Abstract
Objective
To better understand the relationship between knee pain and bilateral knee lesions, we compared focal knee lesions in knee pairs of subjects with no, unilateral, and bilateral knee pain, and risk factors for knee osteoarthritis (OA), but no radiographic knee OA.
Materials and Methods
We examined both knees of 120 subjects from the Osteoarthritis Initiative database. We randomly selected 60 subjects aged 45–55 years with OA risk factors, no knee pain (WOMAC pain score =0) and no radiographic OA (KL-score ≤1) in both knees. We also selected two comparison groups with OA risk factors and no radiographic OA in both knees, but with knee pain (WOMAC pain score ≥5): 30 subjects with right only knee pain and 30 subjects with bilateral knee pain. All subjects underwent 3T MRI of both knees and focal knee lesions were assessed.
Results
Statistically significant associations between prevalence of focal lesions in the right and left knee with odds ratios up to 13.5 were found in all three subject groups. Focal knee lesions were generally not associated with pain in analyses comparing knee pairs of subjects with unilateral knee pain (p>0.05). The prevalence and severity of focal knee lesions were not significantly different in knee pairs of subjects with no knee pain and those with bilateral knee pain (p>0.05).
Conclusion
Focal knee lesions in the right and left knee of subjects with OA risk factors were positively associated with each other independent of knee pain status, and were not statistically significant different between knees in subjects with unilateral knee pain.
Keywords: Osteoarthritis, MRI, WORMS, WOMAC, knee pairs
Introduction
Osteoarthritis (OA) is an increasingly prevalent global health problem, with estimated 27 million US adults having clinical signs of OA [1]. OA is characterized by the progressive loss of hyaline articular cartilage, which can be evaluated by using magnetic resonance imaging (MRI) [2]. Pain secondary to osteoarthritic changes is the predominant clinical symptom [3]. The source of pain in subjects with OA is still not well understood. Subchondral bone and synovium may be responsible for nociceptive stimuli in OA and not the cartilage itself, since it does not contain nerve fibers and therefore cannot directly generate pain [4].
One of the best established measures of pain is the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index, a multi-dimensional health status instrument that quantifies pain, stiffness, and limited function of subjects with OA of the knee [5]. Previous studies found an inverse relationship between MRI-based knee cartilage volume measurements and knee pain assessed by using the WOMAC pain score [6;7]. Furthermore knee pain was associated with elevated MRI-based knee cartilage T2 relaxation times, prevalent bone marrow edema pattern, synovitis, joint effusion, meniscal tears, and denuded subchondral bone [8–12].
However, little is known about the symmetry of OA associated focal knee lesions in a subject’s two knees and whether knee pairs that differ on pain also show a difference in the degree of focal knee lesions. Since a subject’s inherent anatomy and environmental stresses are likely similar for both knees, significant associations may be hypothesized between prevalent focal knee lesions in knee pairs. The question whether (a)symmetry in knee pain between a subject’s two knees is associated with (a)symmetric prevalence of focal knee lesions has been investigated only in subjects with unilateral knee trauma and unilaterally suspected meniscal injuries [13;14]. These studies found ligamentous lesions, bone marrow edema pattern, and specific meniscal tears (radial, longitudinal, and complex) almost exclusively in the symptomatic knee, in contrast to horizontal meniscal tears and joint effusion, which were often observed in the symptomatic as well as the asymptomatic contralateral knee. Therefore it may be important to study the symmetry of focal knee lesions in knee pairs and its relationship with knee pain status in asymptomatic and symptomatic subjects in the early phase of OA, since they may most benefit from treatment or behavioural interventions.
Radiologic imaging techniques, in particular MRI, are essential to investigate this clinically important research topic. Radiologists may also face the question of (a)symmetric prevalence of focal knee lesions and knee pain status in knee pairs by referring physicians in clinical day life. In this study, we used data from the NIH Osteoarthritis Initiative (OAI), an observational multi-center cohort study with 4,796 participants, who have or are at risk for developing knee OA (http://www.oai.ucsf.edu/). The OAI database contains bilateral knee radiographs and MRIs as well as clinical data, including the WOMAC pain score [15].
The purpose of this study was to compare focal knee lesions in knee pairs of subjects with no, unilateral, and bilateral knee pain, and risk factors for knee OA, but no radiographic knee OA. We hypothesized that focal lesions would be symmetric in knee pairs of subjects with bilateral asymptomatic and bilateral symptomatic knees, while they would be more prevalent in the single symptomatic knee compared to the contralateral asymptomatic knee.
Materials and Methods
Subjects
Data used in the preparation of this article were obtained from the Osteoarthritis Initiative (OAI) database, which is available for public access at http://www.oai.ucsf.edu/. Specific OAI datasets used were baseline clinical dataset 0.2.2 and baseline imaging datasets 0.E.1 and 0.C.2.
We selected 120 subjects from the OAI incidence cohort (n=3,284) for this study. Subjects from the OAI incidence cohort did not have symptomatic knee OA, defined as having both frequent symptoms (“pain, aching, or stiffness in or around the knee” on most days of a month in the past 12 months) and radiographic OA in the same knee. However, these subjects had at least one of the following OA risk factors at baseline: knee symptoms in the past year that did not occur on most days of a month, overweight or obesity, history of knee injury, history of knee surgery, family history of total knee replacement, Heberden’s nodes, or engaging in frequent knee bending activities.
