Abstract
Purpose
To assesse circulating levels of Anti-Müllerian hormone (AMH) as a predictor of oocyte number and their potential to mature in vitro in both normo-ovulatory (NO) women and in women with Polycystic Ovary Syndrome (PCOS) undergoing in vitro maturation (IVM) treatments.
Methods
We prospectively studied NO women and women diagnosed with PCOS, (age range 21–39 years) underwent IVM treatments at our center. Serum AMH levels were quantified before each cycle and correlated to oocytes number, maturation and fertilization during in vitro maturation.
Results
104 NO and 30 PCOS IVM cycles were followed with retrieval of a total of 672 and 491 oocytes, respectively. In NO women, the serum AMH level positively correlated with the number of oocytes retrieved, (R = 0.6; P <0.0001) the number of M2 oocytes at 24 and 48 h (R = 0.4; P <0.01; R = 0.26 p < 0.007, respectively) and with the total number of M2 oocytes (R = 0.47; P < 0.0001). In the PCOS group, the serum AMH level positively correlated only with the number of oocytes retrieved (R = 0.43; P <0.03). Receiver operating characteristic (ROC) analyses showed that a cutoff AMH level of 1.56 (ng/ml) could identify patients with 5 or more oocytes at OPU with a sensitivity of 83 % and a specificity of 75 %. An AMH level of 1.63 (ng/ml) was the threshold for 5 or more matured oocytes (sensitivity = 81 %, specificity = 53 %).
Conclusions
Serum AMH may be used as a marker to identify candidates for IVM treatment in both NO and PCOS women.
Keywords: Anti-Müllerian hormone, In vitro maturation, Oocyte maturation, PCOS
Introduction
In vitro maturation (IVM) of immature oocytes is an emerging technology providing a hormone-free or mild hormonal stimulation environment for fertility treatment. The aspiration of immature oocytes from small- and medium-sized antral follicles followed by their maturation in vitro, presents an attractive alternative to the hormonal stimulation of patients undergoing in vitro fertilization (IVF). Currently, the IVM technique remains controversial in humans because of its suboptimal pregnancy rates when compared with regular IVF treatments and as such its use is restricted to specific indications. These include women at risk of hyperstimulation syndrome [1–4, 16, 18, 24], those women with cancer requiring immediate fertility preservation and those patients who cannot receive hormonal stimulation [6, 17]. In view of the low pregnancy rate with IVM, a reliable and available tool needs to be discovered which is capable of predicting a subgroup of patients most likely to benefit from this novel treatment. Recent studies have shown that the number of retrieved oocytes is the most significant predictive factor of successful IVM treatments [9].
Anti- Müllerian hormone (AMH) is a dimeric glycoprotein and a member of the transforming growth factor-beta superfamily, [6] being exclusively produced by granulosa cells from the primary to the large antral follicle stage [25] and secreted by the ovarian follicles into the circulation. It has been suggested that AMH levels may effectively represent the ovarian follicular pool where recently, several large prospective studies have reported that its measurement may have potential clinical application in the prediction of the quantitative and qualitative ovarian response during assisted reproductive technologies [7, 11, 14, 15, 21, 23, 25]. As a result, AMH is currently being used as a novel clinical marker of ovarian reserve as well as a guide to the response to gonadotropin [8]. Our recent data would suggest that AMH expression by granulosa cells and its secretion level within the follicular fluid of small- and medium-sized follicles is strongly correlated with the serum AMH (unpublished results). Moreover, our recent studies have suggested that there is also a correlation between follicular fluid (FF) AMH levels and oocyte development during IVF cycles [13].
From this data it is proposed that serum AMH might not only be a useful tool for the prediction of the number of retrieved oocytes, but also that it may be a potential marker of their capacity to mature in-vitro. In spite of the large number of studies investigating the role of AMH as a marker of ovarian reserve and response during IVF, there are limited data concerning its role in IVM, where recently, Fadini et al. have suggested that it may be used to identify suitable normo-ovulatory (NO) IVM candidates with sufficient oocyte retrieval [8]. As far as we are aware, there are no available data concerning the measurement of serum AMH levels in those women with PCOS undergoing IVM treatment. The aim of this study was to investigate the role of serum AMH as a predictor of oocyte number and their potential to mature in vitro in both NO and PCOS patients undergoing IVM treatments.
