Abstract
Itch is the most common symptom described by our patients. Treating this symptom can be challenging. A revolution is ongoing in understanding the pathophysiology of itch and will allow this challenge to be met. The present authors review and update the current understanding of the pathophysiology of itch.
Keywords: itch, mediator, neuron, pruritus
Introduction
Itch is the dominant symptom of a vast variety of diseases from cutaneous inflammatory conditions such as atopic dermatitis to systemic, neurologic, and autoimmune disorders such as hepatic or renal failure, multiple sclerosis, or celiac disease. Recent studies have indicated that this frequently ignored symptom can have a major impact on health-related quality of life (1). It is recognized that antihistamines are not effective to treat most itches. These observations have paralleled a much needed increase of investigation into the mechanisms underlying both acute and chronic itch and will ultimately lead to new and effective therapies. Itch has been the least understood and researched somatosensory modality. This is changing as the growth of dermatologic and neuroscience-based research in the last decade has allowed for a better understanding of the neuro- and physioanatomical bases of itch. The importance of the immune system in mediating cutaneous and neurogenic inflammation also contributes to itch but is beyond the scope of this article. By understanding the pathophysiology of itch, clinicians are better equipped to manage and treat patients with itch. This article describes our current understanding of the pathophysiology of itch.
Types of itch
Itch has been classified into four different clinical categories. These include neurogenic, psychogenic, neuropathic, and pruritoceptive (2) (Table 1). These categories were developed based on anatomical, pathophysiological, and psychological factors. A given patient can have one or more types of itch. These four categories form the structure of this article. Emphasis is placed on pruritoceptive itch following brief discussions of the other types.
Table 1.
Clinical categories of itch
| Four main clinical categories of itch |
|---|
| 1. Neurogenic: Generated in the central nervous system in response to pruritogens but without any evidence of neural pathology. |
| 2. Psychogenic: Itching caused by a psychological disorder. |
| 3. Neuropathic: Results from neuronal pathology along the afferent pathway. |
| 4. Pruritoceptive: Generated in the skin, usually by inflammation or other visible pathological processes involving the skin. |
Neurogenic and systemic itch
Neurogenic and systemic itch result from disorders that affect organ systems other than the skin. These disorders include chronic renal failure, liver disease, hematologic, and lymphoproliferative conditions and malignancies. These itches are transmitted via the central nervous system, but there is no evidence of neural pathology. The administration of opioids in epidural anesthesia frequently results in itch. This observation has led to the hypothesis that neurogenic itch may result, at least in part, from a response to intraspinal endogenous opioids (3). It follows that the administration of opioid antagonists might be expected to be at least partially effective in treating neurogenic itch. Recent advances in itch research have raised the possibility that itch-specific or itch-selective neurons in the spinal cord may provide targets for future therapies.
Psychogenic itch
Psychogenic itch is associated with psychological abnormalities and is considered psychiatric in origin. It typically presents with excessive impulses to scratch or pick at otherwise normal skin (4). Psychogenic pruritus involves brain or psychiatric abnormalities that are not yet well defined, but multiple psychiatric diagnoses including depression, obsessive compulsive disorder, anxiety, somatoform disorders, mania, psychosis, and substance abuse have been associated with itch (5). The incidence of patients in dermatology clinics with psychogenic itch is estimated to be 2% (6). Psychogenic itch is generally a diagnosis of exclusion and requires ruling out other causes of pruritus (4,7).
The most well-known diagnosis with a major psychogenic itch component is delusions of parasitosis. Delusion of parasitosis is a rare psychiatric disorder in which patients have fixed, false beliefs that their body is infested with an insect or parasite. The skin lesions seen in these patients are in response to these delusions (8). Patients typically engage in self-mutilating behavior by scratching and picking their skin in an effort to remove the insect or parasite. In evaluating a patient with psychogenic itch, clinicians should rule out systemic, neuropathic, and dermatologic causes of itch. The mechanism or pathophysiology of psychogenic pruritus is unclear.
Neuropathic itch
Because of the extensive nature of this topic, the present authors refer interested readers to the article in this issue of Dermatologic Therapy, “Neuropathic itch diagnosis and management.” The present authors limit our discussion here to the known pathophysiology of some of the more common causes of neuropathic itch. Neuropathic itch results from damage to central or peripheral sensory neurons, which leads to the firing of pruritic neurons without any cutaneous pruritogenic stimuli (9). Neuropathic itch can be caused by primary lesions or dysfunction at any point along the afferent pathway of the nervous system (4). As the location of the underlying neural damage can be located away from the actual itchy area, scratching a neuropathic itch is rarely effective. Neuropathic itch is often accompanied by other sensory abnormalities such as paresthesia, hyperesthesia, or hypoesthesia. Patients whose neural damage causes both sensory loss as well as neuropathic itch can self-inflict lesions upon themselves via repetitive, painless scratching (9). Of note, many neurological diseases that cause neuropathic itch can also cause neuropathic pain.