First, we identified asymptomatic subjects (WOMAC pain score of zero in both knees) aged 45–55 years from the OAI incidence cohort. The age range of 45–55 years was used to focus on younger subjects, who may most benefit from treatment or behavioural interventions. From 331 eligible subjects we identified those, who were right side dominant and had no radiographic OA (KL-score ≤1) in both knees based on an additional reading done for the present study, and randomly selected 60 of these. The sample was designed to have an equal number of men and women. There was no specific inclusion criteria based on body mass index (BMI).
Subsequently we identified two comparison groups with symptomatic subjects aged 45–55 years from the OAI incidence cohort, who had either a WOMAC pain score ≥5 in the right knee and score of zero in the left knee or a WOMAC pain score ≥5 in both knees. A WOMAC pain score threshold of 5 was used similar to previous studies [9;16]. From 49 eligible subjects with right only knee pain and 90 subjects with bilateral knee pain we identified those, who were right side dominant and had no radiographic OA (KL-score ≤1) in both knees based on an additional reading done for the present study, and randomly selected for each group 30 subjects (15 males, 15 females).
All subjects included in this study provided informed consent. The study protocol, amendments and informed consent documentation were reviewed and approved by the local institutional review boards.
WOMAC Questionnaire
The Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index is a well-established tool to evaluate clinical symptoms of OA in the knee, including pain, stiffness, and physical function over the last seven days [5]. All subjects completed the WOMAC questionnaire for the right and left knee on the day knee radiographs and MR images were acquired. They were asked five activity questions and had to provide a pain score for each activity (0 = none; 1 = mild; 2 = moderate; 3 = severe; 4 = extreme pain). Using this grading system, summed scores ranged from 0 to 20 for each knee.
Imaging
Bilateral standing posterior-anterior fixed flexion knee radiographs were acquired. Knees were positioned in a plexiglas frame (SynaFlexer, CCBR-Synarc, San Francisco, CA, USA) with 20°–30° flexion and 10° internal rotation of the feet. Bilateral knee radiographs were graded by two radiologists (R.C. and L.N. both with 4 years of experience) in consensus by using the Kellgren-Lawrence (KL) scoring system [17].
All subjects underwent 3T MRI (Trio, Siemens, Erlangen, Germany) of both knees. The following four sequences were used in this study as described in the OAI MRI protocol [15]:
a sagittal two-dimensional intermediate-weighted turbo spin echo (TSE) sequence with fat suppression (TE / TR = 30 / 3200 ms, field of view (FOV) = 16 cm, slice thickness = 3 mm, in-plane spatial resolution = 0.357 × 0.511 mm2, flip angle = 180, bandwidth = 248 Hz / pixel),
a coronal two-dimensional intermediate-weighted turbo spin-echo (TSE) sequence (TE / TR = 29 / 3850 ms, field of view (FOV) = 14 cm, slice thickness = 3 mm, in-plane spatial resolution = 0.365 × 0.456 mm2, flip angle = 180, bandwidth = 352 Hz / pixel),
a sagittal three-dimensional dual-echo steady-state (DESS) sequence with water excitation and coronal and axial reformations (TE / TR = 4.7 / 16.3 ms, field of view (FOV) = 14 cm, slice thickness = 0.7 mm, in-plane spatial resolution = 0.365 × 0.456 mm2, flip angle = 25, bandwidth = 185 Hz / pixel), and
a coronal three-dimensional T1-weighted fast low-angle shot (FLASH) sequence with water excitation (TE / TR = 7.57 / 20 ms, field of view (FOV) = 16 cm, slice thickness = 1.5 mm, in-plane spatial resolution = 0.313 × 0.313 mm2, flip angle = 12, bandwidth = 130 Hz / pixel).
WORMS Grading
MR images of both knees were transferred to picture archiving communication system (PACS) workstations (Agfa, Ridgefield Park, NJ, USA) and assessed for the presence and grade of meniscal, cartilage, and ligamentous lesions as well as bone marrow edema pattern (BMEP) and joint effusion using a modified whole organ MRI score (WORMS) as previously described [9;18–23]. Three radiologists (R.C. with 4 years, L.N. with 4 years, and T.M.L. with 22 years of experience) analyzed the MRI studies of 30 subjects in consensus to calibrate thresholds for grading abnormalities. The MRI studies of the remaining 90 subjects were read by two radiologists (R.C. and L.N.) independently. In case of disagreement, consensus reading was performed with the third, most experienced radiologist (T.M.L.). The radiologists were blinded to the WOMAC pain scores of the subjects.
Cartilage lesions and BMEP were not assessed by using the original 15 regions, but six condensed regions (patella, trochlea, medial/lateral femur, and medial/lateral tibia) as previously reported [9;18–23].
BMEP were defined as poorly marginated areas of increased T2 signal intensity and graded using a 4-point scale: 0, none; 1, <25% of the region; 2, 25%-50% of the region; 3, >50% of the region.
