Abstract
Purpose
This study compared the impact of using the Steiner-Tan pseudo double lumen needle for antral follicle oocyte retrieval to using a conventional non-flushing needle. The Steiner-Tan needle has a much smaller dead space than the needles commonly used for IVM oocyte retrievals.
Methods
This was a retrospective cohort study. The patient population was determined by the time period in which a patient underwent IVM in a single physician’s IVF practice. The following data was abstracted from clinical and embryology records: oocytes retrieved, oocytes matured, early maturing oocytes, oocytes fertilized, embryo quality measures, retrieval time, needle punctures, clot formation, and clinical pregnancy rate.
Results
The Steiner-Tan needle did not increase the number of oocytes retrieved. It also did not increase the time required for retrieval. However, flushing of antral follicles significantly decreased clot formation in fluid aspirates. Use of the Steiner-Tan needle also significantly decreased the number of vaginal needle punctures during each case. There was a trend toward improved embryo quality, but statistical power was inadequate to show a difference.
Conclusions
The primary benefit of the Steiner-Tan needle was on the embryological aspects of IVM. Decreased blood and blood clots in the aspirates made an IVM retrieval more like conventional IVF for the embryologist. The patient also experienced less tissue trauma without increasing anesthesia or surgical time. There was no improvement in the number of oocytes retrieved, but based on the results, we hypothesized that oocytes were more commonly retrieved from slightly large follicles than when using a routine needle.
Keywords: IVM, In vitro maturation, Follicle flushing, Oocyte retrieval, In vitro oocyte maturation techniques
Introduction
In vitro maturation (IVM) is a variant of IVF in which oocytes are retrieved from the antral follicles (AFs) of a patient before FSH stimulation enables most oocytes to undergo reach maturity (polar body extrusion). For most practitioners of IVM, the process is relatively natural, although estrogen, FSH and/or hCG may be used. Retrieved oocytes are cultured in the laboratory and after they achieve maturity, oocytes are treated as in conventional IVF. Usually 60–80 % of oocytes will become mature and the fertilization rate is similar to that of conventional IVF [1, 2, 7, 11, 17]. Some studies suggest that successful pregnancy with IVM is correlated with the number oocytes retrieved [3].
Oocytes are usually retrieved using a single lumen needle (19 to 17 gage). Because the volume of most antral follicles is less than the internal volume of the needle (Tables 1 and 2), several AFs are aspirated before the needle is removed from the vagina and rinsed with media to clear the needle of follicular fluid, blood and oocytes [11]. Because of the blood that has been sitting in the needle for a period of time, the specimen is more difficult for the embryologist to work with, requires additional equipment, and oocyte identification is more time consuming than in conventional IVF [11]. If the follicle could be flushed without removing the needle from the ovary and vagina, all of these issues might be improved upon. Flushing may also enable the physician to more quickly learn how effective he or she was in obtaining oocytes.
Table 1.
Antral follicle volumes
| Antral follicle diameter (mm) | Calculated volume (ml) |
|---|---|
| 2 | 0.003 |
| 4 | 0.025 |
| 6 | 0.085 |
| 8 | 0.201 |
| 10 | 0.393 |
| 12 | 0.679 |
Table 2.