The mechanisms of neuropathic itch are poorly understood, but some hypotheses have been proposed. One such hypothesis suggests that local nerve damage to pain- and itch-transmitting C-fiber neurons could result in misfiring of itch-specific C-fibers. In addition, the loss of C-fiber neurons and thus loss of afferent input to the central neurons can lead to uninhibited signaling of centrally located itch neurons, leading to the sensation of itch. Another hypothesis suggests that loss of itch-inhibiting neurons in the spinothalamic tract could result in unwanted activation of the itch sensation (4).
Postherpetic neuralgia (PHN) is a common complication associated with severe cases of shingles (herpes zoster), a condition caused by the reactivation of a dormant varicella zoster virus in the sensory ganglia. PHN is considered to be a type of neuropathy. About half of the patient with this disease also develop postherpetic itch (PHI) (10). Patients with PHN can experience pain and itch simultaneously in the affected area. Skin biopsies for cutaneous nerve density studies from patients with PHN, some of whom also with PHI, have demonstrated loss of cutaneous neurons (11,12). The pain from PHN is believed to be from the loss of nociceptive nerve fibers, and thus, the putative cause of PHI could be damage to the itch-encoding neurons.
Other diseases with a neuropathic itch component include notalgia paresthetica (NP) and brachioradial pruritus (BP). NP is a sensory neuropathy resulting in localized pain, itch, hyperesthesia, or paresthesia. The most common explanation for NP is thoracic nerve root compression. This may be due to degenerated vertebrae or vertebral discs (13). Patients with NP experience itch accompanied by burning pain, paresthesia, and/or hyperesthesia (14). BP is a localized neuropathic pruritus of the dorsolateral arms (15,16). It has been proposed to be due to compression of the cervical nerve root in the level of C5–C8 (17). However, there are cases of BP where there is no evidence of nerve root compression. Prolonged sun exposure is the putative cause of such non-compression presentations. This condition is exacerbated in the summer, and skin biopsies from these patients have demonstrated decreased epidermal and dermal nerve fiber density (15). The exact mechanism of neuropathic itch remains unknown, but studies on postherpetic neuralgia, brachioradial pruritus, and notalgia paresthetica are shedding light on the pathophysiology of this distressing condition.
Pruritoceptive itch
Pruritoceptive itch is the type most frequently encountered by dermatologists. It is generated in the skin either through inflammation or skin damage, and is typically visualized by clinical examination. Age-related changes in the barrier function of the skin can also lead to pruritoceptive itch. This type of itch accounts for the majority of the cases of clinical pruritus because everything from endogenous mediators and exogenous allergens that come into contact with the skin can induce pruritoceptive itch (18).
Neuroanatomy of itch
A discussion of pruritoceptive itch would not be complete without mention of pain. These two sensations may not seem related, yet both pruritoception and nociception (detection of noxious stimuli, such as pain) exist as physiological sensations prompting avoidance of the sensation-causing stimulus. Along with the inherent discomfort associated with itch and pain, both induce protective behaviors (scratching and escape, respectively) in an attempt to reduce one’s exposure to potential adverse outcomes.
