Abstract
The rate of phosphocreatine (PCr) resynthesis following physical exercise, has been extensively studied with 31P-MRS. Previous studies have used small surface coils that were limited to measuring one superficial muscle per experiment.
Our present work focuses on the development and implementation of a spectrally selective 3D turbo-spin-echo sequence at 3T and 7T with temporal resolution of 24s, using two geometrically identical volume coils. We acquired imaging data of PCr recovery from four healthy volunteers and one diabetic patient, who performed plantar flexions using resistance bands. We segmented the anatomical regions of six different muscles from the lower leg, namely the gastrocnemius (lateral and medial), the tibialis (anterior and posterior), the soleus and the peroneus and measured the local PCr resynthesis rate constants. During the same examination, we also acquired unlocalized 31P-MRS data at a temporal resolution of 6s.
At 3T, the PCr resynthesis rate constants were measured at 25.4±3.7s (n=4, mean±standard deviation) using the MRS method and 25.6±4.4s using the MRI method. At 7T, the measured rates were 26.4±3.2s and 26.2±4.7s for MRS and MRI. Using our imaging method, we measured the local PCr resynthesis rate constants in six individual muscles of the lower leg (min/max 20.2/31.7s). The recovery rate constants measured for the diabetic patient were 55.5s (MRS) and 52.7s (MRI).
The successful implementation of our 3D-method suggests that imaging is possible at both fields with relatively high spatial resolution (voxel size: 4.2 ml at 3T and 1.6 ml at 7T) using volume coils and that local PCr resynthesis rates can be obtained in a single measurement. The advantage of the imaging method is that it can highlight differences in PCr resynthesis rates between different muscles in a single measurement in order to study spatial gradients of metabolic properties of diseased states for which very little is currently known.
Keywords: Phosphocreatine resynthesis, 31P MRI, TSE MRI, human calf muscle, lower leg muscles, phosphorus MRI
INTRODUCTION
Phosphorus Magnetic Resonance Spectroscopy (31P-MRS) offers the ability to detect important metabolites in a non-invasive manner, and therefore has become the standard method for studying mammalian metabolism (1-7). More specifically, 31P-MRS is a well-established method for measuring the rate of phosphocreatine (PCr) resynthesis after exercise, which is a valid index of mitochondrial oxidative metabolism in the muscle (8-10), and has been used extensively to differentiate between normal and pathological states (11-14).
Human skeletal muscles vary in fibre type and composition, which can result in variable functional properties among different muscle groups (15) that can affect the oxidative capacity of muscles and their response to exercise. Increased muscle functional variability can result from certain disease states, such as diabetes, that can create heterogeneous patterns of altered metabolic function (16). The vast majority of 31P-MRS studies of dynamic recovery of muscles after physical exercise employ surface coils, which are placed adjacent to the muscle of interest (10). These methods often make use of simple pulse-acquire sequences, and provide high signal-to-noise (SNR) spectra with adequate temporal resolution (typically on the order of seconds) to follow the dynamics of muscle recovery. However, these techniques are limited to measuring only superficial muscle regions, and they cannot differentiate between signals that originate from different muscle groups.
Imaging methods that can provide simultaneous measurement of PCr resynthesis rates in several muscles of the exercising body part can potentially bring new insights into the mechanisms of muscle function, or reveal patterns of disease propagation. Forbes and co-workers (17) used a low-intensity gated exercise protocol (18) and 31P chemical shift imaging (19,20). Low intensity exercise protocols allowed the measurement of PCr recovery, with little or no muscle acidification, but they also required long acquisition times. In addition, the use of a surface coil required careful repositioning of the coil and repetition of the exercise in order to acquire a cross-section of the muscles of the lower leg. It has been shown that more rapid acquisitions can be achieved when a single 31P metabolite is imaged with the use of spectrally selective pulse sequences. Such methods have been increasingly used to study 31P metabolites at rest (21-26). Very recently, Greenman et al. (27,28) demonstrated the feasibility of using spectrally selective imaging sequences to study the recovery of a cross-section of the human arm muscle after exercise at 3T, with results very similar to those obtained with the more established 31P-MRS methods.
This preliminary work focuses on the development of a spectrally selective three-dimensional turbo spin echo (3D-TSE) sequence for the simultaneous measurement of PCr resynthesis rates across several muscles of the lower leg, following a single exercise experiment. We implemented the sequence both at 3T and 7T, to prove its feasibility and accuracy.
