Abstract
Adolescence is the transition from childhood to adulthood, with onset marked by puberty and the offset by relative independence from parents. Across species, it is a time of incredible change that carries increased risks and rewards. The ability of the individual to respond adequately to the mental, physical and emotional stresses of life during this time is a function of both their early environment and their present state. In this article, we focus on the effects that acute threat and chronic stress have on the brain and behavior in humans and rodents. First, we highlight developmental changes in frontolimbic function as healthy individuals transition into and out of adolescence. Second, we examine genetic factors that may enhance susceptibility to stress in one individual over another using translation from genetic mouse models to human neuroimaging. Third, we examine how the timing and nature of stress varies in its impact on brain and behavior. These findings are discussed in the context of implications for adolescent mental health and illness.
Keywords: stress, adolescence, anxiety, BDNF, fear, emotion regulation
Introduction
Recent reports in the US suggest that stress is taking a toll on the physical and mental health of Americans and their families (APA, 2010), with reports of stress levels exceeding what families consider to be healthy in the majority of homes. It should come as no surprise then that stress related disorders including anxiety and depression affect as many as 10% of our youth, making them the most prevalent of the developmental psychiatric disorders (Newman et al., 1996, Pollack et al., 1996, Kim-Cohen et al., 2003, Kessler et al., 2005, Merikangas et al., 2010) This article highlights recent studies on the impact of stress on the brain and behavior across development and across species that may help to explain the high incidence of anxiety and stress related disorders during adolescence.
Overview
Stress occurs when mental, emotional and or physical demands exceed the regulatory capacity of the organism. Situations that are highly unpredictable or uncontrollable are examples of highly stressful environments (e.g., institutionalization, warfare) (Koolhaas, 2011)). Such environments threaten the survival or coping ability of the organism. When such threats occur, the nervous system responds by releasing stress hormones that help put the organism in an alert state and ready for action. While moderate stress can be adaptive in helping the animal respond to the situational demands, chronic stress can have lasting negative consequences (Shonkoff et al., 2009). Thus, stress can vary along dimensions of frequency, duration (e.g. acute or chronic), and magnitude, each of which has different implications for the stability of the animal. We focus on the adaptive and maladaptive effects of psychological stress across different points in development in this paper.
We highlight three approaches that examine the impact of emotionally charged or environmentally demanding events that can lead to stress. In the context of this review, a potential threat can be a stressor depending on how one perceives that threat. Since threat is invariably associated with negative emotions, how well we can regulate those emotions can influence whether we perceive it as a psychologically stressful event. We begin with a brief review of threat related brain circuitry. We then present findings from recent human imaging and mouse studies that illustrate developmental differences in response to potential threat highlighting changes during the period of adolescence. Second, we describe mouse and human genetic studies that illustrate individual variability in response to threat. We end by providing an example of prolonged early stress in humans; specifically those reared in institutions abroad and illustrate how such challenging environments impact later behavioral and neural responses to potential threat.
Stress effects on the brain
Major circuits involving the amygdala/hippocampal complex together with the prefrontal cortex support behaviors related to threat processing and vigilance (Lupien et al., 2009). Threat results in the release of stress hormones that target regions of the brain and major muscles key for flight or flight. Under non-stressful conditions, these hormones help to support growth and development (De Kloet et al., 1998). However, under conditions of challenge the release of hormones suppress growth and repair in order to support functions necessary for survival. Especially key to the stress response is the release of glucocorticoids that redistribute glucose to the body to help the individual overcome the threat or challenge. Failure to activate the stress response places the organism in a fragile state. Yet, failure to inhibit the stress response can result in disease and lasting adverse effects on growth and development.
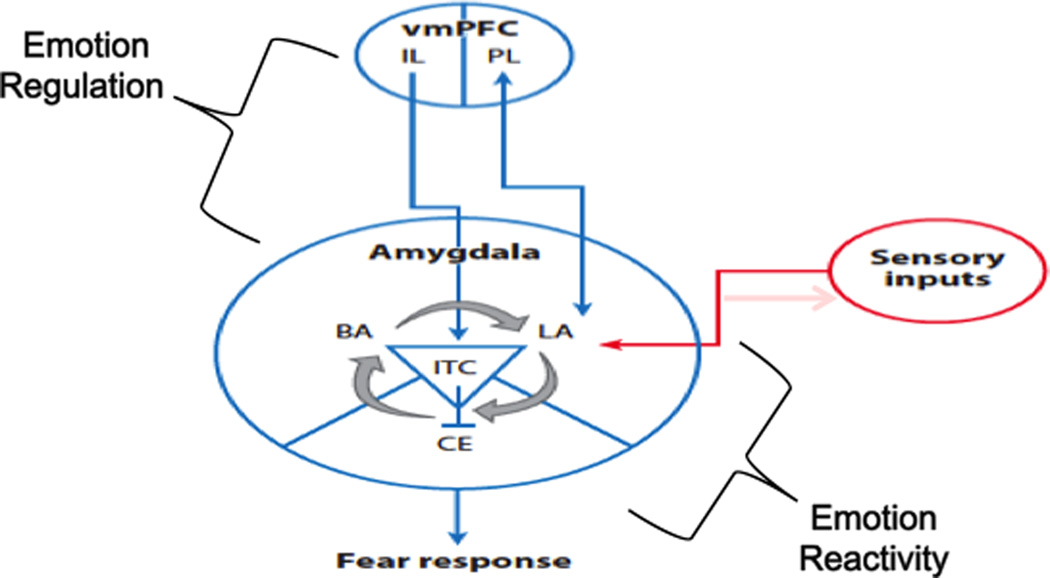
This article focuses on the effects of psychological stressors. Psychological stressors, in contrast to physiological ones (e.g., hypoxia), require higher order processing and interpretation of sensory information, thus making connections with limbic and cognitive circuitry crucial for reacting to this type of stressor. Specifically the amygdala (Davis, 1992) appears to be critical in activating the stress response to cognitive-emotional challenge and threat while hippocampal and prefrontal regions appear critical in the regulation of the stress response. For the purposes of this review we will focus predominantly on frontoamygdala circuitry implicated in threat processing (see Figure 1).