Needle characteristics
| Measured | Cook 19 gage needle | Steiner-Tan 21 gage needle |
|---|---|---|
| Dead space volume (SD) | 0.53 ml (0.08) | 0.096 ml (0.002) |
| Needle tip to ultrasound mark | 5 mm | 4 mm |
| Distance from tip to top of lumen | 3 mm | 3 mm |
The problem of large differences in volumes of antral follicles and the aspiration needle dead space volume was solved by Dr. Hans-Peter Steiner [15]. He invented a pseudo double lumen needle where the volume of the fluid remaining in the needle after flushing was smaller than most visualizable antral follicles (Table 2). The ST needle is composed of three parts: a 7 cm long 21 gage needle which penetrates the vagina and ovary, an adjacent rigid 17 gage tube which carries aspirates and flush media back to a collection tube, and a plastic sheath surrounding this tube for carrying flush media which connects to a flush syringe on one end and extends down to the top of the 21 gage needle on the other end. There are holes drilled in the 21 gage needle so that flush media goes through it into the ovary (Fig. 1). The suction on the longer 17 gage tube pulls the aspirate and flush media away from the patient and into the media collection tube. The process significantly dilutes the aspirate so that it is generally not bloody enough to form clots in the needle or in the collection tube. A major difference between the ST needle and a standard 19 gage single lumen needle that is important for IVM retrievals is the amount of dead space. The dead space in a 19 gage needle will hold the fluid contained in more than four 6 mm follicles; whereas, the dead space in the ST needle will only hold the fluid from only one (Tables 1 and 2).
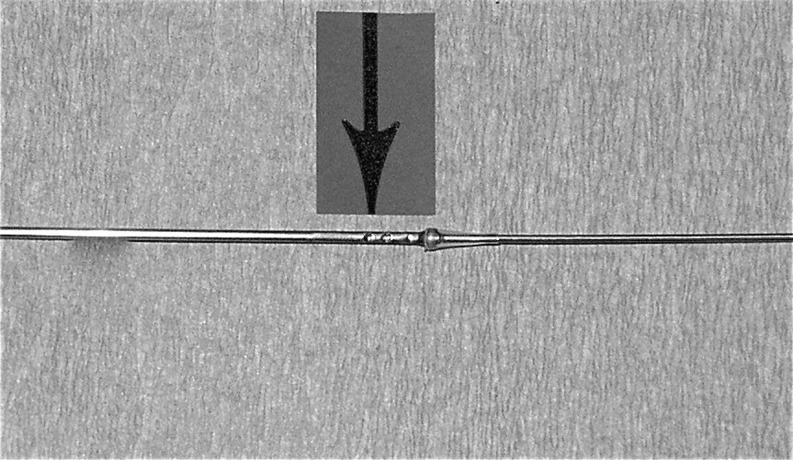
Fig. 1.
Steiner-Tan needle with the plastic outer sheath removed. The arrow indicates the hole through which flush fluid passes flowing in both directions in the needle
The objective of this study was to retrospectively compare the ST needle (www.ivfetflex.com, Graz, Austria) to the needle we had used previously for IVM retrieval: the 19 gage Immature IVM Ovum Aspiration Needle (K-OPS-7035-RWH-ET, Cook Australia, Queensland, Australia) which we will call the Cook needle. We were interested in the impact of these needles on oocyte retrieval effectiveness. We were also concerned about any other impacts on the IVM process that this change in needles might entail. The ST needle used with flushing has not previously been compared to a traditional needle used for IVM. However, follicle flushing with a 16 or 17 gage double lumen needle has been studied in conventional IVF. Flushing with this needle in conventional IVF has convincingly been shown in recent meta-analysis to be ineffective in increasing the number of oocytes retrieved [9, 12]. Additionally, flushing often almost doubles the time required for oocyte collection and may decrease embryo competence [9].
Methods
Our program began using the ST needle exclusively for IVM case retrievals in September of 2011. Embryology and clinical records were reviewed on this first series of patients (through October, 2012). If a patient had more than one retrieval using the ST needle, only the last case was reviewed (18 unique patients). Patients who had IVM retrievals using the Cook needle in the immediate time period preceding the use of the S-T needle were used as a comparison group. This group consisted of all (19) unique patients undertaking IVM between July, 2010 and August, 2011. If a patient had more than one retrieval, only the last retrieval was utilized for this study and if a patient had retrievals using both needles, her records were only used in the ST needle group. Patients (one using the ST needle and three using the Cook needle) without transfers were excluded from this study.
