Abstract
Purpose
Telomere length plays a significant role in various disorders; however, its role in idiopathic recurrent pregnancy loss (iRPL) is not known. The objective of this study was to assess telomere length in peripheral blood leukocytes in couples experiencing unexplained recurrent pregnancy loss (iRPL).
Methods
The study included 25 couples experiencing iRPL and 20 controls. The mean relative telomere length was measured by quantitative Real Time PCR (Q-PCR) based assay, which measures the average ratio of telomere repeat copy number to a single copy gene (36B4) copy number (T/S ratio) in each sample.
Results
The relative leukocyte mean telomere length (T/S) in both men and women from iRPL group was significantly lower (p < 0.05) when compared to controls. A significant (P < 0.05) negative correlation was found between age and leukocyte telomere length (T/S ratio). Among the sperm parameters seminal volume was found to be negatively (r = −0.4679) associated with the telomere T/S ratio. The DNA fragmentation index of sperm showed positive correlation (r = 0.4744) with telomere length. In this preliminary study, we found that shorter telomere length in both men and women may be associated with early pregnancy loss.
Conclusion
In conclusion, shorter telomere length in both male and female partners appears to play a role in the idiopathic recurrent pregnancy loss. Loss of telomeric DNA due to oxidative stress needs further analysis. Analysis of telomere length in germ cells are needed to further substantiate the findings of this study.
Keywords: Telomere length; Recurrent pregnancy loss; Q-PCR, Sperm chromatin structure assay; Reactive oxygen species
Introduction
At least 1–3 % of married couples experience idiopathic recurrent pregnancy loss (iRPL) (>3 consecutive pregnancy losses before 20–24 weeks of gestation) [1, 2]. It is estimated that the incidence of spontaneous abortions is between 10 and 20 % among clinically recognized pregnancies [3]. Though there are numerous factors such as anatomical defects, endocrinological, immunological and genetic factors which can lead to iRPL but the exact cause is not known in majority of cases and such cases are classified as idiopathic recurrent pregnancy loss (iRPL) [2]. Telomere are enriched with CpG islands and subtelomeric regions are believed to have the highest gene density [4] and have specialized functions in maintaining chromosome integrity and perform capping function, protect chromosome from degradation and end-to-end fusion. With each cell division due to end replication problem telomere are shortened and this results in cell senescence [5]. In germ cells they aid in meiotic recombination and pairing of homologous chromosomes. [6] reported that shortened telomere resulted in apoptosis, replicative senescence, decreased recombination, meiotic arrest, impair embryonic viability and fetal development and cause chromosome missegregation (aneuploidy) and instability. Further shortened telomere loose their capping function at the end of the chromosomes which results in nonreciprocal translocations, (damaged chromosome captures a telomere from another chromosome) thus resulting in chromosomal instability or rearrangements [7] deletions, aneuploidy & DNA damage [8].
Telomeres in the genome are highly prone to oxidative damage as they are G rich and these non coding buffer zones flanking chromosomal ends determines cellular and genomic integrity. Their function depends not only on their length, but also on their structure, where the length of telomere is dictated by several factors (both genetic and lifestyle). Though shortened telomere length plays a significant role in various diseases its role in iRPL has not been evaluated. Chromosomes having sufficient telomere length have probable role in fertilization and shortened telomeres either from sperm or oocyte may contribute to abnormal cleavage and impaired development, as demonstrated in knock-out mice [9].
In human embryos telomere length in oocytes predicts cytoplasmic fragmentation, related to apoptosis [10]. Telomeres might be amongst the first structures in the sperm nucleus that respond to oocyte signals for male pronucleus development at fertilization [11]. Short telomere length in telomerase-deficient mice was found to be associated with premature aging, impaired oogenesis, poor quality of oocyte, increased rate of apoptosis, impaired chromosome synapsis and aneuploidy [6]. Critically short telomeres are associated with sperm DNA fragmentation in mice and abnormal embryonic development in man [12, 13]. From these observations it is suspected that telomeres might play an important role in chromosomal localization within the sperm nucleus [14], where abnormal chromosome localization leads to meiotic errors which results in sperm borne aneuploidies [15]. Moreover telomere length in human unfertilized oocytes has been correlated with the morphological appearance of embryo and embryo quality in sibling fertilized oocytes as well as the probability of pregnancy [10, 16], surprisingly, there are few reports showing association of telomere length with various reproductive disorders. Hence this study was planned with the aim to assess the role of telomere length in the leukocytes of couples (both male and female partner) experiencing iRPL.