Fig. 1. Frontoamygdala circuit of Fear.
A simplified cartoon of the brain circuitry involved in emotion reactivity and regulation related to threat processing. The amygdala receives multimodal sensory signals that may initiate a fear response, while top-down input from the infralimbic prefrontal cortex to the amygdala can dampen or extinguish fear responses generated there. Abbreviations: BA, basal amygdala; CE, central amygdala; IL, infralimbic prefrontal cortex; ITC, intercalated; LA, lateral amygdala; PL, prefrontal cortex; vmPFC, ventromedial prefrontal cortex. Adapted from Casey et al 2013.
The majority of human and animal stress studies have focused on the effects of stress on the hippocampus, but more attention has been given to the amygdala and prefrontal cortex in recent years (Liston et al., 2009). A large body of research, beyond the scope of this paper, has documented detrimental effects of stress on the hippocampus. In brief, these studies show that repeated threat or chronic stress leads to decreased hippocampal volume, dendritic spine density, and a remodeling of synaptic terminals of this region (Magarinos et al., 1997). If the stressor is short-lived, these effects are reversible (McEwen, 1998). However, if stress occurs over a period of months or years it can result in irreversible apical dendritic atrophy and even cell death (Uno et al., 1989). The amygdala, in contrast to the hippocampus, shows proliferative effects with stress. Specifically, stress and/or the administration of stress hormones leads to enhanced dendritic arborization and increased spine density (Vyas et al., 2002, Vyas et al., 2003, Mitra et al., 2005) that may be less reversible than the hippocampus (Vyas et al., 2004). Human imaging studies show parallel results of stress on these regions, with individuals who have experienced high levels of stress showing smaller hippocampal volume (Bremner et al., 1995, Gurvits et al., 1996, Bremner et al., 1997), larger amygdala volume (Tottenham et al., 2010), and elevated amygdala activity to cues of threat relative to non-stressed individuals (Liberzon et al., 1999, Rauch et al., 2000, Shin et al., 2005).
Repeated stress has been shown to have profound effects on prefrontal functions too (e.g., Arnsten, 1999, Mizoguchi et al., 2000, Bland et al., 2004, Cook and Wellman, 2004, Moghaddam and Jackson, 2004, Maroun, 2006, Radley et al., 2006, Del Arco et al., 2007), by diminishing the ability to flexibly regulate attention, actions and affect (Phelps et al., 2004, Liston et al., 2006b). Whereas the amygdala and hippocampus are involved in learning about cues and contexts that signal threat, the prefrontal cortex has been suggested to be involved in “un-learning” these associations (Morgan and LeDoux, 1999, Nair et al., 2001, Herry and Garcia, 2002, Gottfried and Dolan, 2004, Phelps et al., 2004, Santini et al., 2004, Mickley et al., 2005, Akirav and Maroun, 2006, Kalisch et al., 2006, Corcoran and Quirk, 2007, Milad et al., 2007). Failure to recognize when environmental cues and contexts are no longer threatening (Quirk and Gehlert, 2003) has been suggested to be at the very core of anxiety and stress related disorders that peak in diagnosis around adolescence.
Adolescence: A time of stress
By definition, adolescence poses new environmental demands on the organism, as the individual moves from dependence on parents to relative independence. As such, the adolescent must rapidly adapt to new social, sexual, and intellectual challenges (Romeo, 2010, Spear, 2010). In a series of recent experiments we examined changes in the brain and behavior to threat during adolescence. Our work uses two distinct approaches. The first approach involves the use of naturalistic cues (e.g., a frightened face) that over a lifetime become associated with potential threat in the environment. The second behavioral paradigm involves experimentally manipulating a neutral stimulus to take on aversive associations using Pavlovian fear conditioning. This conditioning involves pairing a neutral cue (e.g., tone) repeatedly with an aversive stimulus (e.g., shock), until the neutral cue takes on noxious properties that mimic the aversive stimulus through associative learning. Both approaches have been used to determine how well an individual can suppress a fear response when danger cues and contexts are no longer a source of threat, but differ in behavioral validity when considering developmental and species differences that we will discuss. We present converging evidence for developmental variation in fear regulation using both of these approaches below.
Transitional Studies of Threat
In a series of neuroimaging studies of adolescents, we have examined inflections in behavior as the individual transitions into and out of adolescence (Galvan et al., 2006, Hare et al., 2008, Somerville and Casey, 2010). In the most relevant of these studies, we examined responses to threat cues (fearful faces) in 60 children, adolescents, and adults with functional magnetic resonance imaging (fMRI). This study went beyond examining the magnitude of brain activity that has been shown by several groups to be higher in adolescents than in adults to such cues (Monk et al., 2003b, Ernst et al., 2005, Rich et al., 2006, Williams et al., 2006, Guyer et al., 2008a, Guyer et al., 2008b, Guyer et al., 2009) to show specific changes in adolescents to these threat cues relative to both adults and children. We examined not only transient patterns of frontolimbic activity, but changes in activity as a function of repeated exposure (Hare et al., 2008).