Cartilage lesions were graded using an 8-point scale: 0, normal thickness and signal intensity; 1, normal thickness or swelling with abnormal signal on fluid sensitive sequences; 2, partial-thickness focal defect <1 cm in greatest width; 2.5, full-thickness focal defect <1 cm in greatest width; 3, multiple areas of partial thickness (grade 2) defects intermixed with areas of normal thickness, or a grade 2 defect wider than 1 cm but <75% of the region; 4, diffuse (>75% of the region) partial-thickness loss; 5, multiple areas of full thickness loss (grade 2.5) or a grade 2.5 lesion wider than 1 cm but <75% of the region; 6, diffuse (>75% of the region) full-thickness loss. Condensing the anatomical regions from 15 to 6 would have potentially affected the frequency of grade 4 and 6 lesions. However, grade 4 lesions are very rare and usually if there is >75% partial thickness cartilage loss, full thickness lesions are present and grade 6 lesions are not expected in this cohort with KL-scores ≤1.
Meniscal lesions were graded separately in six regions (medial/lateral and anterior/body/posterior) using the following 5-point scale: 0, normal; 1, intra-substance abnormal signal; 2, non-displaced tear; 3, displaced or complex tear; 4, complete destruction/maceration. Compared to the original WORMS system, grade 1 was added to better reflect presence of early degenerative meniscal disease.
ACL (anterior cruciate ligament), PCL (posterior cruciate ligament), MCL (medial collateral ligament), LCL (lateral collateral ligament), patellar tendon and popliteal tendon were evaluated using a 4-point scale: 0, no lesion; 1, signal changes around the ligament; 2, partial tear; 3, complete tear.
Joint effusion was graded using a 4-point scale: 0, normal; 1, <33% of maximum potential distention; 2, 33–66% of maximum potential distention; 3, >66% of maximum potential distention).
A WORMS maximum score (WORMS Max) was assigned to each knee for each joint structure by the greatest WORMS score in any compartment similar to previous studies [9;18–23]. WORMS Max was used to express the severity of focal knee lesions. WORMS Max >0 in any joint structure was defined as presence of a lesion. A meniscal WORMS Max >1 indicated a non-displaced tear or worse, while a cartilage WORMS Max >1 identified subjects with at least one partial thickness defect. Cartilage WORMS Max >1 was also used to exclude lesions characterized only by signal abnormalities, i.e. grade 1 lesions.
The WORMS grading system semi-quantitatively defines focal knee lesions with a relatively sensitive threshold, in particular for meniscal and cartilage lesions (WORMS grade 1). It has been controversially discussed whether these MRI findings are clinically relevant and prognostic of further tissue degeneration. We used this methodological definition of focal knee lesions in our study, since longitudinal studies underlined the relevance of these early degenerative changes with respect to the development of OA [19;23;24].
Statistical Analysis
The statistical analyses were performed with SPSS software using a two-sided 0.05 level of significance. Pearson chi-square test and ANOVA were used to compare frequencies of OA risk factors, age, BMI, and WOMAC pain scores between the three subject groups. The association of prevalent focal knee lesions in the right and left knee in each group was expressed as odds ratio and corresponding 95% confidence interval (CI). McNemar’s test was used to evaluate whether the two marginal frequencies of focal knee lesions in knee pairs, i.e. the prevalence of right only and left only focal knee lesions differed significantly in each of the three groups. Severity of bilaterally prevalent focal knee lesions in knee pairs was compared by using Wilcoxon signed-rank tests in each group.
Reproducibility
To assess intra- and inter-reader reproducibility of the WORMS grading, 24 knees were randomly selected and WORMS grading was performed two times by two readers (R.C. and L.N.) independently. Intra-class correlation coefficients (ICC) were calculated to compare WORMS Max for meniscal and cartilage lesions and BMEP. Reproducibility for ligamentous lesions and joint effusion was not performed due to their low prevalence in the study population.
An intra-reader (inter-reader) reproducibility for meniscal WORMS Max of 0.93 and 0.93 (0.96) was calculated, for cartilage WORMS Max of 0.97 and 0.96 (0.98), and for BMEP WORMS Max of 0.96 and 0.98 (0.97).
Results
Mean ± standard deviation (SD) of age, BMI, and WOMAC pain scores as well as frequencies of gender and OA risk factors are listed for each group in Table 1.
Table 1.
Characteristics of subjects without knee pain (A), with right knee pain (B), and with bilateral knee pain (C). Age, BMI, and WOMAC pain scores are displayed as mean ± SD. Frequencies of gender and OA risk factors are given in absolute numbers and on percentage basis.
| A: Subjects without knee pain (n=60) | B: Subjects with right knee pain (n=30) | C: Subjects with bilateral knee pain (n=30) | |
|---|---|---|---|
| male | 30 (50%) | 15 (50%) | 15 (50%) |
| age [years] | 50.8±3.0 | 50.3±2.9 | 51.5±2.9 |
| BMI [kg/m²] | 27.6±3.6 | 28.8±4.8 | 28.3±4.8 |
| WOMAC pain score right knee | 0.0±0.0* | 6.8±2.2* | 8.6±2.5* |
| WOMAC pain score left knee | 0.0±0.0* | 0.0±0.0* | 6.8±3.1* |
| any pain, aching, or stiffness in or around either knee in the past 12 months | 53 (88.3%)* | 30 (100%)* | 30 (100%)* |
| history of knee injury in either knee | 32 (53.3%) | 16 (53.3%) | 13 (43.4%) |
| history of knee surgery in either knee | 9 (15.0%) | 6 (20.0%) | 8 (26.7%) |
| family history of total knee replacement | 10 (17.2%)* | 8 (27.6%)* | 0 (0%)* |
| Heberden‘s nodes | 13 (21.7%) | 4 (13.3%) | 5 (16.7%) |
| frequent knee bending activities | 45 (47.4%) | 24 (25.3%) | 26 (27.4%) |
indicates statistically significant differences between the subject groups (p<0.05).