Methods
Patients~
We prospectively studied women who underwent IVM protocols in our fertility center (the IVF Unit, Sheba Medical Center Israel) between March 2008 and October 2010. Approval for the conduct and analysis of the study was provided by the local hospital Ethics Committee with written informed consent being obtained from all study participants. The study included NO and PCOS patients with patient demographics represented in Table 1. Inclusion criteria for IVM patients were: Age ≤ 38 years, body mass index < 31. Patients included in the NO group underwent IVM to avoid hormonal therapy and patients treated for fertility preservation. The inclusion criteria for NO patients were: Regular menstrual cycles (25–34 days) and morphologically and endocrinologically normal ovaries. Patients in the PCOS group were diagnosed according to the Rotterdam criteria (Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS)) [19]. All PCOS women must had two of the following three manifestations: Irregular or absent ovulation, elevated levels of androgenic hormones, and/or enlarged ovaries containing at least 12 follicles each.
Table 1.
Characteristics of 100 IVM patients included in the study
| Norm-ovulatory | PCOS | |
|---|---|---|
| Number of patients | 80 | 20 |
| Number of cycles | 104 | 30 |
| Age (years) | 31.5 ± 5 | 29 ± 4 |
| BMI | 22 ± 2 | 23 ± 2 |
| FSH (IU/l) | 8.2 ± 3 | 6.7 ± 3 |
| Serum AMH (ng/ml) | 2.7 ± 2.5 | 8.4 ± 4 |
Values given as mean ± St. Dev.
IVM protocol
Patients underwent an IVM cycle according to a previously described protocol [9]. Briefly, a baseline evaluation including an hormonal profile (AMH, FSH, estradiol and progesterone levels) and an ultrasound scan were performed on day 2–3 of the menstrual cycle. On day three, 150 IU/day of recombinant follicle-stimulating hormone (FSH) was commenced for 3 days. A second evaluation was then performed on day 6 of the menstrual cycle. Following this, an injection of 10,000 IU of chorionic gonadotropin (hCG) (Pregnyl; Organon, Oss, Holland) was administered when the endometrial thickness was at least 6 mm and when the dominant follicle was 12 mm in diameter. Transvaginal oocyte retrieval was scheduled 36–38 h after hCG injection. Metaphase II oocytes underwent intracytoplasmic sperm injection (ICSI) as previously described [22]. Immature oocytes were incubated in IVM medium (Sage; Trumbull, CT) supplemented with 75 IU FSH and 75 IU Luteinizing Hormone (LH) (Ferring, Keil, Germany). Oocyte maturation was assessed after 24 and 48 h in culture and mature oocytes were fertilized by ICSI. Eighteen hours after the ICSI procedure, fertilization was assessed using the established pronuclei (PN) scoring system [20]. Laboratory outcome measurements included the number of retrieved oocytes, the number of mature oocytes on the day of the aspiration, the number of newly mature oocytes after 24 and 48 h, the fertilization rates and the number of embryos appropriate for transfer. Maturation rates were calculated as the number of newly matured oocytes divided by the number of the immature oocyte pool over a defined period of time.
AMH measurements in serum
Serum AMH levels were measured on the third day of menstruation. The serum was separated and frozen in aliquots at −80 °C for future analysis with measurement being performed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions [Diagnostic Systems Laboratory, Webster, TX]. The intra- and inter-assay coefficients of variations (CVs) were 4.6 and 8.0 %, respectively.
Statistics
All statistical procedures were conducted with PASW Statistics, Release Version 18.0.0 software (SPSS, Inc., 2009, Chicago, IL). Paired comparisons were made using the paired two sided Student’s t-test assuming unequal variances. The relationship between any two continuous variables was assessed by correlation when they were independent from each other and by simple regression when there was dependency. ROC curves were calculated in an attempt to determine a cutoff value for the serum AMH level with the first criterion of analysis being the retrieval of at least 5 immature oocytes. This oocyte number was considered as critical based on previous recent studies which have suggested that recovery of at least 5 immature oocytes are necessary to ensure the chances of achieving a pregnancy [9]. P-values < 0.05 were considered significant.
Results
Patient demographics~
One hundred IVM patients met our inclusion criteria, including 80 in the NO group undergoing 104 IVM cycles with an average AMH serum level of 2.7 ± 2.5 ng/ml. Twenty PCOS patients underwent 30 IVM cycles with an average AMH serum level of 8.4 ± 4 ng/ml where the stimulation dose and duration of the IVM protocols were both fixed in accordance with the treatment protocol. All stimulation cycles were successfully reaching the egg retrieval criteria.