Our adult human results showed that reaction times to threat cues were longer than to neutral ones (Hare et al 2005). Reaction times were positively associated with amygdala activity and negatively associated with the ventromedial prefrontal activity. Adolescents showed an initial exaggerated amygdala response to cues that signal threat (fearful faces) relative to children and adults (see Figure 2, Hare et al., 2008). This initial heightened response in amygdala activity was age-dependent and did not correlate with symptoms of anxiety. Although several groups have shown similar elevated amygdala activity to emotional pictures in adolescents relative to adults (Monk et al., 2003a, Guyer et al., 2008b) few studies have shown a distinct pattern in adolescents from both children and adults. This response was attenuated with repeated presentation of the fearful face (i.e., exposure to empty threat) across experimental trials. The extent to which activation of the amygdala diminished with time was correlated with ratings of everyday anxiety as measured by the Spielberger trait anxiety rating scale (Spielberger et al., 1988). These findings suggest that initial emotional reactivity as indexed by elevated amygdala activity may be typical of or normal for adolescents, but that failure of this response to subside over time with no impending threat is atypical and may be indicative of heightened anxiety during this period. During adolescence, when the amygdala response is heightened relative to that observed in children and adults, more top down prefrontal control may be needed to effectively attenuate the fear response. Failure to dampen this response may lead to symptoms and ultimately diagnosis of anxiety and stress related disorders.
Fig. 2. Amygdala response to empty threat as a function of age and symptoms of anxiety.
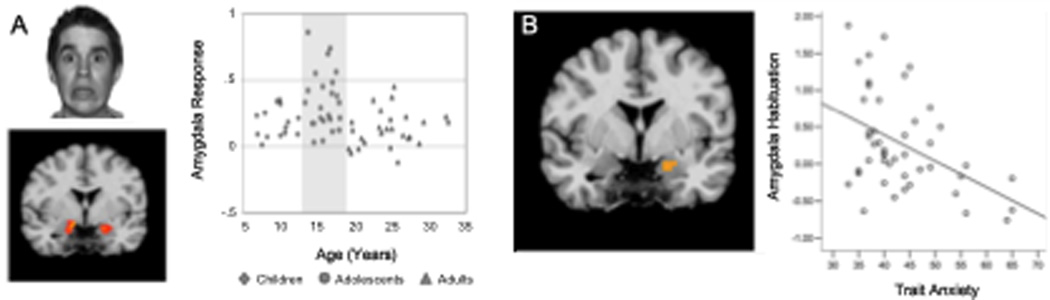
(A) Depiction of threat stimulus and location of activation in the amygdala. Middle: Amygdala activity to empty threat (fearful faces) plotted as a function of age. (B) Scatter plot of the correlation between Spielberger trait anxiety scores and habituation (decrease from early to late trials) of amygdala activity for teens and adults (note: anxiety scale was not appropriate for under 13 years) r = −.447, p < 0.001. Adapted from Hare et al., 2008.
Translational Studies of Threat
In a recent parallel study of adolescent humans and mice, we examined sensitivity to threat cues. In contrast to the previous studies, instead of using naturalistic cues (fearful faces) we used neutral cues to control for the amount of history with the cue of threat. Naturalistic threat cues come to be associated with danger over a lifetime. However, our experiences over a lifetime are not equivocal and are limited by our age and opportunity for such experiences. For example, a child may have fewer experiences of dangerous situations or threats than an adult, and an anxious child may have many more experiences of threat than a non-anxious child. These experiences will differentially impact threat-related circuitry. Fear learning paradigms are thus advantageous in that they can assess fear learning equivalently in typically and atypically developing humans. Second, because there is a high degree of neural and behavioral conservation across species, fear learning can be assessed equivalently in humans and mice. The translation of findings from human to mouse provides the added opportunity of delineating mechanisms of change in mice that would be more difficult in developing humans.
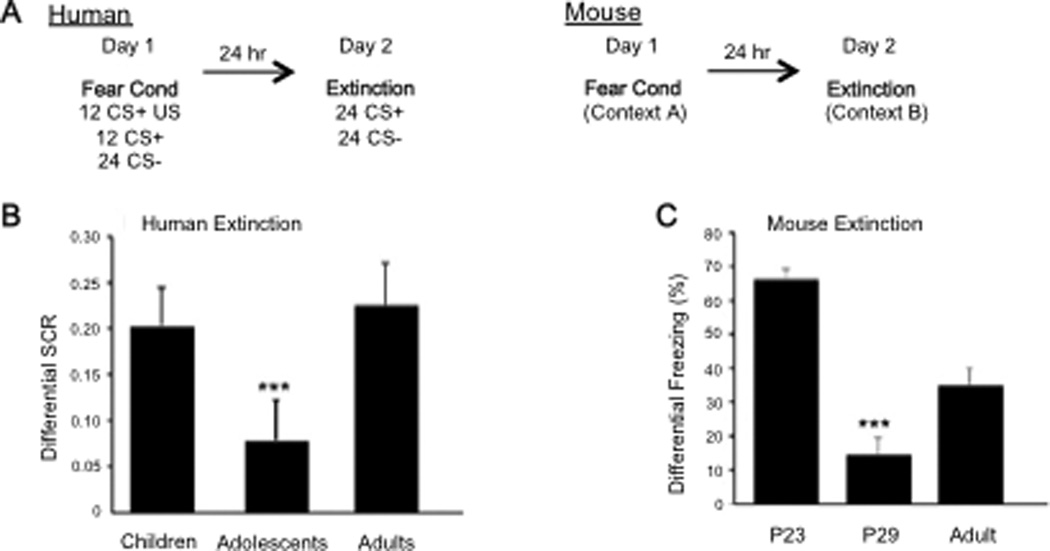
In our experiments we used a Pavlovian fear conditioning paradigm to more directly examine how responses to threat change during the period of adolescence (Lau et al., 2008, Lissek et al., 2009, Pine, 2009, Waters et al., 2009, Britton et al., 2011, Lau et al., 2011, Pattwell et al., 2012). Specifically we wanted to examine the ability of the adolescent to regulate fear once the threat of fear was removed (i.e., extinction learning). We tested over 80 individuals between the ages of 5 and 28 using skin conductance response (SCR) to measure physiological responses of arousal during both fear conditioning and extinction (Fere, 1888, Cacioppo et al., 2007). Because of the developmental nature of this study we used an aversive sound for the human subjects rather than shock as our aversive stimulus and paired it repeatedly with a neutral stimulus (yellow or blue square). Our results indicated no effect of age on fear acquisition, but a significant effect on fear extinction (Pattwell et al., 2012). Adolescents showed attenuated fear extinction relative to both children and adults (Figure 3). This effect remained when co-varying for both gender and trait anxiety in the humans.