Materials and methods
Study subjects
A total of 25 iRPL couples (more than 3 consecutive pregnancy losses before 20–24 weeks of gestation) and 20 fertile proven couples were included in this study after written informed consent and ethical approval from Institute Ethics Committee (IEC, AIIMS). All the male cases included in the study were cytogenetically normal and had no Y chromosome microdeletion. Detailed medical, family history and life style parameters of both partners were recorded in a predesigned proforma. Female partners were evaluated for complete gynaecological, clinical investigation to rule out cervical infection, endocrine disorder, haematological disorder, cytogenetic abnormality, anatomical defects etc. The female partners of the couples were less than 40 years old and had normal haemograms. Control subjects (18–40 year) who fathered child in last 1 year and had no history of infertility or miscarriages were enrolled as controls.
Semen analysis, and measurement of DFI and ROS
Standard semen analysis was done according to WHO guidelines 1999 [17]. The sperm DFI in iRPL men was measured by sperm chromatin structure assay as described earlier [18]. The ROS levels in the neat semen were estimated by luminal depended chemiluminesence assay as described by Venkatesh et al. [2].
Telomere length assay by Q-PCR
DNA was isolated using the standard phenol chloroform method. Relative mean telomere length was measured from leukocyte DNA by a quantitative polymerase chain reaction (qPCR) that compares mean telomere repeat sequence copy number (T) to a reference single-copy gene copy number (36B4) (SCG) in each sample as previously described [19]. Amplification of the telomeric repeat region was expressed relative to amplification of 36B4 encoding acidic ribosomal phosphoprotein located on chromosome 12. Bio-Rad CFX 96 (Hercules, CA, USA) was used to perform the quantitative real-time PCR with primer sequences obtained from [19] as reported earlier [19]. Real time kinetic quantitative PCR determines, for each sample well, the Ct, i.e. the fractional cycle number at which the well’s accumulating fluorescence crosses a set threshold that is several standard deviations above baseline fluorescence. A plot of Ct versus log (amount of input target DNA) is linear, allowing simple relative quantitation of unknowns by comparison to a standard curve derived from amplification, in the same plate, of serial dilutions of a known reference DNA sample. For this study, telomere (T) PCR and single copy gene (SCG) PCR was performed in separate 96-well plates. The primers for the telomere PCR were Telo F (Forward): 5′ CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT 3′ Telo R (Reverse): 5′ GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT 3′. The primers for the single copy gene PCR were 36B4F: 5′ CAGCAAGTGGGAAGGTGTAATCC 3′, 36B4R: 5′ CCCATTCTATCATCAACGGGTACAA 3′. Two master mixes of PCR reagents were prepared, one with the Telomere (T) primer pair, the other with the single-copy gene (SCG) primer pair. QPCR was performed as described by Cawthon [19]. Amplifications were carried out in triplicate in 10 μl reaction with 20 ng/μl of genomic DNA, and 1XSYBR Green (SsoFastTM Evagreen supermix, BioRad).
Thermal cycling profile
The T PCR thermal cycling profile consisted of 10 min at 95 °C followed by 35 cycles of 95 °C for 30 s, 57.5 °C for 30 s, followed by a melt curve at 65 °C −95 °C 5 s. The SCG PCR thermal cycling profile consisted of 10 min at 95 °C followed by 40 cycles of 95 °C for 30s, 57.5 °C for 30 s, followed by a melt curve 65 °C to 95 °C for 5 s. DNA samples were serially diluted (10 fold) to obtain the required concentrations (ng/μl) of reference DNA in each PCR assay to determine the standard curve. The purpose of the standard curve was to assess and compensate for interplate variations in PCR efficiency. The T/S ratio was used to assess the relative length of telomere, while Ct is the fractional cycle number for a threshold fluorescence level to be reached during quantitative real-time PCR. All samples were run in triplicate along with a no-template control. Dissociation melting curves were run after each sample to ensure amplification of a single species. Replicates of each plate were done to ensure reliable values.