Patients were candidates for IVM if they met routine criteria for IVF, were under 38 years old, and had a total AF count of 20 or greater. AFs were cysts between 2 and 12 mm in diameter. Ovulatory and anovulatory polycystic ovarian patients were not distinguished and were treated identically. Patients self-selected IVM as an alternative to conventional IVF.
All patients were treated with oral contraceptives for cycle timing. Most received low dose gonadotropins for priming1 and all received hCG for priming. Patients with a thin endometrial lining were managed with supplemental estrogen. When the lead follicle averaged 10–12 mm in diameter and the endometrial lining was at least 6 mm in diameter, 10,000 U of HCG was given and a retrieval was performed 38 h later. The retrieval was performed under IV sedation using propofol. Retrievals utilized an Acuson Sequoia 512 ultrasound machine and a Rocket of London suction device (Model 1641-3, Herts, England) set at a pressure of 100 to 120 mmHg. Media used were DPBS (Gibco 14287-080, Grand Island, NY, US), Oocyte Washing Medium with Hepes (ART-1600-A, Sage, Trumbull, CT, US), Oocyte Maturation Medium (ART-1600B, Sage, Trumbull, CT, US) with 75 U/L of Menopur (Ferring Pharmaceuticals, Parsippany, NJ, US) and Embryo Maintenance Medium (ART-1600-C, Sage, Trumbull, CT, US)
When the Cook needle was used, AFs were visualized by ultrasound. The needle was passed through the vagina and into the ovary and follicle. The follicle was aspirated as the needle was gently rotated from side-to-side before the needle was moved within the ovary to another AF. After three or four follicles were aspirated, the needle was removed from the ovary and vagina. The needle tip was placed in a 14 ml polystyrene round bottom collection tube (BD Falcon, Franklin Lakes, NJ) and media was flushed thru the needle tubing and needle to clear it. The tube was then passed to the embryologist for oocyte identification. While this was being done, the needle was again passed through the vagina and into the ovary to try to obtain additional oocytes.
Although the media contained heparin (2 IU/ml), almost all tubes contained blood and clots. As is relatively routine [11] for IVM, a 70 μm nylon cell strainer filter (BD Falcon 352350, Franklin Lakes, NJ, US) was used to aid in oocyte identification. Aspirates were retained for additional review at the end of the surgical case or during breaks between new tubes. About 10 to 20 % of the oocytes retrieved were found in these tubes in later checks. Oocytes retrieved were kept in a holding dish without an oil overlay (oil interfered with the effective use of the filter) on the heated flow hood covered with an air atmosphere.
When the ST needle was used, the needle was passed through the vagina and into the ovary and follicle. The surgeon controlled both the needle movement and flush fluid injection. Flush fluid was contained in a hand held 10 ml syringe (Norm-Ject, Tuttlingen, Germany) that was replaced with a new syringe by an assistant whenever all the fluid in the syringe had been used. With the needle in the follicle, a small amount of fluid was injected into the follicle to confirm correct placement within the AF by visualization of turbulence, before aspiration. Only a small fraction of a milliliter of media was required to produce turbulence. With good placement within the follicle, the AF was rinsed by gently inflating it with media before aspirating the contents. As with the Cook needle, the ST needle was gently rotated from side-to-side during aspiration to enhance the free flow of fluid between the opening in the needle and the follicle wall. The tubes were then passed to the embryologist who provided feedback about its cellular content to the surgeon. The number of flushes, which usually varied from 3 to 12, depended on continued good visualization, good placement in the AF, and feedback from the embryologist that an oocyte had been found or that the fluid was repeatedly acellular.
The embryologist identified oocytes using the same protocols and equipment used in conventional IVF. Oocytes retrieved were kept in a holding dish with an oil overlay on the heated flow hood covered with an air atmosphere.
Needles were removed from the vagina only when needle replacement was deemed necessary or there was a change in the side of the patient’s ovary on which aspiration was being done. About once per case, flushing would impair optimal visualization of the AFs in an ovary and the surgeon would switch ovaries and return later to that side of the patient after visualization cleared which would necessitate another puncture in the vagina.