Fig. 3. Developmental variation in fear extinction learning.
(B) Extinction learning is attenuated during adolescence in the human as measured by less change in galvanic skin response with repeated presentation of the conditioned stimulus alone during extinction trials. (C) This finding is paralleled in the mouse as measured by less change in freezing behavior. Reproduced with permission from (Pattwell et al 2012).
In a parallel study with mice postnatal days (P)23, 29 and 70, we used freezing behavior to measure the fear response, electric shock as the unconditioned aversive stimulus, and a tone as the conditioned stimulus. We observed a similar developmental pattern. The adolescent (P29) mice, like human subjects, showed diminished fear extinction learning compared to the preadolescent and adult mice. These findings are consistent with rodent studies that show adolescent rats require twice as many extinction trials as adults, or prolonged duration of the conditioned stimulus to achieve reductions in conditioned fear behavior comparable to those seen in adult rats (McCallum et al., 2010, Lai et al., 2012). Thus, adolescence is a time when threat cues appear to be highly salient and more resistent to exinction than at any other time in development.
The mouse model provides the opportunity to assess the mechanism underlying the conserved age differences in fear extinction learning across species. As such, we used immunohistochemical and electrophysiological methods to assess neurobiological changes in frontolimbic circuitry in the mice across developmental stages. We focused on the infralimbic cortex because of its role in extinction learning (Santini et al., 2004) and because of the behavioral findings indicating diminished extinction learning in adolescents. We measured activity-induced expression of the immediate early gene c-Fos in the infralimbic cortex. Consistent with previous studies, the density of c-Fos-labeled cells in the infralimbic cortex of adult mice was significantly higher than non-extinguished, fear-conditioned controls. In contrast, there was no change in density of c-Fos labeling in the adolescent (29-day-old) mice. These data suggest that the neural circuit engaged by fear extinction learning in adults is not active during adolescence, providing a likely neural substrate for the inefficiency of cortical control of fear responses during adolescence.
To further delineate changes in frontoamygdala circuitry with age, we performed electrophysiological recordings in ventromedial prefrontal cortex (vmPFC) brain slices of mice after both fear acquisition and fear extinction. Previously it has been shown that fear conditioning involves a decrease in intrinsic excitability of infralimbic cortex whereas fear extinction reversed this decrease in excitability (Santini et al., 2008). Electrophysiological recordings at infralimbic and prelimbic cortex synapses across age showed a fear-conditioning-induced potentiation of prelimbic synapses present in adult mice that was absent in adolescent mice. Extinction-induced enhancement of infralimbic cortex synaptic plasticity in adult mice was lacking in adolescent mice (Pattwell et al., 2012)
Together, these studies suggest blunted regulation of amygdaladependent fear responses during fear extinction in adolescents. These findings may help provide novel insights into the heightened prevalence and treatment of anxiety disorders during adolescence, as the main form of cognitive behavioral therapy relies on principles of extinction learning.
Genetic Factors
The previous work is consistent with different developmental trajectories of distinct limbic brain regions being involved in adaptive fear responses. However, within any developmental stage there is marked individual variability. An important source of variability is that of genetic variation. The main avenues for understanding gene function in anxiety and stress related disorders have been human genetic association studies on one end and genetically engineered mouse models on the other. We have used brain imaging to link structural and functional abnormalities seen in knockout/transgenic mouse models to abnormal patterns of brain activity seen in humans to bridge these (Casey et al., 2010). In an effort to implement a translational approach to human genetic variability, we focused on a common polymorphism in the human gene for brain-derived neurotrophic factor (BDNF). BDNF is a growth factor that plays a central role in neuronal survival, growth, and synaptic plasticity---all core aspects of associative learning in the central nervous system and adaptive fear learning in particular. Human populations contain a common single nucleotide polymorphism (SNP) that causes a valine-to-methionine substitution at codon 66 (Val66Met). This polymorphism leads to decreased trafficking of BDNF into the regulated secretory pathway, which in turn leads to impaired activity-dependent release of BDNF. The BDNF gene is highly conserved from mouse to human, and wild-type mice naturally express the ancestral valine form of the BDNF peptide. To study the effects of the human Val66Met polymorphism in mice, we created a knock-in mouse with a BDNF protein identical to the wild type except it contains a methionine in codon 66 (BDNFmet). Hippocampal neurons obtained from these BDNFmet mice have impaired activity-dependent BDNF secretion and show reductions in dendritic arborization. These mice also exhibit hippocampaldependent learning deficits similar to the findings in humans with the variant human BDNF, validating this mouse as a model of the human Val66Met polymorphism.