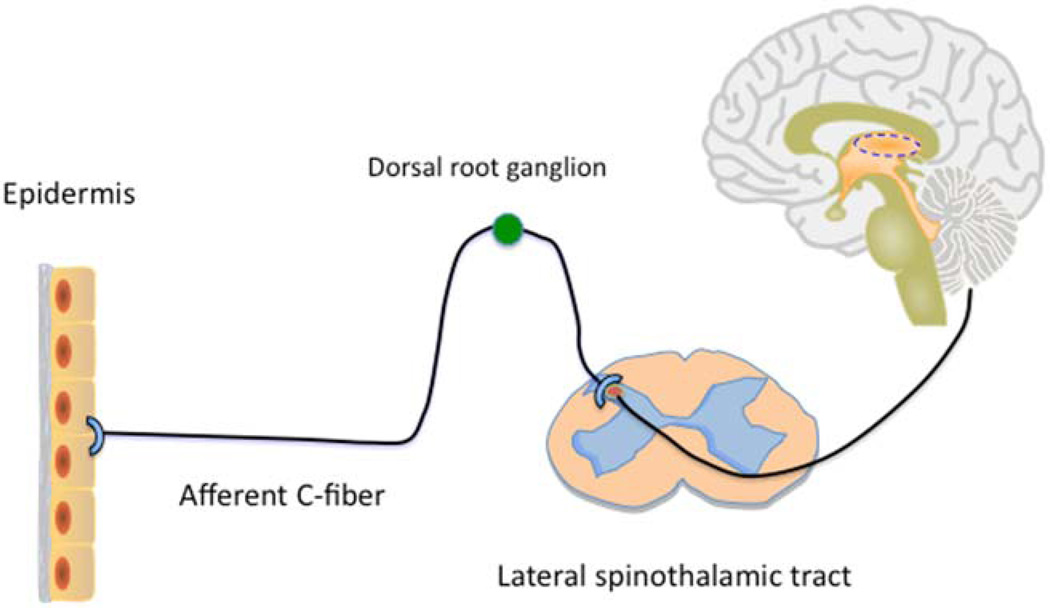
The primary sensory nerve fibers that innervate the skin are categorized into three groups based on the degree of myelination, diameter, and conduction velocity. The thick myelinated Aβ fibers transmit tactile sensation, whereas the thinly myelinated Aδ and unmyelinated C-fibers are mainly involved in the conduction of thermal and pain/itch sensation (19). Itch is transmitted predominately by these unmyelinated, slow conducting C-fibers (FIG. 1). These fibers extend to the dermo-epidermal junction with free endings penetrating into the epidermis where sensation is detected (20). The cell bodies for these fibers are in the dorsal root ganglia (DRG), just outside the spinal cord. From here, both sensations involve secondary transmission neurons that ascend via the contralateral spinothalamic tract to the thalamus (21). These similarities led to past speculation that itch was a low-intensity form of pain transmitted by the same C-fiber neurons. This theory is no longer in favor as ongoing research supports other concepts.
FIG. 1.
Itch pathway from the skin to the brain.
Recent advances, including a variety of genetic models in mice, studies in other mammals, microneurography in humans, and the identification of new itch mediators, have led to insights that are moving the field ahead. Current findings support two hypotheses regarding how the sensation of itch is passed from the skin to the brain. Our view is that elements of both are likely to be correct. The selectivityh theory posits that certain neurons are relatively selective for both itch and pain. The labeled line theory posits that there are itch-specific neuronal fibers. These extend from the skin to the DRG. There, they associate with itch-specific neurons in the spinal cord that send a signal onto the brain. Evidence for itch-specific peripheral neurons has just been reported, whereas itch-specific spinal neurons have been known for some time (22). In addition, recent data suggest that spinal interneurons influence these pathways. These interneurons likely play an important role in determining whether the brain interprets a signal as itch versus pain (23). These, or other spinal interneurons, are likely responsible for the effectiveness of scratching to diminish itch. With respect to the brain, functional magnetic resonance imaging studies are leading to an understanding of how the itch sensation is processed centrally (24).
In the selectivity theory (25,26), there exist overlapping populations of itch and pain fibers. Most fibers respond only to painful stimuli, but some respond to both pain and itch stimuli. The much greater population of pain-related C-fibers exerts an inhibitory influence on the smaller population of itch-sensitive C-fibers. Itch is only perceived when the itch-transmitting C-fibers are selectively activated. If a stimulus activates both itch and pain, then the itch input will be masked by the large population of C-fiber neurons transmitting the pain signal, according to this hypothesis (23). When the pain pathway is activated, it serves to inhibit any itch sensation from the dual-modal pathway. One can infer the evolutionary advantage of this theory in that when afflicted by both pain- and itch-inducing stimuli, this inhibition allows humans to focus on the more dire sensation – pain. The observation that strong pain and itch are not simultaneously perceived and that slightly painful scratching suppresses itch supports this hypothesis. When one scratches itchy stimuli, the pain-only nerves are activated and the itching is blocked (27).
Regarding the labeled line theory, there is evidence for spinal neurons that specifically transmit itch signals. As noted above, it is not clear that there are at least some peripheral neurons that respond to itch stimuli alone.
Cellular anatomy of itch
Are neurons the only cells responsible for detecting itch? Do we know what the nature is of a “free nerve ending”? It is known that keratinocytes and perhaps other skin cells express molecules that can interact with pruritogens. Stimulation of these molecules elicits signals in the keratinocytes. Free nerve endings are certainly present near keratinocytes. These fibers may interact directly with keratinocytes. Together, it is possible that keratinocytes, not just nerve fibers, serve as outposts that detect sensory stimuli. The nature of the interaction between keratinocytes and nerve fibers is an active area of investigation.