Statistical analysis
The telomere to single copy gene (T/S) ratio between iRPL group and controls was compared by ANCOVA test after adjusting the age of the subject. The correlation between the T/S ratio and the other parameters were calculated by spearman correlation co-efficient test since the data is not normally distributed. All the statistical analysis was performed using Medcalc trial version software. P < 0.01 was considered as significant.
Results
Q-PCR efficiency
The slope of the standard curve for both telomere and 36B4 reactions were −2.99 and −3.34 and acceptable linear correlation coefficient (R2) value for both telomere and housekeeping gene reactions were 0.93 and 0.97 respectively. Leukocyte telomere length in male partners of iRPL group
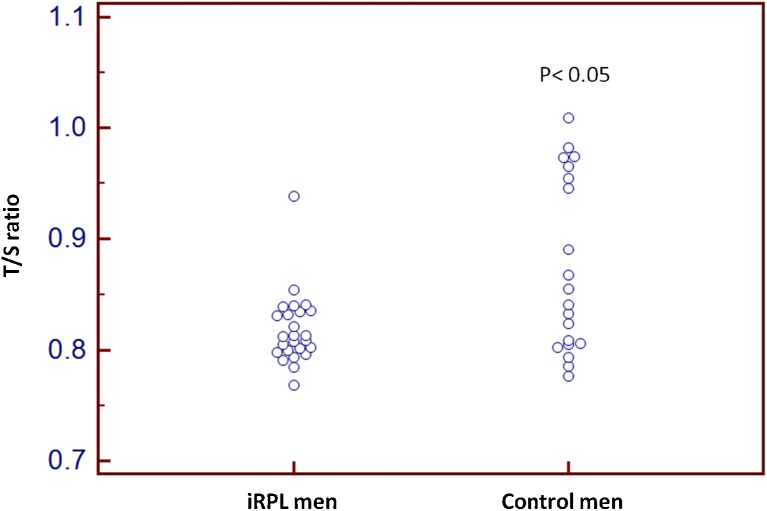
The relative leukocyte mean telomere length (T/S) in the men from iRPL group was significantly lower (p < 0.05) when compared to controls (Fig. 1) (Table 1). No significant difference in the average mean age of iRPL and control men was observed (33.17 ± 5.16 Vs 31.50 ± 5.28 year). However, a significant negative correlation was found between age and the leukocyte telomere length (T/S ratio) of iRPL men (Fig. 2) (Table 2). Semen parameters did not show any correlation with telomere length except sperm count and seminal pH that showed non-significant positive correlation (Table 2).
Fig. 1.
Dot plot of relative leukocyte telomere length (T/S) of control and iRPL men, p < 0.05 is considered as significance
Table 1.
Comparison of leukocyte telomere length (T/S ratio) between recurrent pregnancy loss (RPL) and control subjects
| Case | Control | p-value | Adjusted p-value | |
|---|---|---|---|---|
| Mean±SD (Male) | 0.81 ± 0.032 | 0.87 ± 0.078 | <0.001 | <0.004 |
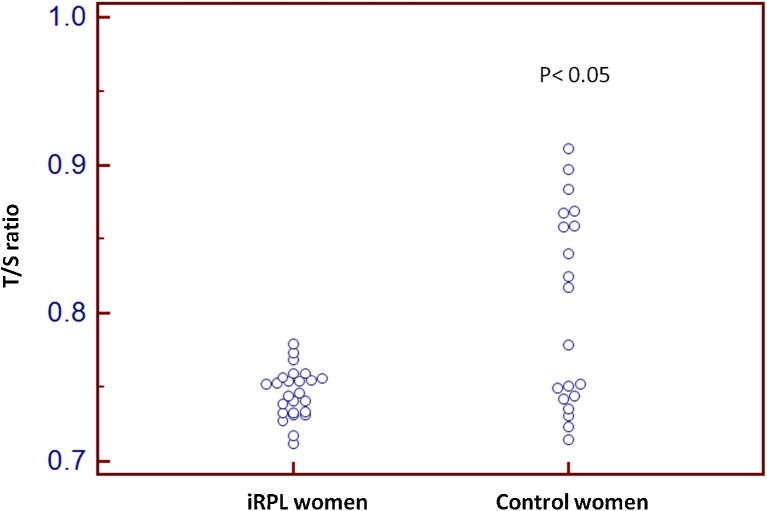
| Mean±SD (Female) | 0.74 ± 0.016 | 0.80 ± 0.066 | <0.001 | <0.000 |
Fig. 2.