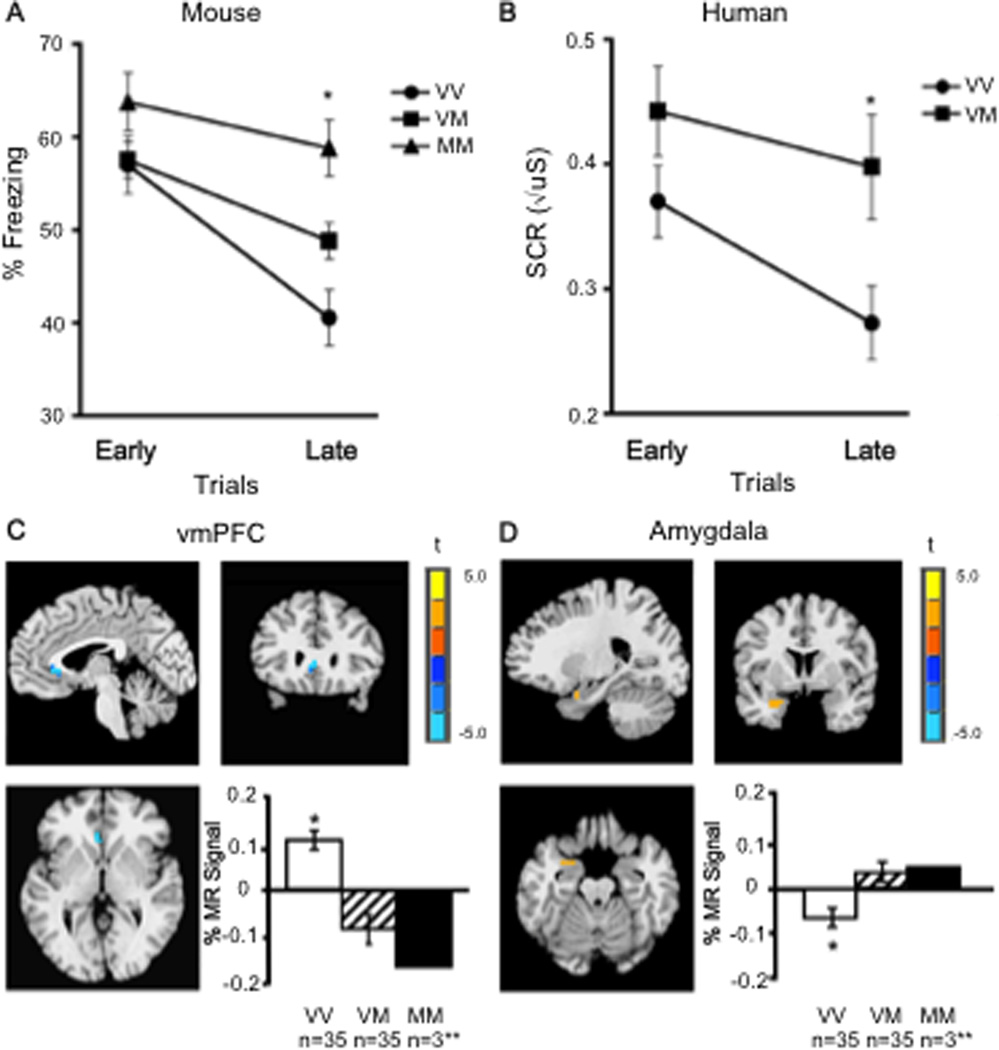
We examined the impact of the variant BDNF on fear regulation using similar fear conditioning and extinction paradigms in mice and humans as those described above. In adult humans and mice, we observed less extinction in Met allele carriers than in Val allele carriers, as shown in Figure 4 (Soliman et al., 2010). Moreover, human functional neuroimaging data provided neurobiological validation of the cross-species translation. Specifically, we showed alterations in frontoamygdala circuitry, as a function of BDNF genotype. During extinction, Met allele carriers showed less vmPFC activity (Figure 4c) but greater amygdala activity (Figure 4d) than non-carriers. These findings suggest that cortical regions essential for extinction in animals and humans are less responsive in Met allele carriers. Moreover, amygdala recruitment which should show diminished activity during extinction was elevated in Met allele carriers, suggesting less dampening by vmPFC and more fear response as generated by amygdala output to neuromodulatory systems, the hypothalamus, periaqueductal gray, and vagus (LeDoux, 2000, Phelps et al., 2004).
Fig. 4. Genetic variation in fear extinction learning and limbic activity.
(A) Extinction learning is attenuated in mice with the BNDF Met (M) allele relative to non-Met allele (V) carriers as measured by less change in freezing behavior with repeated presentation of the conditioned stimulus alone during extinction trials. (B) This finding is paralleled in the human as measured by less change in galvanic skin response. (C) Brain activity as indexed by percent change in magnetic resonance (MR) signal during extinction in the ventromedial prefrontal cortex (vmPFC) by genotype (x, y, z = −4, 24, 3), with Met allele carriers having significantly less activity than Val/Val homozygotes (VM < VV is blue), image threshold P < 0.05, corrected. (D) Genotypic differences in left amygdala activity during extinction (x, y, z = −25, 2, −20) in 70 humans, with Met allele carriers having significantly greater activity than Val/Val homozygotes (VM > VV is orange), image threshold P < 0.05, corrected. *P < 0.05. **MM were included in the analysis with VM, but plotted separately to see the dose response. All results are presented as mean +/− SEM. VV, Val/Val; VM, Val/Met; MM, Met/Met. Adapted from Soliman et al 2010.
These genetic findings provide an example of bridging human behavioral and imaging genetics with a molecular mouse model to suggest a role for BDNF in anxiety and stress. Individuals with the BDNF Met allele may be more vulnerable to developing symptoms of anxiety as teens, in that they show higher and prolonged patterns of amygdala activity and less vmPFC activity in response to threat. During a period when evaluating social cues from peers is essential in forming and maintaining healthy peer relationships, the failure to suppress heightened emotional responses to empty threat (e.g., failure of a peer to notice or smile at a teenager, without any negative intent) could lead to over interpretation and ruminations of self-doubt (Guyer et al., 2008a, Monk et al., 2008). The genetic data provide an example of how an imbalance in amygdalavmPFC coupling during typical development could predispose the adolescent to anxiety and, when exacerbated by an individual factor such as the BDNF Met66 allele, lead to clinical levels of anxiety.