Molecular anatomy of itch: channels, receptors, and mediators
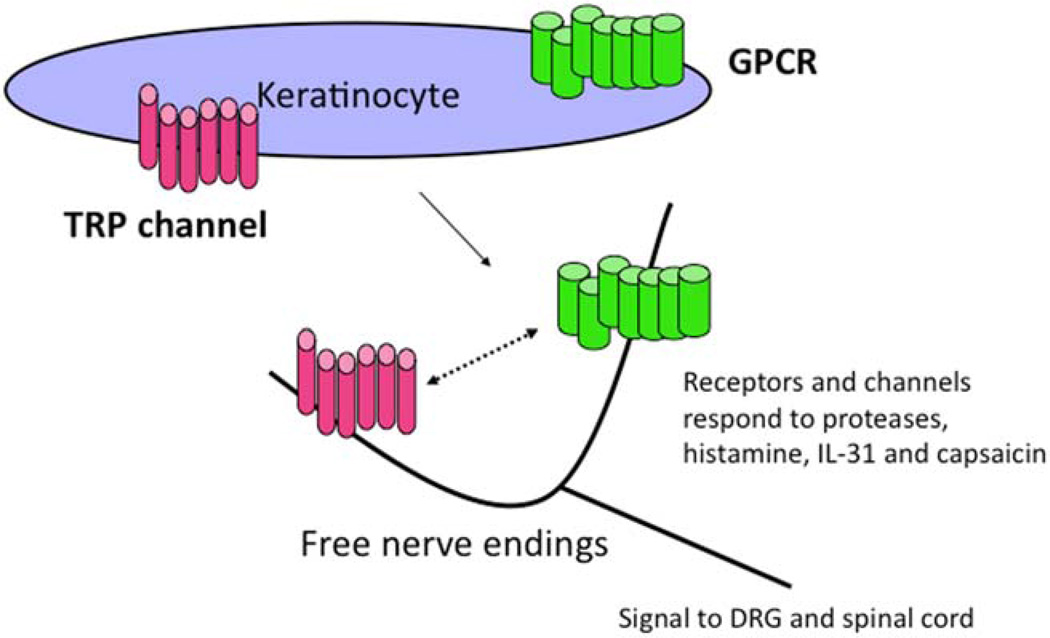
A mediator of itch, a pruritogen, can be defined as a substance that, after induction into the skin, elicits both the sensation of itch and an urge to scratch. These mediators interact with molecular detectors (FIG. 2). These detectors can be receptors or ion channels present on nerve fibers or, as noted earlier, even keratinocytes. The receptors are usually part of the G-protein coupled receptor (GPCR) family. GPCRs detect and respond to a diverse range of ligands or stimuli and are the target of many drugs. GPCRs that are relevant to itch respond to histamine, prostaglandins, neuropeptides, and proteases. The ion channels that appear to be primarily involved are members of the transient receptor potential (TRP) family. As an example, TRPV1 detects capsaicin, the active ingredient in chili peppers. Other TRPs detect heat and cold. Whereas it is not known if TRPs directly detect pruritogens, it is clear that TRPs can be a necessary part of the pathways in which transmission of an itch signal occurs. For example, whereas histamine is detected by a GPCR, the presence of TRPV1 is necessary for an itch signal to be sent along the spinal cord and onto the brain. Certain cytokine receptors may also be involved in itch signaling. Interleukin-31 (IL-31) appears to be a mediator of itch. This cytokine signals via the IL-31 receptor. Itch is thus driven by a variety of mediators that interact with receptors and channels present on the surface of nerve fibers and possibly on keratinocytes. Drugs that block mediators, receptors, or channels have the potential to lead to targeted therapy of peripheral itch independent of blocking inflammation. A brief list of mediators is listed in Table 2.
FIG. 2.
Molecular anatomy of itch. Channels and receptors on the surface of keratinocytes and nerve fibers respond to various mediators. Several examples are provided. The mechanisms by which keratinocytes and neurons communicate with each other are under investigation. DRG, dorsal root ganglia; IL-31, dorsal root ganglia; GPCR, G-protein coupled receptor; TRP, transient receptor potential.
Table 2.
Selected mediators of pruritoceptive itch
| Mediator | Receptor(s) |
|---|---|
| Histamine | H1, H4 |
| Tryptase, cowhagea, cathepsin S, kallikreins, cockroach, and dust mite protease allergens | PAR2 |
| Interleukin-31 | IL-31R |
| Leukotriene B4 | LTB4 |
| Substance P | NK1 |
Cowhage refers to the itchy spicules that cover a plant found throughout the tropics. It is the prototypic mediator of histamine-independent itch.