MATERIALS AND METHODS
Study Subjects
We recruited four healthy volunteers (two male and two female, mean ± standard deviation age: 29.8 ± 4.0 yr). The recruited female volunteers are professional dancers, while the male volunteers are amateur basketball players, all of whom were scanned both on a 3T and a 7T Siemens MRI scanner (Siemens Medical Solutions, Erlangen, Germany). For each volunteer, the two scans were performed on different days. We also recruited one diabetic patient (51 year-old male) who was scanned on the 7T magnet. The New York University School of Medicine Institutional Review Board approved the examination protocol for the study, and all the subjects provided written informed consent for their participation.
Exercise Protocol
During each examination, the subject performed the exercise twice. We collected MRS data for the first performance of the exercise, which was followed by a 10 min interval during which no physical exercise was performed, to ensure full PCr recovery for all subjects. During the second execution of the exercise, we collected MRI data.
For the exercise protocol, the subject lay supine on the scanning table with the right leg inside the volume coil. After we gave a signal, the subject began the exercise, which consisted of repeated plantar flexions using resistance bands at a frequency of one repetition per second until fatigue was experienced (typically after 1 - 2 min), and we took note of the exact time when each subject stopped exercising. Data were collected continuously before the exercise (baseline), during, and after the exercise for the MRS and MRI experiments, both at 3T and 7T. It has to be noted that for the scope of this feasibility study the force exerted by each subject was not quantified.
NMR
Setup and Coils
The coils at 3T and 7T were geometrically identical, dual-tuned 31P/1H quadrature volume (Rapid MRI, Ohio) with an 18 cm inner diameter and 20 cm resonator length. At the beginning of all measurement sessions, we used the iterative first and second order shimming algorithm provided by the manufacturer, on the entire volume of the calf muscle, using the proton channel. In addition, we performed manual pulse calibration for each volunteer using the phosphorus channel.
MRS
We used a product unlocalized pulse-acquire sequence with a repetition time of 6 s. We acquired 2048 points of the free induction decay, with a spectral width of 5000 Hz, at 7T. At 3T, the same pulse sequence was used with the same repetition time and a reduced spectral width of 3000 Hz. In both fields, we used a non-selective 90° pulse with duration of 400 μs at 3T and 200 μs at 7T respectively.
MRI
The 3D-TSE method we developed has several modifications compared to more traditional TSE methods (29). The pulse sequence, a schematic of which is shown in Fig.1a, used a spectrally selective excitation pulse and sampled k-space in a fully centric manner. Phase encoding in the y-direction was segmented. Within each repetition, 24 ky lines were acquired with centric ordering (so that the center of ky space was acquired first). Equal area crushers were applied on either side of the refocusing pulses to remove free induction decay signals produced by imperfections of the 180° refocusing pulses. The gradients were optimized to induce a minimum of 8π phase dispersion along the pixel-size as described elsewhere (30). The center of k-space was sampled at 26 ms, and the echo-spacing was set to 26 ms which minimized any possible contamination from ATP signal (21,25). Data were acquired at each readout with a 2.5 kHz bandwidth (dwell time: 400 μs). A spectrally selective 90° Gaussian pulse (16 ms duration at 3T and 8 ms at 7T) excited a single resonance peak of the 31P spectrum (i.e. PCr), without the use of a slice selective gradient. A train of 24 non-selective square refocusing (180°) pulses, each of 1 ms duration, was used with the phase of each pulse held constant at 90° relative to the initial excitation pulse (Carr-Purcell-Meiboom-Gill condition). To avoid any wrap around aliasing artifacts due to lack of slab-selective gradients, the field of view (FOV) in the slice direction was equal to the sensitive region of the coil (200 mm). The FOV at 3T was 220 × 220 × 200 mm with matrix size of 24 × 24 × 4 that resulted in voxel size of 4.2 ml. The TR was 6 s with 24 s per image acquisition. At 7T, the FOV was 192 × 192 × 200 with matrix size of 24 × 24 × 8 and voxel size of 1.6 ml, with TR of 3 s and 24 s per image acquisition. We have characterized the point-spread function (PSF) of our imaging sequence along the y-direction (where the echo-train was sampled) in our previous work (31), where we estimated it at 1.6 pixels at 3T and 2.8 pixels at 7T.
Fig.1.

a) Schematic diagram of the 3D-TSE sequence. The moment for the z-gradient (Gz) is kept constant for a given echo train, while the y-phase (Gy) encoding gradient is stepped to cover kz space in segmented fashion. The echo train length (ETL) is 24. Equal area crushers were applied on either side of the refocusing pulses to remove free induction decay signals produced by imperfections of the 180° refocusing pulses.