Effects of Early Adversity
The variability observed in both our developmental studies of fear regulation may in part be due to genetic variation, but clearly individual experiences impact behavior. A number of studies have shown the significance of environmental factors such as early adversity and stress on the brain and on behavior (Liston et al., 2006a, Liston et al., 2009, Tottenham et al., 2009, 2010, Tottenham et al., in press). Individuals who experience adversity or multiple traumas during development, may be especially vulnerable for developing symptoms of anxiety or depression as teens or adults. Non-human animal studies have shown that early rearing conditions can have long-term consequences on emotional behavior and the effects of early experience can be more significant than later experiences (Sabatini et al., 2007). Many of these behavioral outcomes are associated with changes in limbic circuitry. Within this circuitry, the region of the amygdala is particularly sensitive to early life rearing conditions (Plotsky et al., 2005, Sabatini et al., 2007, Kikusui and Mori, 2009) and its growth and hyperactivity under such stress, have been shown to mediate the expression of hyperemotionality as measured by increased anxiety-like behaviors in animals (Vyas and Chattarji, 2004).
We recently examined the effects of suboptimal early rearing conditions on human development by examining threat related behavior in children and adolescents reared in an orphanage before being adopted to the U.S. These children exhibit elevated emotional reactivity (Colvert et al., 2008) more anxiety (Casey et al., 2009, Zeanah et al., 2009), internalizing problems (Juffer and van Ijzendoorn, 2005) and difficulty regulating behavior in emotionally arousing contexts (Tottenham et al., 2009) - a profile that can persist for many years. Our question was to what extent these children could suppress threat responses with repeated presentation of an empty threat such as a fearful face. We used the same paradigm as that described earlier by Hare et al. (2008) and collected both structural and functional MRI data. Behavioral and imaging data were collected from nearly 60 children (28 adopted and 27 non-adopted) with an average age of 10 years (Tottenham et al., 2010).
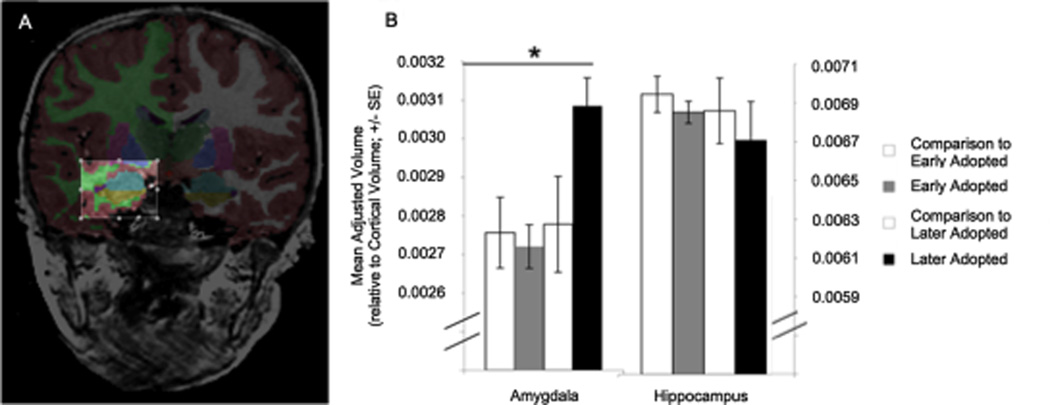
Our structural MRI results revealed larger amygdala volumes for children adopted from institutions abroad, specifically for those adopted at ages older than 15 months (see Figure #5). No differences were observed in the hippocampus, however. Given that several years have passed between the offset of early institutionalization and when children visit the lab for an MRI, it is possible that hippocampal volumes have recovered from any stress effects, while their amygdalae have not. These findings would parallel animal studies showing that changes in the hippocampus and amygdala following termination of stress often result in recovery of the hippocampus but not the amygdala (Vyas et al., 2004).
Fig. 5. Structural changes in limbic structures with early adversity.
(A) Anatomical segmentation of the amygdala. (B) Children institutionalized for more than 15 months had larger amygdala volumes than those institutionalized for less than 15 months, or control children. No differences in hippocampal volume were observed between groups. Adapted from Tottenham et al., 2010.
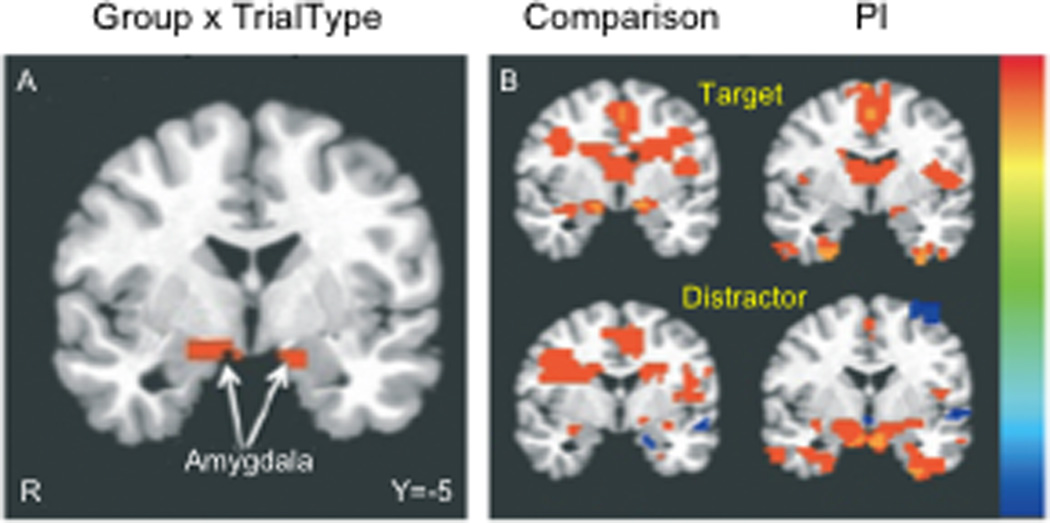
Most striking of the imaging findings were those from the functional imaging experiment. Specifically, children reared in orphanges showed elevated amygdala activity to emotional distractors, relative to children reared with their biological families (see Figure 6). The enhanced amygdala activity in the adopted children may suggest that they were less able to suppress irrelevant emotional information relative to the comparison group when performing the task. Examination of prefrontal regions involved in modulating the amygdala (Phelps et al., 2004, Quirk and Beer, 2006) showed atypical activity. Unlike the comparison children, the adopted children showed little to no change in prefrontal regions. In healthy populations, the amygdala and ventromedial prefrontal cortex showed inverse patterns of activity during performance of such tasks (Phelps et al., 2004, Hare et al., 2008), which might be mediated by the integrity of the white matter tracts between them (Kim and Whalen, 2009). Populations with anxiety and stress related disorders show less inverse coupling between theses two regions (Shin et al., 2006, Marsh et al., 2008). Therefore the findings are consistent with less top down prefrontal control of amygdala related fear responses in the children raised in the orphanage, which is supported by reports of reduced white matter between these regions in an independent sample of previously institutionalized children (Govindan et al., 2009).