Dot plot of relative leukocyte telomere length (T/S) of control and iRPL wommen, p < 0.05 is considered as significance
Table 2.
Correlation of leukocyte telomere length (T/S) ratio and semen parameters, reactive oxygen species (ROS) levels and sperm DNA fragmentation index (DFI) in men from RPL group
| Parameters | r value | P value |
|---|---|---|
| Age | −0.3375 | 0.0410 |
| Seminal pH | 0.3352 | 0.1739 |
| Sperm count | 0.2709 | 0.2929 |
| Sperm motility | 0.1138 | 0.6330 |
| ROS | 0.09590 | 0.7553 |
| DFI | 0.4744 | 0.0740 |
Sperm DFI and ROS in iRPL men
The sperm DNA fragmentation index (DFI) showed non-significant positive correlation (r = 0.4744) with leuckocyte telomere length and no significant correlation (p = 0.7553) was found with sperm ROS levels of iRPL men (Table 2).
Leukocyte telomere length in female partner of iRPL women
The relative telomere length (T/S) was significantly shorter in iRPL women compared to controls (Table 1). No significant difference in the age between the female cases and controls was observed in the study (27.95 ± 4.08 Vs 26.25 ± 3.96 year)
Discussion
Telomeres play critical role in cell maintenance and preserve chromosome integrity, guide chromosome arrangement and their proper alignment and segregation in meiosis and mitosis. Telomere shortening has been reported in many diseases such as diabetes [20], hypertension [21, 22], dementia [23] infections and inflammation [24], and psychological stress [25–28]. Further, telomere shortening has also been reported in many types of cancer due to rapid cell proliferation [29]. However their role in reproductive disorders has not been evaluated. In the current study telomere-specific qPCR was used to assess telomere length in couples experiencing idiopathic recurrent pregnancy loss (iRPL). Q-PCR is most economical, versatile, less time consuming and highly sensitive method, which generates a T/S ratio that is proportional to a cell’s average telomere length. We used single copy acidic ribosomal phosphoprotein P0 expressing 36B4 gene to compare the telomere repeat sequence, because, its cDNA nucleotide sequence has highly conserved in the 5-prime end of its open reading frame among the tissues and species making it as most preferred reference gene [30]. Research is ongoing to understand the function of telomere biology at molecular and cellular level, but very little is known about the causes of telomere erosion in iRPL.
The shortened telomere length observed in the leukocytes of both male and female partners of couples experiencing iRPL is the first such study, though shorter telomere have been reported only in female partner experiencing recurrent miscarriages [16]. It is well known that telomere is a marker for cellular aging because their length declines with each cell division, though it exhibits inter-individual variation [31]. It is possible that telomere shortening may limit the mitotic capacity of primordial germ cells during fetal growth and therefore restrict the size of the follicular pool [32]. There is high probability that shortened telomere in both sperm and ova may have additive effect in limiting the replicative potential growth of embryo, and their mechanisms need detailed investigations. Wang and their colleagues in [33] proposed that moderate accumulation of oxidized bases may induce telomere lengthening while severe acute oxidative DNA damage leads to telomere shortening due to alternative capping, recombination, replication resolution of DNA strand breaks [33]. Telomeres that fail to hide their ends trigger a DNA damage response that leads to cell-cycle arrest and/or apoptosis [34]. However, we have not observed any significant correlation between telomere shortening and ROS or DFI levels in men. It may be possible that shorter telomeres are associated with replicative senescence and may lead to pre or post implantation losses or results in meiotic segregation abnormalities in germ cells that may lead to aneuploid fetus which may itself result in RPL [35–37]. Aneuploidies are the major cause of RPL and telomeres shortening associated with impaired microtubular movement may also impair chromosome segregation.
Studies have reported that women experience an age-dependent increase in various adverse reproductive events such as infertility, pregnancy complications and perinatal maternal morbidity and mortality, as well as an impaired perinatal and post-natal outcome of the offspring [38–41] which could be correlated with telomere shortening. It is known that telomere DNA shortening is associated with genomic instability and may play a role in development of aneuploidy commonly found in female germ cells and human embryos [42]. Telomere length has been both positively and negatively associated with different measures of reproductive aging and contradictory results are reported [16, 43, 44]. Telomere shortening, therefore, could explain many of the manifestations of reproductive senescence.