Histamine had been the paradigm for in vivo modeling of pruritoceptive itch. Although histamine remains important, the itch field is now much broader than this one mediator. Mediators other than histamine had to be important in itch for at least three reasons. First, antihistamines have limited effectiveness. Second, as histamine causes a wheal-and-flare, and most itches are not associated with urticaria, other mediators must be important (28). Third, it has been found that C-fibers can be classified into two overlapping types. One type does not respond to mechanical stimuli but is predominately responsive to histamine. The other type does respond to mechanical stimuli and also to cowhage (see the following paragraphs). It is not yet known if these C-fibers can be distinguished by morphology or by markers. It is now clear that although some pruritogens are derived from mast cells, many, and perhaps most, are not. The present authors will restrict our focus to histamine, proteases, IL-31, and the neuropeptides CGRP and substance P.
Histamine is released from the granules of mast cells in a response to exogenous and endogenous stimuli. Four distinct histamine GPCRs have been indentified, H1, H2, H3, and H4, with the first and last having a role in itch (3,29). The H4 receptor was discovered relatively recently and was shown to be important in mast cell and eosinophil function, and in allergic inflammation in vivo (30,31). H4 receptor activation was also shown to produce acute itching separate from H1 activation (32). H4 receptor antagonists have been receiving attention as a potential new antipruritoceptive target as the use of a H4 antagonist was shown to be superior to traditional antihistamines in the attenuation of experimental pruritus in mice (33).
The mechanism of nonhistaminergic itch was established by coalescing findings. Cowhage evokes a strong itch independent of histamine. Cowhage is the common name for the tropical bean plant, Mucuna pruriens, the spicules that cover its pods, or the cysteine protease which is the active component of the spicules (34). This protease is closely homologous to a series of human proteases called cathepsins, particularly cathepsin S, which also causes itch. Additional human proteases implicated in itch are tryptase, derived from mast cells, and kallikreins, produced by keratinocytes, and available when the epidermal barrier is disrupted (35). All of these proteases can lead to the activation of protease-activated receptor-2 (PAR2). PAR2 is expressed on afferent neuron terminals and keratinocytes (36–38). Cockroach and dust mite allergens are proteases that also stimulate PAR2. PAR2 upregulation has been shown in patients with atopic dermatitis (37). PAR2 activation of the primary spinal afferent neurons leads to the release of proinflammatory neuropeptides including CGRP and substance P, which have been linked to the sensation of itch (39). Substance P and CGRP-mediated itch might also be related to their ability to activate mast cells (40). The activation of PAR2 by so many protease pruritogens suggests promising clinical applications by means of protease or PAR2 antagonists.
The transient receptor potential vanilloid receptor-1 (TRPV1) is a nonselective cation channel that has been referred to as the “capsaicin receptor” because of its ability to bind capsaicin, the active component of chili peppers (41,42). TRPVI is expressed on sensory neurons, keratinocytes, mast cells, and endothelial cells (43–45). TRP channels were originally presumed to be nociception-specific due to their activation by both the burning pain of capsaicin and the noxious temperatures (46).However, they have been implicated in pruritoceptive pathways because studies in TRPV1-deficient mice have shown diminished scratching in response to histamine or trypsin (47) and TRPV1 has been found to be required in histamine and serotonin-induced itch (48). Further supporting TRPV1’s role in itching is the fact that its wide expression is even more emphasized in patients with prurigo nodularis (43). Activation of TRPV1-expressing sensory neurons by pruritogens appears to utilize multiple different intracellular signal-transducing mechanisms to mediate itch signals (48).
Clinically, there are several anti-pruritic drugs that target TRP channels. Repeated topical applications of capsaicin have been shown to desensitize sensory nerves and reduce pruritus via the depletion of relevant neuropeptides (43). However, a recent review of controlled trials involving topical capsaicin as an anti-pruritogen found a much less conclusive result and determined that there is no suitable evidence for use of capsaicin to treat pruritus (49). Finally, another TRP channel, TRPM8, transduces cold sensation (50) and allows for the inhibition of pruritus by menthol and cold (51).
