7. doi: 10.1099/00221287-136-2-311. [DOI] [PubMed] [Google Scholar]
- Dalle F, et al. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010;12:248–71. doi: 10.1111/j.1462-5822.2009.01394.x. [DOI] [PubMed] [Google Scholar]
- Davies JM, et al. Candida albicans hyphal invasion: thigmotropism or chemotropism? FEMS microbiology letters. 1999;171:245–9. doi: 10.1111/j.1574-6968.1999.tb13439.x. [DOI] [PubMed] [Google Scholar]
- Davis D, et al. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect Immun. 2000;68:5953–9. doi: 10.1128/iai.68.10.5953-5959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falgier C, et al. Candida species differ in their interactions with immature human gastrointestinal epithelial cells. Pediatr Res. 2011;69:384–9. doi: 10.1203/PDR.0b013e31821269d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale C, et al. Candida albicans Int1p interacts with the septin ring in yeast and hyphal cells. Mol Biol Cell. 2001;12:3538–49. doi: 10.1091/mbc.12.11.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerami-Nejad M, et al. Cassettes for PCR-mediated construction of green, yellow, and cyan fluorescent protein fusions in Candida albicans. Yeast. 2001;18:859–64. doi: 10.1002/yea.738. [DOI] [PubMed] [Google Scholar]
- Gerami-Nejad M, et al. Cassettes for the PCR-mediated construction of regulatable alleles in Candida albicans. Yeast. 2004;21:429–36. doi: 10.1002/yea.1080. [DOI] [PubMed] [Google Scholar]
- Gow NA. Growth and guidance of the fungal hypha. Microbiology. 1994;140(Pt 12):3193–205. doi: 10.1099/13500872-140-12-3193. [DOI] [PubMed] [Google Scholar]
- Gow NA. Germ tube growth of Candida albicans. Curr Top Med Mycol. 1997;8:43–55. [PubMed] [Google Scholar]
- Gow NA, et al. Investigation of touch-sensitive responses by hyphae of the human pathogenic fungus Candida albicans. Scanning Microsc. 1994;8:705–10. [PubMed] [Google Scholar]
- Hausauer DL, et al. Hyphal guidance and invasive growth in Candida albicans require the Ras-like GTPase Rsr1p and its GTPase-activating protein Bud2p. Eukaryot Cell. 2005;4:1273–86. doi: 10.1128/EC.4.7.1273-1286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatilake JA, et al. An ultrastructural and a cytochemical study of candidal invasion of reconstituted human oral epithelium. J Oral Pathol Med. 2005;34:240–6. doi: 10.1111/j.1600-0714.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- Kang PJ, et al. Interactions among Rax1p, Rax2p, Bud8p, and Bud9p in marking cortical sites for bipolar bud-site selection in yeast. Mol Biol Cell. 2004;15:5145–57. doi: 10.1091/mbc.E04-07-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. A Candida albicans temperature-sensitive cdc12-6 mutant identifies roles for septins in selection of sites of germ tube formation and hyphal morphogenesis. Eukaryot Cell. 2012;11:1210–18. doi: 10.1128/EC.00216-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merson-Davies LA, Odds FC. A morphology index for characterization of cell shape in Candida albicans. J Gen Microbiol. 1989;135:3143–52. doi: 10.1099/00221287-135-11-3143. [DOI] [PubMed] [Google Scholar]
- Odds FC. Pathogenesis of Candida infections. J Am Acad Dermatol. 1994;31:S2–5. doi: 10.1016/s0190-9622(08)81257-1. [DOI] [PubMed] [Google Scholar]
- Pulver R, et al. Rsr1 focuses Cdc42 activity at hyphal tips and promotes maintenance of hyphal development in Candida albicans. Eukaryot Cell. 2012 doi: 10.1128/EC.00294-12. Epub 12/7/2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichart PA, et al. Pseudomembranous oral candidiasis in HIV infection: ultrastructural findings. J Oral Pathol Med. 1995;24:276–81. doi: 10.1111/j.1600-0714.1995.tb01182.x. [DOI] [PubMed] [Google Scholar]
- Reijnst P, et al. Dual-colour fluorescence microscopy using yEmCherry-/GFP-tagging of eisosome components Pil1 and Lsp1 in Candida albicans. Yeast. 2011;28:331–8. doi: 10.1002/yea.1841. [DOI] [PubMed] [Google Scholar]
- Saiman L, et al. Risk factors for candidemia in neonatal intensive care unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J. 2000;19:319–24. doi: 10.1097/00006454-200004000-00011. [DOI] [PubMed] [Google Scholar]
- Sanderson IR, et al. Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci U S A. 1996;93:7717–22. doi: 10.1073/pnas.93.15.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger S, et al. Formation and stability of eisosomes in the filamentous fungus Ashbya gossypii. J Cell Sci. 2011;124:1629–34. doi: 10.1242/jcs.082487. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting Started with Yeast. In: Fink GR, Guthrie C, editors. Methods Enzymol.; Guide to Yeast Genetics and Molecular Biology. Vol. 194. Academic Press, Inc.; San Diego, CA: 1991. pp. 3–20. [DOI] [PubMed] [Google Scholar]
- Sherwood J, et al. Contact sensing in Candida albicans: a possible aid to epithelial penetration. J Med Vet Mycol. 1992;30:461–9. doi: 10.1080/02681219280000621. [DOI] [PubMed] [Google Scholar]
- Vangelatos I, et al. Eisosome organization in the filamentous ascomycete Aspergillus nidulans. Eukaryot Cell. 2010;9:1441–54. doi: 10.1128/EC.00087-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TC, et al. Eisosomes mark static sites of endocytosis. Nature. 2006;439:998–1003. doi: 10.1038/nature04472. [DOI] [PubMed] [Google Scholar]
- Wilson RB, et al. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol. 1999;181:1868–74. doi: 10.1128/jb.181.6.1868-1874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]