Comparison of ligamentous lesions and joint effusion in knee pairs was limited due to their low prevalence. The prevalence of ligamentous lesions in both knees combined ranged from 1.7% in subjects with no and bilateral knee pain to 10.0% in subjects with right knee pain. Joint effusion in both knees combined was diagnosed in 0 (0.0%), 1 (1.7%), and 3 (5.0%) subjects with no, right, and bilateral knee pain, respectively.
Subjects with right knee pain showed odds ratios of 13.5 (2.0–93.2) and 6.4 (1.0–40.3) for the association of prevalent meniscal lesions (WORMS Max >0) and meniscal tears (WORMS Max >1) in the right and left knee (Table 2 and 3). The association of prevalent cartilage lesions and BMEP in the right and left knee of subjects with right knee pain was not statistically significant (p>0.05; Table 2 and 3). The calculated odds ratios for the association of prevalent meniscal and cartilage lesions as well as BMEP in the right and left knee in subjects without knee pain were statistically significant with odds ratios up to 7.5 (Table 2 and 3). In subjects with bilateral knee pain, only the association of grade 2 or higher cartilage lesions (WORMS Max >1) and BMEP in the right and left knee were statistically significant with an odds ratio (95% CI) of 11.2 (1.7–72.3) and 11.0 (2.0–60.6), respectively (Table 2 and 3).
Table 2.
Frequencies of focal knee lesions WORMS Max >0 (none, right only, left only, and bilateral) in knee pairs of subjects with no, right, and bilateral knee pain. Frequencies are given in absolute numbers and on percentage basis.
| Subjects with right knee pain (n=30) | left WORMS Max =0 | left WORMS Max >0 | p-value * | Odds ratio (95% CI) for association of findings in right and left knees ** | |
|---|---|---|---|---|---|
|
|
|||||
| Meniscus | right WORMS Max =0 | 6 (20.0%) | 2 (6.7%) | 0.687 | 13.5 (2.0–93.2) |
| right WORMS Max >0 | 4 (13.3%) | 18 (60.0%) | |||
| Cartilage | right WORMS Max =0 | 2 (6.7%) | 0 (0.0%) | 0.500 | NA *** |
| right WORMS Max >0 | 2 (6.7%) | 26 (86.6%) | |||
| BMEP | right WORMS Max =0 | 9 (30.0%) | 4 (13.3%) | 0.754 | 4.1 (0.9–19.3) |
| right WORMS Max >0 | 6 (20.0%) | 11 (36.7%) | |||
|
|
|||||
| Subjects without knee pain (n=60) | |||||
| Meniscus | right WORMS Max =0 | 8 (13.3%) | 8 (13.3%) | 0.815 | 3.4 (1.0–11.4) |
| right WORMS Max >0 | 10 (16.7%) | 34 (56.7%) | |||
| Cartilage | right WORMS Max =0 | 5 (8.3%) | 13 (21.7%) | 0.021 | 5.0 (1.0–23.9) |
| right WORMS Max >0 | 3 (5.0%) | 39 (65.0%) | |||
| BMEP | right WORMS Max =0 | 21 (35.0%) | 13 (21.7%) | 0.523 | 3.1 (1.1–8.8) |
| right WORMS Max >0 | 9 (15.0%) | 17 (28.3%) | |||
|
|
|||||
| Subjects with bilateral knee pain (n=30) | |||||
| Meniscus | right WORMS Max =0 | 6 (20.0%) | 8 (26.7%) | 0.388 | 2.3 (0.5–10.6) |
| right WORMS Max >0 | 4 (13.3%) | 12 (40.0%) | |||
| Cartilage | right WORMS Max =0 | 0 (0.0%) | 1 (3.3%) | 0.219 | NA *** |
| right WORMS Max >0 | 5 (16.7%) | 24 (80.0%) | |||
| BMEP | right WORMS Max =0 | 12 (40.0%) | 3 (10.0%) | 1.000 | 11.0 (2.0–60.6) |
| right WORMS Max >0 | 4 (13.3%) | 11 (36.7%) | |||
P-values are calculated using McNemar’s test to evaluate whether the two marginal frequencies of imaging findings in knee pairs, i.e. the prevalence of right only and left only focal knee lesions, differ significantly.
Odds ratios and 95% confidence intervals (CI) express the association of prevalent focal knee lesions in the right and left knee.
Odd ratio cannot be calculated due to zero cell.
P-values of McNemar’s test and odds ratios with statistical significance (p<0.05) are printed in bold.
Table 3.