All data was obtained retrospectively from clinical and laboratory records. The following data was recorded: oocytes retrieved, oocytes matured, oocytes matured at first assessment, oocytes fertilized, the number of patients with at least one good embryo on day 3, the total number of good embryos on day 3, surgical retrieval time, embryology retrieval time, needle punctures, number of aspirates evaluated, patients with clot formation, and clinical pregnancy rate. A good embryo on day 3 was defined as originating from an oocyte that matured within 10 h of aspiration and by day 3 (64 to 72 h after aspiration) had developed into a 7 or 8 cell embryo with less than 10 % fragmentation and with relatively uniform blastomeres [14]. The surgical time was defined as the time interval from when the embryologist was handed the first tube to when she was handed the last tube as recorded on the embryology record sheet. Embryology time was defined as the time interval from when the embryologist was handed the first tube to when all the oocytes for the case had been placed in the holding dish and the embryologist had given up on finding additional oocytes. The number of needle punctures refers to the number of times a needle was passed through the vagina into an ovary. Clot formation refers to macroscopically visible clots that were large enough to potentially completely obscure an oocyte.
Data was analyzed using the unpaired t-test and the Fisher exact test, as appropriate. The software utilized was Prism for Windows, GraphPad Software, Inc. (www.graphpad.com). All p values quoted were two sided. A probability >0.05 was considered not significant (NS). Power and sample size computations used DSS Research, Fort Worth, TX (www.dssresearch.com).
Results
Age and BMI were not different between the ST needle group and the Cook needle group (Table 3). The number of oocytes retrieved, the number of oocytes matured, and the number of oocytes fertilized (11, 7.7, and 5.4 for the ST needle and 11.7, 6.9, and 4.9 for the Cook needle; Table 3), were not different between the two groups. The surgical retrieval time was not increased with flushing using the ST needle (38.9 min for the ST compared to 40.9 min for the Cook; NS) (Table 3).
Table 3.
Results compared for use of the Cook and Steiner-Tan needles
| Cook needle group | Steiner-Tan needle group | P value | |
|---|---|---|---|
| Age (years) (SD) | 29.7 (3.6) | 30.7 (3.0) | 0.348a |
| BMI (SD) | 24.6 (3.6) | 24.7 (3.0) | 0.956a |
| Oocytes retrieved (SD) | 11.7 (6.3) | 11 (3.8) | 0.695a |
| Oocytes matured (SD) | 6.9 (3.4) | 7.7 (2.9) | 0.445a |
| Average number of oocytes with early maturation | 1.6 | 2.7 | 0.017b |
| Oocytes fertilized (SD) | 4.9 (3.0) | 5.4 (2.3) | 0.617a |
| Cases with at least one good embryo | 36.8 % | 66.7 % | 0.103b |
| Percentage of good embryos | 13.8 % | 20.6 % | 0.253b |
| Clinical pregnancy rate | 36.8 % | 50 % | 0.515b |
| Cases with large clots present in at least one tube | 52.6 % | 33.3 % | 0.325b |
| Percentage aspirates with large clots | 9.2 % | 2 % | <0.0001b |
| Needle punctures (SD) | 12.1 (4.2) | 2.7 (0.8) | <0.0001a |
| Surgical time (minutes) (SD) | 40.9 (8.2) | 38.9 (11.4) | 0.541a |
| Embryology time (minutes) (SD) | 42.9 (8.4) | 39.5 (11.8) | 0.319a |
at-test
bFisher’s exact test
The use of the ST needle similarly did not increase embryology case time (39.5 min for the ST compared to 42.9 min for the Cook; NS) (Table 3). It markedly decreased the number of needle punctures used (2.7 for the ST and 12 for the Cook; p < 0.0001) (Table 3). It also decreased the number of patients with clot formation in their collection tubes (6 for ST compared to 10 for the Cook; NS) (Table 3) and markedly decreased the proportion of aspirates containing large clots (2 % for the ST needle and 9.2 % for the Cook needle; p < 0.0001) (Table 3).