Fig. 6. Frontoamygdala activity to distracting emotional stimuli with early adversity.
(A) Previously institutionalized (PI) children exhibited greater amygdala activity in response to emotional distractors than their typically reared counterparts, suggesting an inability to suppress emotionally laden irrelevant information. (B) Post-hoc t-tests of activity vs. baseline. Adapted from Tottenham et al., 2011.
Conclusion
In this review, we described three sets of experiments showing differential responses to acute threat and chronic stress across development. In the first, set of studies we illustrated developmental differences in the regulation of the fear response to potential threat in both mice and humans. Specifically, we showed that adolescents have diminished ability to suppress fear responses when the threat is no longer present. Second, we described parallel human and mouse genetic experiments that showed striking individual variability in response to threat and the underlying neural circuitry as a function of genetic factors. Finally, we provided evidence for environmental factors such as the early life stress of institutional rearing on the fear response and underlying neural circuitry. Each of these approaches alone provides limited information on developmental, genetic and environmental factors that influence the impact of stress on behavior and later outcomes. Taken together, the findings indicate that increased risk for anxiety and stress related disorders in adolescence may be associated with different developmental trajectories of subcortical emotional systems relative to cortical control regions involved in suppressing emotional responses. This differential development can lead to an imbalance in control by subcortical regions over prefrontal ones leading to heightened emotional reactivity. Although elevated emotional reactivity appears to be a typical part of development during the period of adolescence, failure to suppress that emotional reactivity over time seems to be associated more with individual differences in, or symptoms of, anxiety. Both environmental (e.g., early institutional experience) and genetic (BDNF Val66Met polymorphism) factors can exacerbate the imbalance between limbic and cortical regions resulting is dysregulation of limbic circuitry and sustained rather than transient emotional responses to cues of threat.
Our findings suggest that it is sustainment of the emotional response that leads to anxiogenic feelings and possible risk for anxiety disorders. Important future directions will be to consider how genetic, environmental and developmental factors inter-relate in sufficiently large human samples or in mouse models to directly test these effects from a developmental perspective. Such genetic studies will need to entertain dynamic models that capture the effects of changing environmental and developmental demands on the organism.
Highlights.
The effects that stress has on brain and behavior in humans and rodents
Developmental changes in frontolimbic function during the transition into and out of adolescence
Genetic factors that may enhance susceptibility to stress in one individual over another
We examine how the timing and nature of stress varies in its impact on brain and behavior
Acknowledgments
This work was supported in part by NIMH 1R01 MH73175, NIDA R01 DA018879, NIMH P50 MH62196, the Mortimer D. Sackler family, the Dewitt-Wallace fund, and by the Weill Cornell Medical College Citigroup Biomedical Imaging Center and Imaging Core.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- BDNFmet
methionine in codon 66 of the BDNF protein
- fMRI
functional magnetic resonance imaging
- MRI
magnetic resonance imaging
- Met
methionine
- P
postnatal day
- PI
previously institutionalized
- SCR
skin conductance response
- Val66met
valine-to-methionine substitution at codon 66
- Val
valine
- vmPFC
ventromedial prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Matthew Malter Cohen, Email: mhm2002@med.cornell.edu.
Nim Tottenham, Email: nimtottenham@ucla.edu.