Normo-ovulatory (NO) group~
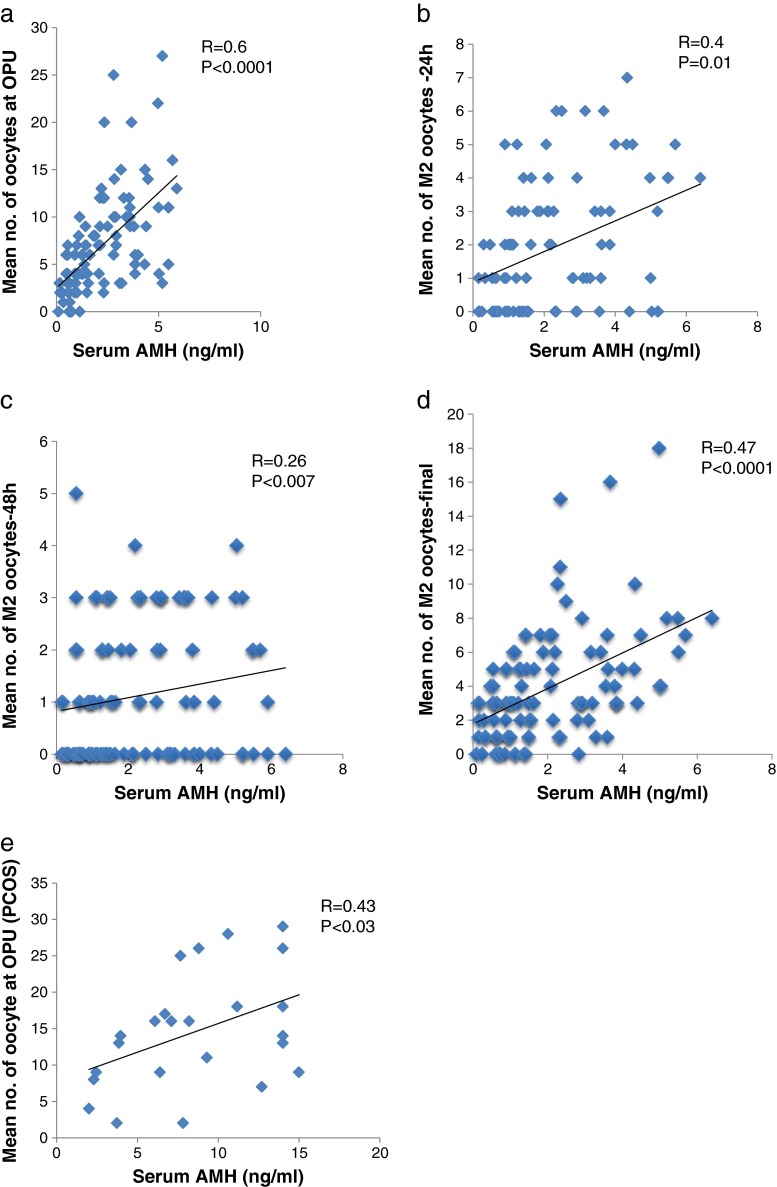
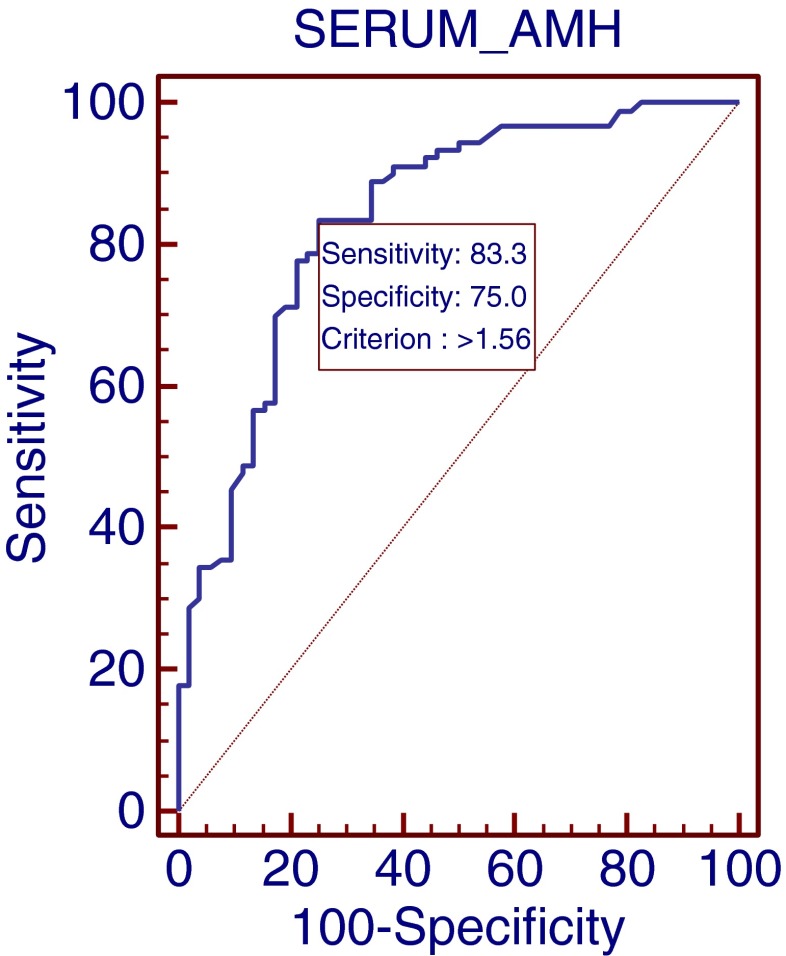
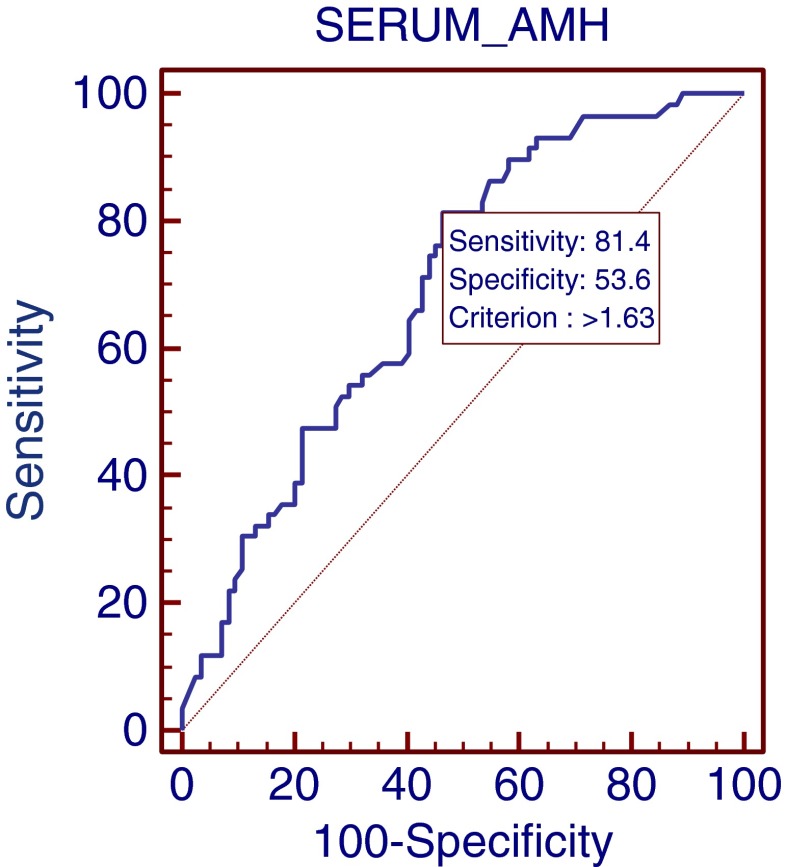
There was a positive correlation between the serum AMH level and the number of oocytes retrieved (R = 0.6; P <0.0001, Fig. 1a) although there was no correlation between the number of mature oocytes on the day of OPU and the serum AMH level. Analysis of the number of newly matured oocytes 24 h after OPU showed a clear correlation with AMH levels (R = 0.40; P <0.01, Fig. 1b) with a weaker but still significant correlation at 48 h (R = 0.26; P <0.007, Fig. 1c). The total number of matured oocytes significantly correlated with the serum AMH level (R = 0.47; P <0.0001, Fig. 1d). In addition there were negative correlations between patient’s age, AMH levels and number of oocytes retrieved (R = 0.23; P <0.01 and R = 0.34; P <0.002 respectively). In an attempt to identify the optimal serum AMH cut-off levels for optimal immature and mature oocyte retrieval, ROC curves were constructed. These showed an AMH threshold level of 1.56 (ng/ml) that identified patients with 5 or more oocytes at OPU (sensitivity = 83 %, specificity = 75 %; Fig. 2) and an AMH level of 1.63 (ng/ml) which defined the threshold for 5 or more matured oocytes ready for fertilization (sensitivity = 81 %, specificity = 53 %; Fig. 3).