Limited data is available on telomere shortening in couples experiencing recurrent spontaneous abortion and our results are consistent with the study by Turner et al. [45]. To our knowledge, this is the first study addressing the potential contributions of relative telomere length in both partners of couple experiencing in iRPL. Thus telomere shortening may be a biological safeguard as shorter telomere length may prevent transmission of mutagenic bases and accumulation of mutagenic load to the developing embryo as such germ cells may result in fertilization but failed to develop into a mature fetus. This may explain for recurrent early pregnancy loss, if such pregnancies are carried to term it may result in birth of offspring with increased risk of development of certain cancers, congenital malformations and has lifelong implications on health. Telomeres are first structures to respond to oocyte signals for male pronucleus development at fertilization and microtubule guided movement [11]. Shortened telomeres in germ cells result in defects in early cleavage and preimplantation and this also has been documented in telomerase knockout mouse [9]. Telomere mediate nuclear reorganization leads to pairing and recombination of homologous chromosomes during meiosis and shortened telomeres result in meiotic arrest or aneuploidy and abnormal embryonic development and decreased rate of blastocyte development [46]. Telomerase knockout mouse exhibit decreases fertility, reduced litter size in subsequent generations and eventual sterility [9]. Hetrogenetiy of mouse and human somatic cell telomere length is also seen in oocytes [47]. Other possible mechanisms such as loss of telomerase activity due to mutation in the gene cannot be ruled out.
In conclusion, shorter telomere length in both male and female partners appears to play a role in the idiopathic recurrent pregnancy loss by limiting the replicative potential of the blastocyst or embryo however, the exact cause for the telomere shortening needs to be elucidated. Studies enrolling larger number of cases and study in germ cells are needed to further substantiate the findings of this study.
Acknowledgements
Authors thank Department of Biotechnology (DBT) (BT/PR4704/MED/12/539/2012), and Indian Council of Medical Research (ICMR) New Delhi, India for their financial support. J. Thilagavathi (ICMR) and S.S. Mishra (DBT) are S.R.F is duly acknowledged.
Footnotes
Capsule
Telomeres the conserved nucleoprotein structures flanking chromosomal ends and confer chromosomal stability and genomic integrity. Telomere length determines the replicative potential of the cell. Telomere length depends on both genetic and life style factors. Oxidative and psychological stress accelerates telomere shortening. Telomere length in both male and female partners determines the telomere length of blastocyst/embryo, but shorter telomere length may impair the replicative potential of embryo and thus may be the underlying aetiology of iRPL.
References
- 1.Jaslow CR, Carney JL, Kutteh WH. Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil Steril. 2010;93(4):1234–43. doi: 10.1016/j.fertnstert.2009.01.166. [DOI] [PubMed] [Google Scholar]
- 2.Venkatesh S, Thilagavathi J, Kumar K, Deka D, Talwar P, Dada R. Cytogenetic, Y chromosome microdeletion, sperm chromatin and oxidative stress analysis in male partners of couples experiencing recurrent spontaneous abortions. Arch Gynecol Obstet. 2011;284(6):1577–84. doi: 10.1007/s00404-011-1990-y. [DOI] [PubMed] [Google Scholar]
- 3.Tummers P, De Sutter P, Dhont M. Risk of spontaneous abortion in singleton and twin pregnancies after IVF/ICSI. Hum Reprod. 2003;18(8):1720–3. doi: 10.1093/humrep/deg308. [DOI] [PubMed] [Google Scholar]