The clinical pregnancy rate was not inferior in the ST group compared to the Cook group (50 % versus 36.8 %; NS). Similarly, the number of patients in the ST group who had at least one good embryo compared to the Cook group (66.7 % versus 36.8 %; NS) and the total number of good embryos in the ST group compared to the Cook group (20.6 % versus 13.8 %; NS) were not inferior (Table 3). Also noteworthy was that approximately one additional oocyte with early maturation was identified in the ST needle group compared to the Cook needle group (p = 0.017, Table 3).
Discussion
Although the original motivation to use the ST needle was to increase the number of oocytes retrieved for IVM, this study demonstrated a similar retrieval rate for both needles. A sample size assessment of what would be required to demonstrate a difference in the number of oocytes obtained (with a 5 % alpha error using the SD of the Cook group) based on Steiner’s results [15] found that sample size was adequate with a statistical power of 95 %. More importantly, the use of the ST needle resulted in much easier IVM cases for both the physician and the embryologist without resulting in any problems that might harm the patient (decreased pregnancy rate, decreased embryo quality, increased anesthesia time, or increased tissue trauma).
Compared to the ST needle, the use of the Cook needle was frustrating for the surgeon. Aspiration was done without a source of feedback about how successful the process was. In retrospect, it was clear that without the ability to flush, the needle sometimes appeared to be in an AF when it was not. In spite of that, occasionally, oocytes were found in that aspirate. With the ST needle, providing conformation of the tip being in the needle by flushing, follicles usually deflated with aspiration. This was not true during use of the Cook needle. With the Cook needle, it was possible to believe that the needle was perfectly placed in a 4 to 6 mm follicle in spite of it not deflating. Without any means to test needle placement and without immediate feedback on whether or not an oocyte was obtained, it was possible to lose the ability to aspirate a given follicle with the Cook needle. With the ST needle, the surgeon could be certain that a well visualized follicle was aspirated.
It is possible that slightly different populations of oocytes were obtained with the use of the different needles. The use of the ST needle may have favored successful oocyte retrieval from larger follicles. Larger follicles (with 6 mm diameters or more) were easier structures in which to perform repeated flushes than smaller follicles and were more easily visualized after flushing because of the extra-follicular fluid introduced into the ovary. Larger follicles were likely to produce more useful oocytes [8, 13]. This could contribute to a better quality embryos being obtained if the ST needle is used rather than the Cook needle. This hypothesis is further supported by the finding that more early maturing oocytes were obtained using the ST needle compared to the Cook needle. Early maturation has been reported to be a positive prognostic factor for IVM [14].
The primary benefit of the use of the ST needle appeared to be its impact on the embryological aspects of the case. The oocyte identification procedure and the equipment used were much closer to that of conventional IVF when the ST needle rather than the Cook needle was used. This was primarily due to a significant reduction in the amount of blood in the specimen, which improved visualization of the specimens. A consequence of decreased blood was a reduction in the number of tubes with aspirates containing large clots (2 % versus 9.2 %; p < 0.0001). Because of the impact of decreased blood, aspirate evaluation was much more like conventional IVF than typical IVM. Easier visualization of the aspirate was found after using the ST needle. It also improved oocyte handling and the oocyte’s environment with the decreased presence of clots and the ability to use an oil overlay for temperature maintenance. These factors could contribute to an improved quality of embryos available for transfer [4, 6].
The increase in surgical time which occurs with flushing in conventional IVF has been the primary argument against routinely using flushing for conventional IVF. This clearly is not the case with IVM using the ST needle where the surgical time was not increased with the ST needle compared to the Cook needle. A statistical power computation using the mean and standard deviation from the Cook group and data from Levy [12] shows that there was a 95 % probability that a statistical difference would have been detected (with an alpha error of 5 %). Flushing likely did not increase the time required for retrieval because it was easier to perform than removing the needle from the vagina to clear it and then re-puncture the ovary. We have not used the foot operated fluid delivery device developed by Steiner for use with this needle which may have further shortened the time required for flushing [15].