Fig. 1.
Correlations of serum AMH levels and laboratory outcomes in NO and PCOS patients. a Mean number of retrieved oocytes on OPU day of NO patients. b Mean number of oocytes that completed their maturation in the first 24 h following OPU (NO patients). c Mean number of oocytes that completed their maturation between 24 and 48 h following OPU (NO patients). d Mean total number of matured oocytes ready for fertilization in NO patients including mature oocytes of OPU. e Mean number of retrieved oocytes on OPU day of PCOS patients
Fig. 2.
ROC curve for the relationship between serum AMH levels and the retrieval of ≥ 5 immature oocytes
Fig. 3.
ROC curve for the relationship between serum AMH levels and the retrieval of ≥ 5 matured oocytes ready for fertilization
On the basis of these findings, an AMH level of 1.6 ng/ml was chosen as the threshold concentration to assess laboratory outcomes in the two patient groups (Table 2). The mean number of retrieved oocytes in the NO group with serum AMH levels > 1.6 (ng/ml) was almost 2.5 times more than that of patients with serum AMH levels < 1.6 (ng/ml) (9 ± 5.5 vs. 3.7 ± 2.3, respectively; P < 0.001). The maturation rate on the day of OPU was significantly higher in patients with an AMH level >1.6 (ng/ml) when compared with patients whose AMH levels < 1.6 (ng/ml) (0.2 ± 0.3 vs. 0.1 ± 0.1 respectively, P < 0.01). There was a significantly higher number of in vitro newly matured oocytes obtained at 24 h when the AMH levels > 1.6 (ng/ml) compared with AMH levels < 1.6 (ng/ml) ( 2.8 ± 2.7 vs. 1.1 ± 1.3, respectively; P < 0.001) and a higher final number of matured oocytes when the serum AMH levels > 1.6 (ng/ml) (1.6 ± 2.1 vs. 0.8 ± 1.1, respectively, P < 0.001). Finally, NO patients with AMH levels exceeding 1.6 ng/ml when compared with those where the serum AMH level was < 1.6 (ng/ml), had a significantly higher number of fertilized oocytes (2PN) (3.5 ± 2.6, vs. 2.2 ± 1.5; P < 0.005) and a greater total number of embryos appropriate for transfer (2.3 ± 2.8 vs. 1.4 ± 1.4, respectively, P < 0.0001).
Table 2.
Laboratory outcomes of 134 IVM cycles in NO and PCOS patients. An AMH threshold of 1.6 ng/ml was identified for obtaining at least 5 matured oocytes
| Normo-ovulatory | PCOS | ||||
|---|---|---|---|---|---|
| AMH < 1.6 ng/ml | AMH > 1.6 ng/ml | P | (n = 20/30) | P | |
| (n = 37/55) | (n = 43/49) | ||||
| Mean serum AMH level (ng/ml) | 0.8 ± 0.4 | 3.2 ± 1 | 0.001 | 8.5 ± 4 | 0.001 |
| Total no. of oocytes retrieved | 200 | 472 | 491 | ||
| Mean no. of oocyte at OPU | 3.7 ± 2.3 | 9 ± 5.5 | 0.001 | 15 ± 9 | 0.001 |
| Mean no. of matured oocytes at OPU: | 0.66 ± 1 | 0.76 ± 1.2 | NS | 1.4 ± 1.7 | 0.01 |
| Maturational rate (OPU) | 0.2 ± 0.3 | 0.1 ± 0.1 | 0.01 | 0.1 ± 0.1 | NS |
| Mean no. Of matured oocytes oocytes (24 h)a | 1.1 ± 1.3 | 2.8 ± 2.7 | 0.001 | 4.2 ± 3 | 0.001 |
| Maturational rate (24 h) | 0.5 ± 0.3 | 0.37 ± 0.3 | NS | 0.36 ± 0.2 | NS |
| Mean no. Of matured oocytes (48 h)b | 0.8 ± 1.1 | 1.6 ± 2.15 | 0.001 | 2.3 ± 3.2 | 0.01 |
| Maturational rate (48 h) | 0.7 ± 0.2 | 0.5 ± 0.2 | NS | 0.5 ± 0.2 | NS |
| Mean final no. of matured oocytes: | 2.5 ± 1.9 | 5.3 ± 4.2 | 0.001 | 7.7 ± 5 | 0.01 |
| Final maturational rate | 0.7 ± 0.3 | 0.55 ± 0.28 | NS | 0.5 ± 0.2 | NS |
| Mean no. of 2PN | 2.2 ± 1.5 | 3.5 ± 2.6 | 0.005 | 5.5 ± 2.8 | 0.01 |
| Fertilization rate | 0.6 ± 0.3 | 0.7 ± 0.2 | NS | 0.74 ± 0.2 | NS |
| No. of cryo-preserved embryos | 1.4 ± 0.7 | 4.1 ± 3.1 | 0.001 | 3.6 ± 2.2 | 0.01 |
| ET | 2.3 ± 1.1 | 2 ± 0.8 | NS | 2.6 ± 0.7 | NS |
| Total Embryo no. | 1.4 ± 1.4 | 2.3 ± 2.8 | 0.0001 | 3.6 ± 2.2 | 0.01 |
Values given as mean ± St. Dev.