Interleukin-31 has emerged as a mediator of pruritus. IL-31 has been detected in the skin of patients with atopic dermatitis and prurigo nodularis (52,53). In addition, genetic mutations in the IL-31 receptor have been implicated in the pathogenesis of a form of localized pruritic disease known as familial primary localized cutaneous amyloidosis (54,55). IL-31 is produced predominantly by Th2 lymphocytes. These T cells contribute to the pathogenesis of atopic dermatitis. It has been postulated that IL-31 exerts its pruritogenic effect by directly binding to IL-31 receptors on cutaneous nerves, but the precise mechanisms remains unknown. Administration of antibodies to IL-31 reduces scratching behavior in a mouse model of atopic dermatitis. This finding supports the possibility that IL-31 is a mediator of pruritus (55).
Substance P, as mentioned before, is a neuropeptide released from mast cells and can mediate itch and neurogenic inflammation (40,56). It is a tachykinin that binds to neurokinin receptors NK1, NKR2, and NKR3 (57). The NKR1 receptor has been implicated in the induction of itch in rats (58). In addition, increased expression of the neurokinin-1 receptor has been reported on keratinocytes in pruritic skin diseases (59).
Conclusion
In closing, there is an ongoing revolution in the understanding of the pathophysiology of itch. This understanding is likely to lead to therapies that specifically target the itch of distinct conditions. Alleviating the symptom of itch will improve the quality of life of patients even if the underlying condition remains active. Keep these concepts in mind as you read through the other articles in this issue.
Acknowledgements
The present authors thank Dr. Sarina Elmariah for helpful comments. This work was supported by NIH grant R01AR057744 to E. A. L.
References
- 1.Kini SP, DeLong LK, Veledar E, McKenzie-Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147(10):1153–1156. doi: 10.1001/archdermatol.2011.178. [DOI] [PubMed] [Google Scholar]
- 2.Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361(9358):690–694. doi: 10.1016/S0140-6736(03)12570-6. [DOI] [PubMed] [Google Scholar]
- 3.Greaves MW. Pathogenesis and treatment of pruritus. Curr Allergy Asthma Rep. 2010;10(4):236–242. doi: 10.1007/s11882-010-0117-z. [DOI] [PubMed] [Google Scholar]
- 4.Yosipovitch G, Samuel LS. Neuropathic and psychogenic itch. Dermatol Ther. 2008;21(1):32–41. doi: 10.1111/j.1529-8019.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan A, Koo J. Psyche, opioids, and itch: therapeutic consequences. Dermatol Ther. 2005;18(4):314–322. doi: 10.1111/j.1529-8019.2005.00038.x. [DOI] [PubMed] [Google Scholar]
- 6.Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs. 2001;15(5):351–359. doi: 10.2165/00023210-200115050-00002. [DOI] [PubMed] [Google Scholar]
- 7.Gupta MA, Gupta AK, Ellis CN, Koblenzer CS. Psychiatric evaluation of the dermatology patient. Dermatol Clin. 2005;23(4):591–599. doi: 10.1016/j.det.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Koblenzer CS. Cutaneous manifestations of psychiatric disease that commonly present to the dermatologist – diagnosis and treatment. Int J Psychiatry Med. 1992;22(1):47–63. doi: 10.2190/JMLB-UUTJ-40PN-KQ3L. [DOI] [PubMed] [Google Scholar]
- 9.Oaklander AL. Common neuropathic itch syndromes. Acta Derm Venereol. 2012;92(2):118–125. doi: 10.2340/00015555-1318. [DOI] [PubMed] [Google Scholar]
- 10.Oaklander AL, Cohen S, Raju SV. Intractable postherpetic itch and cutaneous deafferentation after facial shingles. Pain. 2002;96(1–2):9–12. doi: 10.1016/s0304-3959(01)00400-6. [DOI] [PubMed] [Google Scholar]
- 11.Oaklander AL, Romans K, Horasek S, Stocks A, Hauer P, Meyer RA. Unilateral postherpetic neuralgia is associated with bilateral sensory neuron damage. Ann Neurol. 1998;44(5):789–795. doi: 10.1002/ana.410440513. [DOI] [PubMed] [Google Scholar]
- 12.Rowbotham MC, Yosipovitch G, Connolly MK, Finlay D, Forde G, Fields HL. Cutaneous innervation density in the allodynic form of postherpetic neuralgia. Neurobiol Dis. 1996;3(3):205–214. doi: 10.1006/nbdi.1996.0021. [DOI] [PubMed] [Google Scholar]
- 13.Savk E, Dikicioğlu E, Culhaci N, Karaman G, Sendur N. Immunohistochemical findings in notalgia paresthetica. Dermatology. 2002;204(2):88–93. doi: 10.1159/000051823. [DOI] [PubMed] [Google Scholar]