Another reservation about flushing has been that it might impair the fertilization rate of flushed oocytes [9]. For example, Waterstone and Parsons showed a 43 % fertilization rate for oocytes obtained in the first three flushes and a 24 % fertilization rate for the last three flushes [16]. In contrast, our results comparing the number of oocytes fertilized, the increased number of early maturing oocytes, the presence of high quality embryos and the pregnancy rate, suggest that no harm was done to the embryos by flushing in this setting using the ST needle and that a larger study might find a benefit. A prospective randomized approach would also have provided a more robust approach.
The final beneficiary of the use of the ST needle was the patient, who experienced less tissue trauma with markedly fewer tissue punctures using a smaller gage needle (21 gage compared to 19 gage). Because of the decreased dead space in the needle, it was not necessary to remove the needle from the vagina to clear the oocytes and avoid clots. This reduction in trauma was done without increasing surgery or anesthesia time.
In summary, the ST needle is a major new contribution to the performance of IVM. It should be adopted for IVM retrievals because it reduces the difficulty of the embryology requirements of an IVM case so that they are more similar to a conventional IVF case. It also simplifies the oocyte retrieval procedure for the surgeon and provides him/her immediate information about the effectiveness of the retrieval. The skill sets required by both the surgeon and the embryologist are closer to those of conventional IVF, which should make the IVM procedure more attractive to conventional IVF programs. Although the number of oocytes retrieved was not increased using the ST needle, embryo quality and pregnancy rates were not harmed and for theoretical reasons may have been improved by the use of this needle for retrieval. Unlike some prior results for conventional IVF, antral follicle flushing did not increase the time required for oocyte aspiration.
Footnotes
The choice of using low dose gonadotrophins (50 U FSH for 4 to 6 days) had the objective of increasing antral follicle diameters [8, 13] without pushing past the 12 mm largest AF diameter size target. The value of this approach has been affirmed in recent publications [5, 10].
Capsule The Steiner-Tan needle simplified IVM retrievals, but did not increase the number of oocytes.
References
- 1.Cha KY, Han SE, Chung HM, Choi DH, Lim JM, Lee WS. Pregnancies and deliveries after in vitro maturation culture followed by in vitro fertilization and embryo transfer without stimulation in women with polycystic ovary syndrome. Fertil Steril. 2000;73:978–83. doi: 10.1016/S0015-0282(00)00422-2. [DOI] [PubMed] [Google Scholar]
- 2.Chian RC, Gulekla B, Buckett WM, Tan SL. Priming with human chorionic gonadotropin before retrieval of immature oocytes in women with infertility due to the polycystic ovarian syndrome. N Engl J Med. 1999;341:1624–6. doi: 10.1056/NEJM199911183412118. [DOI] [PubMed] [Google Scholar]
- 3.Child TJ, Phillips SJ, Abdul-Jahil AK, Gelekli B, Tan SL. A comparison of in vitro maturation and in vitro fertilization and embryo transfer for women with polycystic ovaries. Obstet Gynecol. 2002;100:665–70. doi: 10.1016/S0029-7844(02)02193-2. [DOI] [PubMed] [Google Scholar]