ET embryos transfer
aMean no. of oocytes that completed their maturation in the first 24 following OPU (M2 oocytes on OPU day are excluded)
bMean no. of oocytes that completed their maturation between 24 and 48 h following OPU (M2 oocytes of the first 24 h are excluded)
Polycystic ovary syndrome (PCOS) group~
In this group there was a significant positive correlation only between serum AMH levels and the number of oocytes retrieved (R = 0.43, P <0.03; Fig. 1E). In addition there was a negative correlation between patient’s age and number of oocytes retrieved (R = 0.52; P <0.002) and non significant negative correlation between patient’s age and AMH levels (R = 0..32; P <0.08). The laboratory outcomes of oocytes obtained from PCOS patients are presented in Table 2 with a total of 491 oocytes being retrieved. The mean number of oocytes on the day of OPU was 4 times higher than those obtained from the NO patients (15 ± 9) and there were consistently higher numbers of mature oocytes during the different culture periods in the PCOS cases when compared with the NO patients. Despite these findings, the maturational rates between the patient groups were comparable.
Discussion
The part of the study which assessed normo-ovulatory (NO) patients undergoing IVM treatment, showed a direct correlation between measurable serum AMH levels and the number of oocytes retrieved as well as the number of newly matured oocytes. For PCOS patients, AMH levels correlated with the total number of oocytes retrieved with this group producing consistently greater numbers of mature oocytes when compared with the NO group. ROC curves described an AMH cut-off level with high sensitivity and specificity, of 1.6 ng/ml for the critical production of 5 or more matured oocytes. This cut-off provided a higher mean number of oocytes, a higher maturation rate, a higher number of newly matured oocytes, a higher number of fertilized oocytes and a greater total number of embryos appropriate for transfer.
Other studies have also shown that in IVM cycles, the number of available oocytes is the single most important factor correlating with a successful procedural outcome in NO patients [5, 8, 9]. Our findings are in agreement with Fadini et al. [8] who also showed a correlation between AMH levels and the number of oocytes harvested, The final number of matured oocytes suitable for fertilization is dependent not only on their quantity but also upon their quality and maturation potential in in vitro culture systems. In this respect, our study reports for the first time to the best of our knowledge the association between AMH levels and the maturational status of oocytes, where on the day of the OPU, we did not detect any correlation. By 24 h and 48 h, however, a significantly positive correlation was clearly evident. This was unassociated with any correlation between maturational rates and AMH levels with the exception of the OPU day, suggesting that patients with higher AMH levels result in a higher number of retrieved oocytes without any compromise in their final maturational rate and hence, with a significantly higher number of embryos for transfer. This would imply that despite the fact that the serum AMH level predicts ovarian response and may act as a surrogate marker of ovarian reserve, that it is only a limited quality indicator during IVM treatment.
In an attempt to identify a cut-off AMH level, ROC curves were calculated with the principal criterion of analysis being the retrieval of at least 5 immature oocytes. This oocyte number has previously been positively associated with the chances of a pregnancy during IVM therapy [5, 9, 10]. In a further attempt to increase the sensitivity and specificity of AMH, a threshold cut-off level was identified for the retrieval of 5 matured oocytes, where a level of 1.63 ng/ml yielded a sensitivity and a specificity of 81 % and 53 % respectively. This slightly higher AMH level than previously reported is recommended to obtain the critical number of mature oocytes that are ready for fertilization. One of the difficulties with IVM treatments especially when applied to regularly cycling women, is the worse clinical outcome when compared with conventional IVF. In this regard, it is proposed that this stricter criterion for production of mature oocytes primed for fertilization could be crucial for identifying in advance those truly non-responsive cases and hence, could avoid inefficient IVM treatments.