- 4.Saccone S, De Sario A, Della Valle G, Bernardi G. The highest gene concentrations in the human genome are in telomeric bands of metaphase chromosomes. Proc Natl Acad Sci U S A. 1992;89(11):4913–7. doi: 10.1073/pnas.89.11.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baird DM. New developments in telomere length analysis. Exp Gerontol. 2005;40(5):363–8. doi: 10.1016/j.exger.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Franco S, Spyropoulos B, Moens PB, Blasco MA, Keefe DL. Irregular telomeres impair meiotic synapsis and recombination in mice. Proc Natl Acad Sci U S A. 2004;101(17):6496–501. doi: 10.1073/pnas.0400755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murnane JP. Telomeres and chromosome instability. DNA Repair (Amst) 2006;5(9–10):1082–92. doi: 10.1016/j.dnarep.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Krizhanovsky V, Xue W, Zender L, Yon M, Hernando E, Lowe SW. Implications of cellular senescence in tissue damage response, tumor suppression, and stem cell biology. Cold Spring Harb Symp Quant Biol. 2008;73:513–22. doi: 10.1101/sqb.2008.73.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Blasco M, Trimarchi J, Keefe D. An essential role for functional telomeres in mouse germ cells during fertilization and early development. Dev Biol. 2002;249(1):74–84. doi: 10.1006/dbio.2002.0735. [DOI] [PubMed] [Google Scholar]
- 10.Keefe DL, Franco S, Liu L, Trimarchi J, Cao B, Weitzen S, Agarwal S, Blasco MA. Telomere length predicts embryo fragmentation after in vitro fertilization in women--toward a telomere theory of reproductive aging in women. Am J Obstet Gynecol. 2005;192(4):1256–60. doi: 10.1016/j.ajog.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Zalenskaya IA, Bradbury EM, Zalensky AO. Chromatin structure of telomere domain in human sperm. Biochem Biophys Res Commun. 2000;279(1):213–8. doi: 10.1006/bbrc.2000.3917. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez S, Goyanes V, Segrelles E, Blasco M, Gosalvez J, Fernandez JL. Critically short telomeres are associated with sperm DNA fragmentation. Fertil Steril. 2005;84(4):843–5. doi: 10.1016/j.fertnstert.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23(1):25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 14.Scherthan H, Weich S, Schwegler H, Heyting C, Harle M, Cremer T. Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J Cell Biol. 1996;134(5):1109–25. doi: 10.1083/jcb.134.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlow AL, Hulten MA. Combined immunocytogenetic and molecular cytogenetic analysis of meiosis I human spermatocytes. Chromosome Res. 1996;4(8):562–73. doi: 10.1007/BF02261719. [DOI] [PubMed] [Google Scholar]
- 16.Keefe DL, Liu L, Marquard K. Telomeres and aging-related meiotic dysfunction in women. Cell Mol Life Sci. 2007;64(2):139–43. doi: 10.1007/s00018-006-6466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO (ed) (1999) WHO laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction., 4 edn,
- 18.Venkatesh S, Singh A, Shamsi MB, Thilagavathi J, Kumar R, Mitra DK, Dada R. Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod Sci. 2011;18(10):1005–13. doi: 10.1177/1933719111401662. [DOI] [PubMed] [Google Scholar]
- 19.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salpea KD, Humphries SE. Telomere length in atherosclerosis and diabetes. Atherosclerosis. 2010;209(1):35–8. doi: 10.1016/j.atherosclerosis.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aviv A, Aviv H. Reflections on telomeres, growth, aging, and essential hypertension. Hypertension. 1997;29(5):1067–72. doi: 10.1161/01.HYP.29.5.1067. [DOI] [PubMed] [Google Scholar]
- 22.Aviv A. Telomeres, sex, reactive oxygen species, and human cardiovascular aging. J Mol Med (Berl) 2002;80(11):689–95. doi: 10.1007/s00109-002-0377-8. [DOI] [PubMed] [Google Scholar]
- 23.Grodstein F, van Oijen M, Irizarry MC, Rosas HD, Hyman BT, Growdon JH, De Vivo I. Shorter telomeres may mark early risk of dementia: preliminary analysis of 62 participants from the nurses’ health study. PLoS One. 2008;3(2):e1590. doi: 10.1371/journal.pone.0001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilmonen P, Kotrschal A, Penn DJ. Telomere attrition due to infection. PLoS One. 2008;3(5):e2143. doi: 10.1371/journal.pone.0002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–44. doi: 10.1016/S0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 26.Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5(4):325–30. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 27.Raynaud CM, Jang SJ, Nuciforo P, Lantuejoul S, Brambilla E, Mounier N, Olaussen KA, Andre F, Morat L, Sabatier L, Soria JC. Telomere shortening is correlated with the DNA damage response and telomeric protein down-regulation in colorectal preneoplastic lesions. Ann Oncol. 2008;19(11):1875–81. doi: 10.1093/annonc/mdn405. [DOI] [PubMed] [Google Scholar]