Frequencies of focal knee lesions WORMS Max >1 (none, right only, left only, and bilateral) in knee pairs of subjects with no, right only, and bilateral knee pain. Frequencies are given in absolute numbers and on percentage basis.
| Subjects with right knee pain (n=30) | left WORMS Max ≤ 1 | left WORMS Max >1 | p-value * | Odds ratio (95% CI) for association of findings in right and left knees ** | |
|---|---|---|---|---|---|
|
|
|||||
| Meniscus | right WORMS Max ≤1 | 15 (50.0%) | 2 (6.7%) | 0.180 | 6.4 (1.0–40.3) |
| right WORMS Max >1 | 7 (23.3%) | 6 (20.0%) | |||
| Cartilage | right WORMS Max ≤1 | 6 (20.0%) | 2 (6.7%) | 0.109 | 5.3 (0.9–32.4) |
| right WORMS Max >1 | 8 (26.7%) | 14 (46.6%) | |||
|
|
|||||
| Subjects without knee pain (n=60) | |||||
| Meniscus | right WORMS Max ≤1 | 29 (48.3%) | 7 (11.7%) | 0.629 | 5.8 (1.8–18.5) |
| right WORMS Max >1 | 10 (16.7%) | 14 (23.3%) | |||
| Cartilage | right WORMS Max ≥1 | 20 (33.4%) | 8 (13.3%) | 1.000 | 7.5 (2.4–23.6) |
| right WORMS Max >1 | 8 (13.3%) | 24 (40.0%) | |||
|
|
|||||
| Subjects with bilateral knee pain (n=30) | |||||
| Meniscus | right WORMS Max ≤1 | 17 (56.6%) | 5 (16.7%) | 0.727 | 5.7 (0.9–32.4) |
| right WORMS Max >1 | 3 (10.0%) | 5 (16.7%) | |||
| Cartilage | right WORMS Max ≤1 | 7 (23.3%) | 5 (16.7%) | 0.453 | 11.2 (1.7–72.3) |
| right WORMS Max >1 | 2 (6.7%) | 16 (53.3%) | |||
P-values are calculated using McNemar’s test to evaluate whether the two marginal frequencies of imaging findings in knee pairs, i.e. the prevalence of right only and left only focal knee lesions, differ significantly.
Odds ratios and 95% confidence intervals (CI) express the association of prevalent focal knee lesions in the right and left knee.
P-values of McNemar’s test and odds ratios with statistical significance (p<0.05) are printed in bold.
Subjects with no, right, and bilateral knee pain had in the majority either a bilateral absence or prevalence of focal knee lesions (Table 2 and 3; Figure 1 and 2). Differences in frequencies of right only and left only focal knee lesions (WORMS Max >0 and WORMS Max >1, respectively) in knee pairs of each group were not statistically significant (p>0.05; Table 2 and 3) with one exception: the frequency of left only compared to right only cartilage lesions WORMS Max >0) was significantly higher in subjects without knee pain (p=0.021; Table 3).
Figure 1.
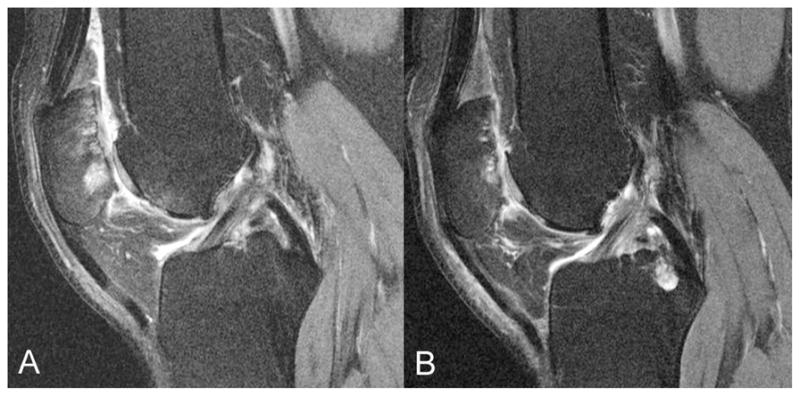
Representative images of an asymptomatic subject with bilateral similar, grade 5 cartilage lesions at the femoro-patellar joint and additional BMEP (A: right knee, B: left knee).
Figure 2.
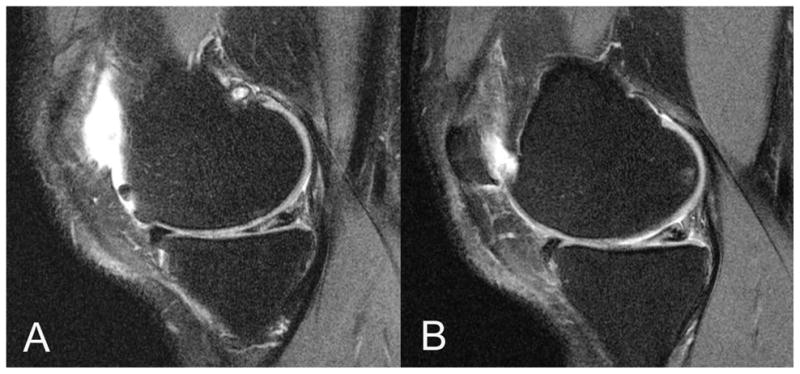
Representative images of an asymptomatic subject with bilateral meniscal grade 2 lesions in the medial posterior horn, which appear similar in appearance and location (A: right knee, B: left knee).
The number of subjects with bilaterally prevalent focal knee lesions and the respective percentage of subjects with higher, lower, and equal severity of focal knee lesions in the right compared to the left knee are listed for each group in Table 4. Severity of bilaterally prevalent meniscal lesions and BMEP in knee pairs was not significantly different in each subject group (p>0.05; Table 4). While severity of bilaterally prevalent cartilage lesions was significantly higher in the right knee of subjects without knee pain (p=0.041; Table 4), similar grades of cartilage lesions were found in subjects with no and bilateral knee pain (p>0.05; Table 4).