- 4.Crane MM, Divine GW, Blackhurst DW, Black CL, Higdon HL, Price TM, et al. Conclusions about the effects on fertilization of time from aspiration to incubation and blood in the aspirate depend on the use of appropriate statistical techniques. Fertil Steril. 2004;81:1548–53. doi: 10.1016/j.fertnstert.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 5.Dal Canto M, Brambillasca F, Renizini MM, Coticchio G, Merola M, Lain M, et al. Cumulus cell-oocyte complexes retrieved from antral follicle in IVM cycles: relationship between COCs morphology, gonadotropin priming and clinical outcome. J Assist Reprod Genet. 2012;29:513–9. doi: 10.1007/s10815-012-9766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daya S, Kohut J, Gunby J, Younglai E. Influence of blood clots in the cumulus complex on oocytes fertilization and cleavage. Hum Reprod. 1990;5:744–6. doi: 10.1093/oxfordjournals.humrep.a137179. [DOI] [PubMed] [Google Scholar]
- 7.Gremeau AS, Andreadis N, Fatum M, Craig J, Turner K, Mcveigh E, et al. In vitro maturation or in vitro fertilization for women with polycystic ovaries? A case–control study of 194 treatment cycles. Fertil Steril. 2012;98:355–60. doi: 10.1016/j.fertnstert.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 8.Guzman L, Ortega-Hrepich C, Albutz FK, Verheyen G, Devroey P, Smitz J, et al. Developmental capacity of in vitro-matured human oocytes retrieved from polycystic ovary syndrome ovaries containing no follicles larger than 6 mm. Fertil Steril. 2012;98:503–7. doi: 10.1016/j.fertnstert.2012.01.114. [DOI] [PubMed] [Google Scholar]
- 9.Hill MJ, Levens ED. To flush or not to flush follicles at oocytes retrieval? In: Sharif K, Coomarasamy A, editors. Assisted reproduction techniques: challenges and management options. Oxford: Wiley Blackwell Publishing Ltd; 2012. pp. 232–5. [Google Scholar]
- 10.Junk SM, Yeap D. Improved implantation and ongoing pregnancy rates after single-embryo transfer with an optimized protocol for in vitro oocyte maturation in women with polycystic ovaries and polycystic ovary syndrome. Fertil Steril. 2012;98:888–92. doi: 10.1016/j.fertnstert.2012.06.055. [DOI] [PubMed] [Google Scholar]
- 11.Jurema MW, Nogueira D. In vitro maturation of human oocytes for assisted reproduction. Fertil Steril. 2006;86:1277–91. doi: 10.1016/j.fertnstert.2006.02.126. [DOI] [PubMed] [Google Scholar]
- 12.Levy G, Hill MJ, Ramirez CI, Correa L, Ryan ME, DeCherney AH, et al. The use of follicle flushing during oocytes retrieval in assisted reproductive technologies: a systemic review and meta-analysis. Hum Reprod. 2012;27:2373–9. doi: 10.1093/humrep/des174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schramm RD, Bavister BD. A macaque model for studying mechanisms controlling oocytes development and maturation in human and non-human primates. Hum Reprod. 1999;14:2544–55. doi: 10.1093/humrep/14.10.2544. [DOI] [PubMed] [Google Scholar]
- 14.Son WY, Lee SY, Lim JH. Fertilization, cleavage and blastocyst development according to the maturation timing of oocytes in in vitro maturation cycles. Hum Reprod. 2005;20:3204–7. doi: 10.1093/humrep/dei195. [DOI] [PubMed] [Google Scholar]
- 15.Steiner HP. Optimizing technique in follicular aspiration and flushing. In: Chavez-Badiola A, Allahbadia GN, editors. Textbook of minimal stimulation IVF—milder, mildest or back to nature. New Delhi: Jaypee Brothers Medical Publishers Ltd; 2011. pp. 98–102. [Google Scholar]
- 16.Waterstone JJ, Parsons JH. A prospective study to investigate the value of flushing follicles during transvaginal ultrasound-directed follicle aspiration. Fertil Steril. 1992;57:221–3. doi: 10.1016/s0015-0282(16)54806-7. [DOI] [PubMed] [Google Scholar]
- 17.Zhao JZ, Zhou W, Zhang W, Ge HS, Huang XF, Lin JJ. In vitro maturation and fertilization of oocytes from unstimulated ovaries in infertile women with polycystic ovary syndrome. Fertil Steril. 2009;91:2568–71. doi: 10.1016/j.fertnstert.2008.03.059. [DOI] [PubMed] [Google Scholar]