References
- Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex. 2006;16:1759–1765. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- APA. 2010 [Google Scholar]
- Arnsten AF. Development of the cerebral cortex: XIV. Stress impairs prefrontal cortical function. J Am Acad Child Adolesc Psychiatry. 1999;38:220–222. doi: 10.1097/00004583-199902000-00024. [DOI] [PubMed] [Google Scholar]
- Bland ST, Schmid MJ, Watkins LR, Maier SF. Prefrontal cortex serotonin, stress, and morphine-induced nucleus accumbens dopamine. Neuroreport. 2004;15:2637–2641. doi: 10.1097/00001756-200412030-00016. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: the role of threat appraisal and fear learning. Depress Anxiety. 2011;28:5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Tassinary LG, Berntson GG. Handbook of psychophysiology. Cambridge England ; New York: Cambridge University Press; 2007. [Google Scholar]
- Casey BJ, Glatt CE, Tottenham N, Soliman F, Bath K, Amso D, Altemus M, Pattwell S, Jones R, Levita L, McEwen B, Magarinos AM, Gunnar M, Thomas KM, Mezey J, Clark AG, Hempstead BL, Lee FS. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience. 2009;164:108–120. doi: 10.1016/j.neuroscience.2009.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Soliman F, Bath KG, Glatt CE. Imaging genetics and development: challenges and promises. Hum Brain Mapp. 2010;31:838–851. doi: 10.1002/hbm.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvert E, Rutter M, Beckett C, Castle J, Groothues C, Hawkins A, Kreppner J, O'Connor TG, Stevens S, Sonuga-Barke EJ. Emotional difficulties in early adolescence following severe early deprivation: findings from the English and Romanian adoptees study. Dev Psychopathol. 2008;20:547–567. doi: 10.1017/S0954579408000278. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr. 2007;12:200–206. doi: 10.1017/s1092852900020915. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Garrido P, de Blas M, Mora F. Stress, prefrontal cortex and environmental enrichment: studies on dopamine and acetylcholine release and working memory performance in rats. Behav Brain Res. 2007;176:267–273. doi: 10.1016/j.bbr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Fere C. Note on changes in electrical resistance under the effect of sensory stimulation and emotion. Comptes rendus des seances de la societe de biologie. 1888;5:217–219. [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with Tract-Based Spatial Statistics (TBSS) Cereb Cortex. 2009;20:561–569. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, Orr SP, Kikinis R, Jolesz FA, McCarley RW, Pitman RK. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJ, Leibenluft E, Fox NA, Ernst M, Pine DS, Nelson EE. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008a;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009;80:1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008b;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci. 2002;22:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juffer F, van Ijzendoorn MH. Behavior problems and mental health referrals of international adoptees: a meta-analysis. JAMA. 2005;293:2501–2515. doi: 10.1001/jama.293.20.2501. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Mori Y. Behavioural and neurochemical consequences of early weaning in rodents. J Neuroendocrinol. 2009;21:427–431. doi: 10.1111/j.1365-2826.2009.01837.x. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry. 2003;60:709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, Grillon C, Leibenluft E, Lissek S, Norcross M, Shiffrin N, Pine DS. Distinct neural signatures of threat learning in adolescents and adults. Proc Natl Acad Sci U S A. 2011;108:4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, Jenness J, Ernst M, Grillon C, Pine DS. Fear conditioning in adolescents with anxiety disorders: results from a novel experimental paradigm. J Am Acad Child Adolesc Psychiatry. 2008;47:94–102. doi: 10.1097/chi.0b01e31815a5f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, McDowell DJ, Dvir S, Bradford DE, Geraci M, Pine DS, Grillon C. Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behav Res Ther. 2009;47:111–118. doi: 10.1016/j.brat.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006a;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006b;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M. Stress reverses plasticity in the pathway projecting from the ventromedial prefrontal cortex to the basolateral amygdala. Eur J Neurosci. 2006;24:2917–2922. doi: 10.1111/j.1460-9568.2006.05169.x. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJ. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley GA, Kenmuir CL, Yocom AM, Wellman JA, Biada JM. A role for prefrontal cortex in the extinction of a conditioned taste aversion. Brain Res. 2005;1051:176–182. doi: 10.1016/j.brainres.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Jackson M. Effect of stress on prefrontal cortex function. Neurotox Res. 2004;6:73–78. doi: 10.1007/BF03033299. [DOI] [PubMed] [Google Scholar]
- Monk CS, Grillon C, Baas JM, McClure EB, Nelson EE, Zarahn E, Charney DS, Ernst M, Pine DS. A neuroimaging method for the study of threat in adolescents. Developmental Psychobiology. 2003a;43:359–366. doi: 10.1002/dev.10146. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluff E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003b;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Contribution of ventrolateral prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Neurobiol Learn Mem. 1999;72:244–251. doi: 10.1006/nlme.1999.3907. [DOI] [PubMed] [Google Scholar]
- Nair HP, Berndt JD, Barrett D, Gonzalez-Lima F. Metabolic mapping of brain regions associated with behavioral extinction in preweanling rats. Brain Res. 2001;903:141–153. doi: 10.1016/s0006-8993(01)02469-6. [DOI] [PubMed] [Google Scholar]
- Newman DL, Moffitt TE, Caspi A, Magdol L, Silva PA, Stanton WR. Psychiatric disorder in a birth cohort of young adults: prevalence, comorbidity, clinical significance, and new case incidence from ages 11 to 21. J Consult Clin Psychol. 1996;64:552–562. [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, Soliman F, Glatt CE, Casey BJ, Ninan I, Lee FS. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pine DS. Integrating research on development and fear learning: a vision for clinical neuroscience? Depress Anxiety. 2009;26:775–779. doi: 10.1002/da.20595. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Pollack MH, Otto MW, Sabatino S, Majcher D, Worthington JJ, McArdle ET, Rosenbaum JF. Relationship of childhood anxiety to adult panic disorder: correlates and influence on course. Am J Psychiatry. 1996;153:376–381. doi: 10.1176/ajp.153.3.376. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: Convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16 doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Ann N Y Acad Sci. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences, USA. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. J Neurosci. 2007;27:3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Van Kempen T, Lee FS, Casey BJ. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:1–6. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. The Behavioral Neuroscience of Adolescence. 2010 [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI-Manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1988. [Google Scholar]
- Tottenham N, Hare T, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated Amygdala Response to Faces Following Early Deprivation. Developmental Science. doi: 10.1111/j.1467-7687.2010.00971.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM, Thomas KM, Freed PJ, Booma ES, Gunnar MR, Altemus M, Aronson J, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2009;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM, Thomas KM, Freed PJ, Booma ES, Gunnar MR, Altemus M, Aronson J, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vyas A, Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav Neurosci. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Waters AM, Henry J, Neumann DL. Aversive Pavlovian conditioning in childhood anxiety disorders: impaired response inhibition and resistance to extinction. J Abnorm Psychol. 2009;118:311–321. doi: 10.1037/a0015635. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, Peduto A, Gordon E. The mellow years? Neural basis of improving emotional stability with age. Journal of Neuroscience. 2006;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, Guthrie D. Institutional rearing and psychiatric disorders in Romanian preschool children. Am J Psychiatry. 2009;166:777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]