Table 4.
Comparison of severity of focal knee lesions (WORMS Max) in knee pairs of subjects with no, right, and bilateral knee pain. Frequencies of subjects with higher, lower, and equal severity of focal knee lesions in the right compared to the left knee are given in absolute numbers and on percentage basis. Severity of bilaterally prevalent focal knee lesions are compared by using Wilcoxon signed-rank tests and displayed as mean ± SD (p-value). P-values indicating statistical significance (p<0.05) are printed in bold.
| Subjects with right knee pain (n=30) | Subjects without knee pain (n=60) | Subjects with bilateral knee pain (n=30) | |
|---|---|---|---|
| Meniscus bilateral WORMS Max >0: | 18 | 34 | 12 |
| right WORMS Max > left WORMS Max | 5 (27.8%) | 10 (29.4%) | 2 (16.7%) |
| right WORMS Max < left WORMS Max | 1 (5.6%) | 8 (23.5%) | 3 (25.0%) |
| right WORMS Max = left WORMS Max | 12 (66.6%) | 16 (47.1%) | 7 (58.3%) |
| mean ± SD right vs. left WORMS Max | 2.2±1.3 vs. 1.9±1.3 (p=0.290) | 2.0±1.0 vs. 1.8±1.0 (p=0.474) | 2.1±1.3 vs. 1.9±1.0 (p=0.680) |
|
| |||
| Cartilage bilateral WORMS Max >0: | 26 | 39 | 24 |
| right WORMS Max > left WORMS Max | 12 (46.2%) | 14 (35.9%) | 5 (20.8%) |
| right WORMS Max < left WORMS Max | 3 (11.5%) | 7 (17.9%) | 4 (16.7%) |
| right WORMS Max = left WORMS Max | 11 (42.3%) | 18 (46.2%) | 15 (62.5%) |
| mean ± SD right vs. left WORMS Max | 2.7±1.2 vs. 2.2±1.2 (p=0.057) | 2.7±1.3 vs. 2.3±1.0 (p=0.041) | 2.5±1.2 vs. 2.5±0.9 (p=0.857) |
|
| |||
| BMEP bilateral WORMS Max >0: | 11 | 17 | 11 |
| right WORMS Max > left WORMS Max | 1 (9.1%) | 3 (17.6%) | 5 (45.5%) |
| right WORMS Max < left WORMS Max | 2 (18.2%) | 3 (17.6%) | 1 (9.0%) |
| right WORMS Max = left WORMS Max | 8 (72.7%) | 11 (64.8%) | 5 (45.5%) |
| mean ± SD right vs. left WORMS Max | 1.8±0.8 vs. 1.9±0.5 (p=0.564) | 1.9±0.5 vs. 1.9±0.5 (p=1.000) | 2.3±0.5 vs. 1.8±0.6 (p=0.096) |
Discussion
Focal knee lesions in the right and left knee of subjects with OA risk factors were positively associated with each other independent of knee pain status. Differences in prevalence and severity of focal knee lesions in knee pairs were not statistically significant not only in subjects with symmetric, but also with asymmetric knee pain status.
The predominant clinical symptom in most knee OA patients is pain [3]. Therefore the pathogenesis of OA related knee pain is an important research topic. Previous studies reported a positive association of prevalence and severity of focal knee lesions with knee pain in subjects with symptomatic and radiographic knee OA [10–12]. We recently investigated the association of focal knee lesions and knee pain status in right knees of subjects without radiographic OA, but with OA risk factors [9]. We demonstrated that only the prevalence of cartilage lesions was associated with knee pain status. In the present study, we compared prevalence and severity of focal knee lesions in knee pairs of subjects with no, right, and bilateral knee pain. We hypothesized that focal lesions would not differ between right and left knees in subjects with bilateral asymptomatic and bilateral symptomatic knees, while focal lesions would differ between knees, and be more common in the symptomatic knee of subjects with one painful and one non-painful knee. However, focal knee lesions were generally not associated with pain in analyses comparing knee pairs of subjects with unilateral knee pain. Furthermore focal knee lesions in the right and left knee of subjects with OA risk factors were positively associated with each other independent of knee pain status.
These findings suggest that there may be an inherent individual propensity to develop focal knee lesions in both knees, which may not necessarily be symptomatic. This seems logical as an individual’s inherent anatomy and environmental stresses are likely similar for both knees. A possible genetic predisposition in developing OA has been postulated previously [25–30]. A twin study, which looked at the prevalence of hand and knee OA found an increased correlation of radiographic OA in monozygotic twins as compared with dizygotic twins, and estimated the genetic influence of radiographic OA in the knee and hand to be between 39% and 65% [29]. Another study that looked at the incidence of hip OA in twins, estimated a heritability of radiographic hip OA among women to be approximately 58% [28].