- 14.Springall DR, Karanth SS, Kirkham N, Darley CR, Polak JM. Symptoms of notalgia paresthetica may be explained by increased dermal innervation. J Invest Dermatol. 1991;97(3):555–561. doi: 10.1111/1523-1747.ep12481889. [DOI] [PubMed] [Google Scholar]
- 15.Wallengren J, Sundler F. Brachioradial pruritus is associated with a reduction in cutaneous innervation that normalizes during the symptom-free remissions. J Am Acad Dermatol. 2005;52(1):142–145. doi: 10.1016/j.jaad.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Cohen AD, Masalha R, Medvedovsky E, Vardy DA. Brachioradial pruritus: a symptom of neuropathy. J Am Acad Dermatol. 2003;48(6):825–828. doi: 10.1067/mjd.2003.494. [DOI] [PubMed] [Google Scholar]
- 17.Goodkin R, Wingard E, Bernhard JD. Brachioradial pruritus: cervical spine disease and neurogenic/neuropathic [corrected] pruritus. J Am Acad Dermatol. 2003;48(4):521–524. doi: 10.1067/mjd.2003.203. [DOI] [PubMed] [Google Scholar]
- 18.Yosipovitch G. Assessment of itch: more to be learned and improvements to be made. J Invest Dermatol. 2003;121(6):xiv–xxv. doi: 10.1111/j.1523-1747.2003.12650.x. [DOI] [PubMed] [Google Scholar]
- 19.Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol. 2002;87(2):239–244. [PubMed] [Google Scholar]
- 20.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17(20):8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4(9):617–622. doi: 10.2165/00128071-200304090-00004. [DOI] [PubMed] [Google Scholar]
- 22.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2012;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handwerker HO. Microneurography of pruritus. Neurosci Lett. 2010;470(3):193–196. doi: 10.1016/j.neulet.2009.06.092. [DOI] [PubMed] [Google Scholar]
- 24.Napadow V, Li A, Loggia ML, Kim J, Schalock PC, Lerner E, Tran TN, Ring J, Rosen BR, Kaptchuk TJ, Pfab F. The brain circuitry mediating antipruritic effects of acupuncture. Cerebral Cortex. 2013 doi: 10.1093/cercor/bhs363. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7(7):535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 26.Patel KN, Dong X. Itch: cells, molecules, and circuits. ACS Chem Neurosci. 2011;2(1):17–25. doi: 10.1021/cn100085g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikoma A, Rukwied R, Ständer S, Steinhoff M, Miyachi Y, Schmelz M. Neurophysiology of pruritus: interaction of itch and pain. Arch Dermatol. 2003;139(11):1475–1478. doi: 10.1001/archderm.139.11.1475. [DOI] [PubMed] [Google Scholar]
- 28.Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135(12):1522–1525. doi: 10.1001/archderm.135.12.1522. [DOI] [PubMed] [Google Scholar]
- 29.Jutel M, Akdis M, Akdis CA. Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy. 2009;39(12):1786–1800. doi: 10.1111/j.1365-2222.2009.03374.x. [DOI] [PubMed] [Google Scholar]
- 30.de Esch IJ, Thurmond RL, Jongejan A, Leurs R. The histamine H4 receptor as a new therapeutic target for inflammation. Trends Pharmacol Sci. 2005;26(9):462–469. doi: 10.1016/j.tips.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Dunford PJ, O’Donnell N, Riley JP, Williams KN, Karlsson L, Thurmond RL. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J Immunol. 2006;176(11):7062–7070. doi: 10.4049/jimmunol.176.11.7062. [DOI] [PubMed] [Google Scholar]
- 32.Bell JK, McQueen DS, Rees JL. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br J Pharmacol. 2004;142(2):374–380. doi: 10.1038/sj.bjp.0705754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunford PJ, Williams KN, Desai PJ, Karlsson L, McQueen D, Thurmond RL. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol. 2007;119(1):176–183. doi: 10.1016/j.jaci.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 34.Shelley WB, Arthur R. The neurohistology and neurophysiology of the itch sensation in man. AMA Arch Dermatol. 1957;76(3):296–323. doi: 10.1001/archderm.1957.01550210020004. [DOI] [PubMed] [Google Scholar]
- 35.Järvikallio A, Naukkarinen A, Harvima IT, Aalto ML, Horsmanheimo M. Quantitative analysis of tryptase- and chymase-containing mast cells in atopic dermatitis and nummular eczema. Br J Dermatol. 1997;136(6):871–877. [PubMed] [Google Scholar]
- 36.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28(17):4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinhoff M, Neisius U, Ikoma A, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23(15):6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinhoff M, Vergnolle N, Young SH, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6(2):151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 39.Hägermark O. Itch mediators. Semin Dermatol. 1995;14(4):271–276. doi: 10.1016/s1085-5629(05)80047-1. [DOI] [PubMed] [Google Scholar]