To the best of our knowledge, this the first study that aims to investigate the possible correlation of serum AMH levels and laboratory outcomes in PCOS patients undergoing IVM treatments. In these cases, the average AMH level was 8.5 ng/ml, which was twice as much as that observed in the high-level AMH NO group, with a consistently higher number of retrieved oocytes and mature oocytes at all stages of culture in the IVM-treated PCOS patients. This was unaccompanied by any effect on the maturational potential of oocytes. The finding of a strong correlation between AMH levels and the number of oocytes retrieved in PCOS patients suggests that even at the higher AMH range, the AMH level remains a relatively sensitive tool in predicting the number of oocytes retrieved. Other oocyte parameters did not correlate with the AMH level in this group and it may be that the narrow concentration range and the small number of study participants contributed to these negative results. We did not consider pregnancy rates as one of our outcome end points, as there may be many confounding variables which affect these rates such as endometrial receptivity which is an extremely variable factor in IVM cycles. Equally, it is accepted that a limitation of our study is that the majority of embryos remained frozen and were not transferred, largely as a result of our recruitment criteria for IVM treatment.
In summary, the identification of cycles able to produce numerous oocytes will considerably improve the efficiency of IVM treatments, where we would propose that women who do not reach the AMH threshold might benefit where possible from standard ovarian stimulation. In the same way that cut-off AMH levels have been proposed for the modulation of ovarian stimulation in IVF cycles, [12] we would suggest that a similar cut-off concentration can guide the appropriateness of IVM therapy in selected cases, reducing the number of recruited cycles where suboptimal oocyte numbers are likely to be retrieved.
Acknowledgments
The authors are greatly indebted to Prof. Andrew Zbar of MEDEDIT, Sheba Medical Center for the English editing.
Footnotes
Capsule
The identification of cycles able to produce numerous oocytes will considerably improve the efficiency of IVM treatments. We would suggest that an AMH cut‐off concentration could guide the appropriateness of IVM therapy in selected cases, reducing the number of recruited cycles where suboptimal oocyte numbers are likely to be retrieved.
References
- 1.Barnes FL, Kausche A, Tiglias J, Wood C, Wilton L, Trounson A. Production of embryos from in vitro-matured primary human oocytes. Fertil Steril. 1996;65(6):1151–6. doi: 10.1016/s0015-0282(16)58330-7. [DOI] [PubMed] [Google Scholar]
- 2.Cha KY, Chung HM, Lee DR, Kwon H, Chung MK, Park LS, et al. Obstetric outcome of patients with polycystic ovary syndrome treated by in vitro maturation and in vitro fertilization-embryo transfer. Fertil Steril. 2005;83(5):1461–5. doi: 10.1016/j.fertnstert.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Chian RC. In-vitro maturation of immature oocytes for infertile women with PCOS. Reprod Biomed Online. 2004;8(5):547–52. doi: 10.1016/S1472-6483(10)61101-7. [DOI] [PubMed] [Google Scholar]
- 4.Child TJ, Abdul-Jalil AK, Gulekli B, Tan SL. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil Steril. 2001;76(5):936–42. doi: 10.1016/S0015-0282(01)02853-9. [DOI] [PubMed] [Google Scholar]
- 5.Dal Canto MB, Mignini Renzini M, Brambillasca F, Cepparo H, Comi R, Villa A, et al. IVM–the first choice for IVF in Italy. Reprod Biomed Online. 2006;13(2):159–65. doi: 10.1016/S1472-6483(10)60610-4. [DOI] [PubMed] [Google Scholar]
- 6.Das M, Gillott DJ, Saridogan E, Djahanbakhch O. Anti-Mullerian hormone is increased in follicular fluid from unstimulated ovaries in women with polycystic ovary syndrome. Hum Reprod. 2008;23(9):2122–6. doi: 10.1093/humrep/den185. [DOI] [PubMed] [Google Scholar]