A small number of studies has previously examined the bilateral prevalence of focal knee lesions in subjects with unilateral symptoms [13;14]. These studies looked at patients with suspected meniscal injuries or post-traumatic abnormalities. Boks et al. studied subjects with unilateral knee trauma and found specific meniscal tears and joint effusion in the symptomatic as well as the asymptomatic contralateral knee [13]. Zanetti et al. reported that subjects with a meniscal tear on the symptomatic side, also had a meniscal tear on the contralateral asymptomatic side in 63% [14]. Therefore they concluded that in particular meniscal lesions appear symmetrically and may not always be related to symptoms, which is consistent with the findings in our study. It remains unclear why knee pairs of subjects with unilateral knee pain showed no statistically significant differences in prevalence and severity of focal knee lesions. Possible reasons may be the selected inclusion criteria, the obtained sample size, or the possibility that differences between symptomatic and asymptomatic focal knee lesion are too subtle to be detected by current MRI analyses.
This study underlines once more the importance of MRI and the central role of radiology to answer clinically important research questions related to knee OA. Radiologists may also face the question of (a)symmetric prevalence of focal knee lesions and knee pain status in knee pairs by referring physicians in clinical day life. Therefore this study may have aspects useful in diagnostic imaging practice.
Our study had some limitations. Firstly, the subject groups with unilateral and bilateral knee pain were relatively small (n=30) due to the available subjects in the OAI database based on our inclusion criteria. Thus, the statistical analyses are limited by the sample size of subjects with unilateral and bilateral knee pain. Our conclusions have to be carefully considered with respect to this limitation. In particular, our conclusion that MRI findings did not differ between subject’s two knees in those with unilateral knee pain may reflect low power for detecting associations in the paired knee analyses. Future studies are needed to investigate the full significance of our findings by using in particular a greater sample size of unilaterally symptomatic subjects. Secondly, reproducibility of WORMS grading is critical. However, we found acceptable intra-reader (inter-reader) reproducibility errors ranging from 0.93 to 0.98 (0.96 to 0.98). Lastly, we used the WOMAC pain score, which is a reliable tool to evaluate OA related knee pain [5]. However, the WOMAC questionnaire focuses only on the last seven days and is not a long-term evaluation tool. Knee OA pain is known to fluctuate over days, weeks and months so knees may be classified differently on pain status at different time points, which could bias associations towards the null. In general, subjects in the OAI had MRI scans the same day on which they completed the WOMAC pain score. Since the WOMAC questionnaire has been used in previous studies with similar purposes [6–12], we based our inclusion criteria regarding knee pain on this questionnaire.
In conclusion, focal knee lesions in the right and left knee of subjects with OA risk factors were positively associated with each other independent of knee pain status, suggesting an inherent individual propensity to develop focal knee lesions in both knees, which may be not necessarily symptomatic.
Acknowledgments
This study was supported by NIH U01AR059507-01 and P50 (project 3, AR060752-01). The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Footnotes
Conflict of Interest
All authors state no financial disclosure and no conflict of interest.
ClinicalTrials.gov identifier: NCT00080171
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Renu Chundru, Email: renu.chundru@ucsf.edu, Musculoskeletal and Quantitative Imaging Research Group, Department of Radiology and Biomedical Imaging, University of California San Francisco, 185 Berry Street, Suite 350, San Francisco, CA 94107, USA, Phone: +1 415 353 9436; Fax: +1 415 353 9425.
Thomas Baum, Email: thbaum@gmx.de, Musculoskeletal and Quantitative Imaging Research Group, Department of Radiology and Biomedical Imaging, University of California San Francisco, 185 Berry Street, Suite 350, San Francisco, CA 94107, USA, Phone: +1 415 353 9436; Fax: +1 415 353 9425;.
Lorenzo Nardo, Email: lorenzo.nardo@ucsf.edu, Musculoskeletal and Quantitative Imaging Research Group, Department of Radiology and Biomedical Imaging, University of California San Francisco, 185 Berry Street, Suite 350, San Francisco, CA 94107, USA, Phone: +1 415 353 9436; Fax: +1 415 353 9425;.
Michael C. Nevitt, Email: MNevitt@psg.ucsf.edu, Department of Epidemiology and Biostatistics, University of California San Francisco, 185 Berry Street, Suite 5700, San Francisco, CA 94107, USA, Phone: +1 415 514 8048; Fax: +1 415 514 8150;.
John Lynch, Email: JLynch@psg.ucsf.edu, Department of Epidemiology and Biostatistics, University of California San Francisco, 185 Berry Street, Suite 5700, San Francisco, CA 94107, USA, Phone: +1 415 514 8092; Fax: +1 415 514 8150;.
Charles E. McCulloch, Email: CMcCulloch@epi.ucsf.edu, Department of Epidemiology and Biostatistics, University of California San Francisco, 185 Berry Street, Suite 5700, San Francisco, CA 94107, USA, Phone: +1 415 514 8027; Fax: +1 415 514 8150;.
Thomas M. Link, Email: tmlink@radiology.ucsf.edu, Musculoskeletal and Quantitative Imaging Research Group, Department of Radiology and Biomedical Imaging, University of California San Francisco, 185 Berry Street, Suite 350, San Francisco, CA 94107, USA, Phone: +1 415 353 2450; Fax: +1 415 476 0616;.