- 40.Weidner C, Klede M, Rukwied R, Lischetzki G, Neisius U, Skov PS, Petersen LJ, Schmelz M. Acute effects of substance P and calcitonin gene-related peptide in human skin – a microdialysis study. J Invest Dermatol. 2000;115(6):1015–1020. doi: 10.1046/j.1523-1747.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- 41.Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51(2):159–212. [PubMed] [Google Scholar]
- 42.Morita A, Iwasaki Y, Kobata K, Iida T, Higashi T, Oda K, Suzuki A, Narukawa M, Sasakuma S, Yokogoshi H, Yazawa S, Tominaga M, Watanabe T. Lipophilicity of capsaicinoids and capsinoids influences the multiple activation process of rat TRPV1. Life Sci. 2006;79(24):2303–2310. doi: 10.1016/j.lfs.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 43.Stander S, Moormann C, Schumacher M, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13(3):129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 44.Bodó E, Kovács I, Telek A, Varga A, Paus R, Kovács L, Bíró T. Vanilloid receptor-1 (VR1) is widely expressed on various epithelial and mesenchymal cell types of human skin. J Invest Dermatol. 2004;123(2):410–413. doi: 10.1111/j.0022-202X.2004.23209.x. [DOI] [PubMed] [Google Scholar]
- 45.Denda M, Fuziwara S, Inoue K, Denda S, Akamatsu H, Tomitaka A, Matsunaga K. Immunoreactivity of VR1 on epidermal keratinocyte of human skin. Biochem Biophys Res Commun. 2001;285(5):1250–1252. doi: 10.1006/bbrc.2001.5299. [DOI] [PubMed] [Google Scholar]
- 46.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 47.Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27(9):2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106(27):11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gooding SM, Canter PH, Coelho HF, Boddy K, Ernst E. Systematic review of topical capsaicin in the treatment of pruritus. Int J Dermatol. 2010;49(8):858–865. doi: 10.1111/j.1365-4632.2010.04537.x. [DOI] [PubMed] [Google Scholar]
- 50.Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol. 2011;21(6):880–887. doi: 10.1016/j.conb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Bromm B, Scharein E, Darsow U, Ring J. Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neurosci Lett. 1995;187(3):157–160. doi: 10.1016/0304-3940(95)11362-z. [DOI] [PubMed] [Google Scholar]
- 52.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, Alenius H, Dieu-Nosjean MC, Meller S, Rieker J, Steinhoff M, Hoffmann TK, Ruzicka T, Zlotnik A, Homey B. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117(2):411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 53.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J, Bort S, Mudri S, Kuijper JL, Bukowski T, Shea P, Dong DL, Dasovich M, Grant FJ, Lockwood L, Levin SD, LeCiel C, Waggie K, Day H, Topouzis S, Kramer J, Kuestner R, Chen Z, Foster D, Parrish-Novak J, Gross JA. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5(7):752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka A, Arita K, Lai-Cheong JE, Palisson F, Hide M, McGrath JA. New insight into mechanisms of pruritus from molecular studies on familial primary localized cutaneous amyloidosis. Br J Dermatol. 2009;161(6):1217–1224. doi: 10.1111/j.1365-2133.2009.09311.x. [DOI] [PubMed] [Google Scholar]
- 55.Grimstad O, Sawanobori Y, Vestergaard C, Bilsborough J, Olsen UB, Grønhøj-Larsen C, Matsushima K. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol. 2009;18(1):35–43. doi: 10.1111/j.1600-0625.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- 56.Amatya B, Nordlind K, Wahlgren CF. Responses to intradermal injections of substance P in psoriasis patients with pruritus. Skin Pharmacol Physiol. 2010;23(3):133–138. doi: 10.1159/000270385. [DOI] [PubMed] [Google Scholar]
- 57.Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martín JD, Candenas ML. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem. 2004;11(15):2045–2081. doi: 10.2174/0929867043364748. [DOI] [PubMed] [Google Scholar]
- 58.Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21(4):303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang SE, Han SS, Jung HJ, Choi JH. Neuropeptides and their receptors in psoriatic skin in relation to pruritus. Br J Dermatol. 2007;156(6):1272–1277. doi: 10.1111/j.1365-2133.2007.07935.x. [DOI] [PubMed] [Google Scholar]