- 7.Eldar-Geva T, Margalioth EJ, Gal M, Ben-Chetrit A, Algur N, Zylber-Haran E, et al. Serum anti-Mullerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum Reprod. 2005;20(7):1814–9. doi: 10.1093/humrep/deh873. [DOI] [PubMed] [Google Scholar]
- 8.Fadini R, Comi R, Mignini Renzini M, Coticchio G, Crippa M, De Ponti E, et al. Anti-mullerian hormone as a predictive marker for the selection of women for oocyte in vitro maturation treatment. J Assist Reprod Genet. 2011;28(6):501–8. doi: 10.1007/s10815-011-9589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fadini R, Dal Canto MB, Mignini Renzini M, Brambillasca F, Comi R, Fumagalli D, et al. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod Biomed Online. 2009;19(3):343–51. doi: 10.1016/S1472-6483(10)60168-X. [DOI] [PubMed] [Google Scholar]
- 10.Fadini R, Dal Canto MB, Renzini MM, Brambillasca F, Comi R, Fumagalli D, et al. Predictive factors in in-vitro maturation in unstimulated women with normal ovaries. Reprod Biomed Online. 2009;18(2):251–61. doi: 10.1016/S1472-6483(10)60263-5. [DOI] [PubMed] [Google Scholar]
- 11.Fanchin R, Schonauer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18(2):323–7. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 12.Gnoth C, Schuring AN, Friol K, Tigges J, Mallmann P, Godehardt E. Relevance of anti-Mullerian hormone measurement in a routine IVF program. Hum Reprod. 2008;23(6):1359–65. doi: 10.1093/humrep/den108. [DOI] [PubMed] [Google Scholar]
- 13.Kedem-Dickman A, Maman E, Yung Y, Yerushalmi GM, Hemi R, Hanochi M, et al. Anti-Mullerian hormone is highly expressed and secreted from cumulus granulosa cells of stimulated preovulatory immature and atretic oocytes. Reprod Biomed Online. 2012;24(5):540–6. doi: 10.1016/j.rbmo.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 14.La Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S, et al. Anti-Mullerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. 2007;22(3):766–71. doi: 10.1093/humrep/del421. [DOI] [PubMed] [Google Scholar]
- 15.La Marca A, Malmusi S, Giulini S, Tamaro LF, Orvieto R, Levratti P, et al. Anti-Mullerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum Reprod. 2004;19(12):2738–41. doi: 10.1093/humrep/deh508. [DOI] [PubMed] [Google Scholar]
- 16.Le Du A, Kadoch IJ, Bourcigaux N, Doumerc S, Bourrier MC, Chevalier N, et al. In vitro oocyte maturation for the treatment of infertility associated with polycystic ovarian syndrome: the French experience. Hum Reprod. 2005;20(2):420–4. doi: 10.1093/humrep/deh603. [DOI] [PubMed] [Google Scholar]
- 17.Maman E, Meirow D, Brengauz M, Raanani H, Dor J, Hourvitz A. Luteal phase oocyte retrieval and in vitro maturation is an optional procedure for urgent fertility preservation. Fertil Steril. 2011;95(1):64–7. doi: 10.1016/j.fertnstert.2010.06.064. [DOI] [PubMed] [Google Scholar]
- 18.Mikkelsen AL, Lindenberg S. Benefit of FSH priming of women with PCOS to the in vitro maturation procedure and the outcome: a randomized prospective study. Reproduction. 2001;122(4):587–92. doi: 10.1530/rep.0.1220587. [DOI] [PubMed] [Google Scholar]
- 19.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47 [DOI] [PubMed]
- 20.Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod. 2000;15(11):2394–403. doi: 10.1093/humrep/15.11.2394. [DOI] [PubMed] [Google Scholar]
- 21.Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77(3):468–71. doi: 10.1016/S0015-0282(01)03201-0. [DOI] [PubMed] [Google Scholar]
- 22.Tesarik J, Mendoza C, Testart J. Viable embryos from injection of round spermatids into oocytes. N Engl J Med. 1995;333(8):525. doi: 10.1056/NEJM199508243330819. [DOI] [PubMed] [Google Scholar]
- 23.Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-mullerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45(1):20–4. doi: 10.1111/j.1479-828X.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 24.Trounson A, Anderiesz C, Jones GM, Kausche A, Lolatgis N, Wood C. Oocyte maturation. Hum Reprod. 1998;13(Suppl 3):52–62. doi: 10.1093/humrep/13.suppl_3.52. [DOI] [PubMed] [Google Scholar]
- 25.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]