References
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Link TM, Steinbach LS, Ghosh S, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226(2):373–381. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 3.Hunter DJ, McDougall JJ, Keefe FJ. The symptoms of osteoarthritis and the genesis of pain. Rheum Dis Clin North Am. 2008;34(3):623–643. doi: 10.1016/j.rdc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965–973. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 6.Hunter DJ, March L, Sambrook PN. The association of cartilage volume with knee pain. Osteoarthritis Cartilage. 2003;11(10):725–729. doi: 10.1016/s1063-4584(03)00160-2. [DOI] [PubMed] [Google Scholar]
- 7.Wluka AE, Wolfe R, Stuckey S, Cicuttini FM. How does tibial cartilage volume relate to symptoms in subjects with knee osteoarthritis? Ann Rheum Dis. 2004;63(3):264–268. doi: 10.1136/ard/2003.007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker K, Grainger A, Niu J, et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann Rheum Dis. 2010;69(10):1779–1783. doi: 10.1136/ard.2009.121426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum T, Joseph GB, Arulanandan A, et al. Association of MRI-based knee cartilage T2 measurements and focal knee lesions with knee pain - data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2012;64(2):248–255. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo GH, McAlindon TE, Niu J, et al. Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2009;17(12):1562–1569. doi: 10.1016/j.joca.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moisio K, Eckstein F, Chmiel JS, et al. Denuded subchondral bone and knee pain in persons with knee osteoarthritis. Arthritis Rheum. 2009;60(12):3703–3710. doi: 10.1002/art.25014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres L, Dunlop DD, Peterfy C, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14(10):1033–1040. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Boks SS, Vroegindeweij D, Koes BW, Hunink MM, Bierma-Zeinstra SM. Magnetic resonance imaging abnormalities in symptomatic and contralateral knees: prevalence and associations with traumatic history in general practice. Am J Sports Med. 2006;34(12):1984–1991. doi: 10.1177/0363546506290189. [DOI] [PubMed] [Google Scholar]
- 14.Zanetti M, Pfirrmann CW, Schmid MR, Romero J, Seifert B, Hodler J. Patients with suspected meniscal tears: prevalence of abnormalities seen on MRI of 100 symptomatic and 100 contralateral asymptomatic knees. AJR Am J Roentgenol. 2003;181(3):635–641. doi: 10.2214/ajr.181.3.1810635. [DOI] [PubMed] [Google Scholar]
- 15.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goggins J, Baker K, Felson D. What WOMAC pain score should make a patient eligible for a trial in knee osteoarthritis? J Rheumatol. 2005;32(3):540–542. [PubMed] [Google Scholar]
- 17.Kellgren J, Lawrence J. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baum T, Stehling C, Joseph GB, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: Data from the osteoarthritis initiative. J Magn Reson Imaging. 2012;35(2):370–378. doi: 10.1002/jmri.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baum T, Joseph GB, Nardo L, et al. Correlation of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with body mass index: Thirty-six-month followup data from a longitudinal, observational multicenter study. Arthritis Care Res (Hoboken) 2013;65(1):23–33. doi: 10.1002/acr.21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan J, Pialat JB, Joseph T, et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology. 2011;261(2):507–515. doi: 10.1148/radiol.11102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stehling C, Liebl H, Krug R, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3. 0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010;254(2):509–520. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stehling C, Souza RB, Hellio Le Graverand MP, et al. Loading of the knee during 3. 0T MRI is associated with significantly increased medial meniscus extrusion in mild and moderate osteoarthritis. Eur J Radiol. 2012;81(8):1839–1845. doi: 10.1016/j.ejrad.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laberge MA, Baum T, Virayavanich W, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects--data from the Osteoarthritis Initiative. Skeletal Radiol. 2012;41(6):633–641. doi: 10.1007/s00256-011-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph GB, Baum T, Alizai H, et al. Baseline mean and heterogeneity of MR cartilage T(2) are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3years - data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012;20(7):727–735. doi: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding C, Cicuttini F, Scott F, Stankovich J, Cooley H, Jones G. The genetic contribution and relevance of knee cartilage defects: case-control and sib-pair studies. J Rheumatol. 2005;32(10):1937–1942. [PubMed] [Google Scholar]
- 26.Jones G, Ding C, Scott F, Cicuttini F. Genetic mechanisms of knee osteoarthritis: a population based case-control study. Ann Rheum Dis. 2004;63(10):1255–1259. doi: 10.1136/ard.2003.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loughlin J. The genetic epidemiology of human primary osteoarthritis: current status. Expert Rev Mol Med. 2005;7(9):1–12. doi: 10.1017/S1462399405009257. [DOI] [PubMed] [Google Scholar]
- 28.MacGregor AJ, Antoniades L, Matson M, Andrew T, Spector TD. The genetic contribution to radiographic hip osteoarthritis in women: results of a classic twin study. Arthritis Rheum. 2000;43(11):2410–2416. doi: 10.1002/1529-0131(200011)43:11<2410::AID-ANR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 29.Spector TD, Cicuttini F, Baker J, Loughlin J, Hart D. Genetic influences on osteoarthritis in women: a twin study. BMJ. 1996;312(7036):940–943. doi: 10.1136/bmj.312.7036.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhai G, Stankovich J, Ding C, Scott F, Cicuttini F, Jones G. The genetic contribution to muscle strength, knee pain, cartilage volume, bone size, and radiographic osteoarthritis: a sibpair study. Arthritis Rheum. 2004;50(3):805–810. doi: 10.1002/art.20108. [DOI] [PubMed] [Google Scholar]