- 28.Kananen L, Surakka I, Pirkola S, Suvisaari J, Lonnqvist J, Peltonen L, Ripatti S, Hovatta I. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010;5(5):e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu H, Belcher M, van der Harst P. Healthy aging and disease: role for telomere biology? Clin Sci (Lond) 2011;120(10):427–40. doi: 10.1042/CS20100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akamine R, Yamamoto T, Watanabe M, Yamazaki N, Kataoka M, Ishikawa M, Ooie T, Baba Y, Shinohara Y. Usefulness of the 5′ region of the cDNA encoding acidic ribosomal phosphoprotein P0 conserved among rats, mice, and humans as a standard probe for gene expression analysis in different tissues and animal species. J Biochem Biophys Methods. 2007;70(3):481–6. doi: 10.1016/j.jbbm.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346(6287):866–8. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 32.Keefe DL, Marquard K, Liu L. The telomere theory of reproductive senescence in women. Curr Opin Obstet Gynecol. 2006;18(3):280–5. doi: 10.1097/01.gco.0000193019.05686.49. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Rhee DB, Lu J, Bohr CT, Zhou F, Vallabhaneni H, de Souza-Pinto NC, Liu Y. Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genet. 2010;6(5):e1000951. doi: 10.1371/journal.pgen.1000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21(4):532–40. doi: 10.1038/sj.onc.1205080. [DOI] [PubMed] [Google Scholar]
- 35.Vogt S, Iking-Konert C, Hug F, Andrassy K, Hansch GM. Shortening of telomeres: Evidence for replicative senescence of T cells derived from patients with Wegener’s granulomatosis. Kidney Int. 2003;63(6):2144–51. doi: 10.1046/j.1523-1755.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 36.Hemann MT, Rudolph KL, Strong MA, DePinho RA, Chin L, Greider CW. Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Mol Biol Cell. 2001;12(7):2023–30. doi: 10.1091/mbc.12.7.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107(1):67–77. doi: 10.1016/S0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 38.Pal L, Santoro N. Age-related decline in fertility. Endocrinol Metab Clin North Am. 2003;32(3):669–88. doi: 10.1016/S0889-8529(03)00046-X. [DOI] [PubMed] [Google Scholar]
- 39.Cleary-Goldman J, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, Saade GR, Eddleman KA, Klugman S, Dugoff L, Timor-Tritsch IE, Craigo SD, Carr SR, Wolfe HM, Bianchi DW, D’Alton M. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 Pt 1):983–90. doi: 10.1097/01.AOG.0000158118.75532.51. [DOI] [PubMed] [Google Scholar]
- 40.Nybo Andersen AM, Hansen KD, Andersen PK, Davey Smith G. Advanced paternal age and risk of fetal death: a cohort study. Am J Epidemiol. 2004;160(12):1214–22. doi: 10.1093/aje/kwh332. [DOI] [PubMed] [Google Scholar]
- 41.de la Rochebrochard E, Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Hum Reprod. 2002;17(6):1649–56. doi: 10.1093/humrep/17.6.1649. [DOI] [PubMed] [Google Scholar]
- 42.Treff NR, Su J, Taylor D, Scott RT., Jr Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011;7(6):e1002161. doi: 10.1371/journal.pgen.1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorland M, van Montfrans JM, van Kooij RJ, Lambalk CB, te Velde ER. Normal telomere lengths in young mothers of children with Down’s syndrome. Lancet. 1998;352(9132):961–2. doi: 10.1016/S0140-6736(05)61516-4. [DOI] [PubMed] [Google Scholar]
- 44.Aydos SE, Elhan AH, Tukun A. Is telomere length one of the determinants of reproductive life span? Arch Gynecol Obstet. 2005;272(2):113–6. doi: 10.1007/s00404-004-0690-2. [DOI] [PubMed] [Google Scholar]
- 45.Turner S, Wong HP, Rai J, Hartshorne GM. Telomere lengths in human oocytes, cleavage stage embryos and blastocysts. Mol Hum Reprod. 2010;16(9):685–94. doi: 10.1093/molehr/gaq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392(6676):569–74. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 47.Zijlmans JM, Martens UM, Poon SS, Raap AK, Tanke HJ, Ward RK, Lansdorp PM. Telomeres in the mouse have large inter-chromosomal variations in the number of T2AG3 repeats. Proc Natl Acad Sci U S A. 1997;94(14):7423–8. doi: 10.1073/pnas.94.14.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]