Measurement of the RF Excitation Pulse Width
In the 31P spectrum, the closest peak to PCr is that of γ-ATP (2.5 ppm), which in the scanners used in this study has a peak separation of 299 Hz at 7T and 124 Hz at 3T. We measured the bandwidth of the spectrally selective Gaussian pulse employed in the TSE sequence by acquiring a series of images of a 1000 ml solution containing 85% phosphoric acid (Fischer Scientific, Pittsburgh, USA). We acquired 61 images by incrementing the scanner’s transmission frequency in steps of 20 Hz from 600 Hz below the resonance frequency of Pi to 600Hz above and measured the mean intensity of all voxels that contained signal from the solution.
To further confirm the selectivity of the pulse, we acquired fully relaxed 3D-TSE images (with TR of 30 s, and the same acquisition parameters as described in the previous section) from three 50 ml buffered solutions containing 50 mM of ATP, PCr, and Pi by centering the transmission frequency on the PCr, Pi and γ-ATP peaks respectively.
Proton Imaging
To verify muscle anatomy, we used a product gradient echo (GRE) sequence to acquire 3D-1H images (with the same FOV and orientation as the 31P images) in all volunteers. Acquisition parameters were: matrix size 128 × 128 × 40, TR: 20 ms, TE 3.5 ms, FA 8°, acquisition time 1 min 44 s.
Data Analysis
MRS-MRI
All spectra were processed using the ‘Matlab NMR-library’ spectroscopy processing software (32). Post-processing included zero-filling to 8192 points, baseline correction to remove broadband background signal from immobile phosphate compounds, and constant phasing for all spectra within a measurement. To assess the spectral quality of the data, we determined the SNR and the linewidth acquired before the beginning of the exercise. We measured spectral SNR as the ratio between the PCr peak amplitude and the peak-to-peak spectral noise in the range from 10 to 15 ppm of the 31P spectrum. We defined linewidth as the full width at half maximum (FWHM) of the PCr peak. For the imaging experiments, we measured SNR for each subject by dividing the average signal of all voxels of the muscle by the standard deviation of the signal of voxels outside the volume of the muscle, in the pre-exercise experiment.
PCr Depletion and Recovery Rate Constant Calculation
In order to quantify the working amplitude for each volunteer, we fitted a linear function to the PCr data acquired during the performance of the exercise (both with MRS and MRI). From the slope of the fit, we estimated the PCr depletion rate for each subject. A 10 min interval with no physical exercise ensured full recovery for all subjects, which was confirmed by comparing fully relaxed spectra (with TR = 30 s at 3T, and TR = 15 s at 7T) acquired prior to the first and the second execution of the exercise.
After normalizing the acquired data (both MRS and MRI) to the pre-exercise value for each subject, we fitted them to a single exponential recovery function according to the following equation, using a least squares minimization algorithm.
| [1] |
In Eq.1, PCr0 is the PCr level at the end of exercise, a is the difference between the steady state level and the PCr after recovery and k is the resynthesis rate constant in seconds. For the MRI data, we fitted the mean signal of all voxels of the muscle to the exponential growth. We estimated the first point of the PCr recovery by extrapolating the fitted line of the depletion phase to the end time of the exercise. In addition, we manually segmented the different muscles and fitted Eq.1 in each volume of interest in order to measure the local PCr resynthesis rate.
Statistical Analyses
To compare the PCr resynthesis rate constants obtained with our newly developed imaging sequence to those obtained with the MRS method, we calculated the population Pearson correlation coefficient between unlocalized MRS and MRI measurements for each volunteer, with significance set at the 0.05 level.
RESULTS
The bandwidth profile of the Gaussian pulses, both at 3T and 7T is shown in Fig.2.a. The FWHM of the pulse was 122 Hz at 3T and 220 Hz at 7T. In both cases the pulse bandwidth is narrow enough to avoid unwanted excitation of other metabolites, especially γ-ATP, which has the closest peak separation (124 Hz at 3T and 299 Hz at 7T). The selectivity of the pulse is shown in Fig.2.b-e. The images acquired at 3T with the excitation centre around the peaks of γ-ATP, PCr and Pi, show the selective excitation of each peak without contamination from another metabolite. This was further confirmed by examining the signal in the voxels within the tubes of the non-selected metabolites that were found to be on the noise level.
Fig.2.
a) Experimentally derived bandwidth profile of the Gaussian excitation pulse (FWHM: 122 Hz at 3T and 220 Hz at 7T). The bandwidth is narrow enough to avoid excitation of spins from unwanted resonances. b) 1H image of all three phantoms that contain 50 mM buffered solutions of PCr, ATP and Pi respectively. c-e) Imaging resulting from fully relaxed (TR = 30 s) 3D spectrally selective TSE centered on the γ-ATP, PCr and Pi peaks respectively.
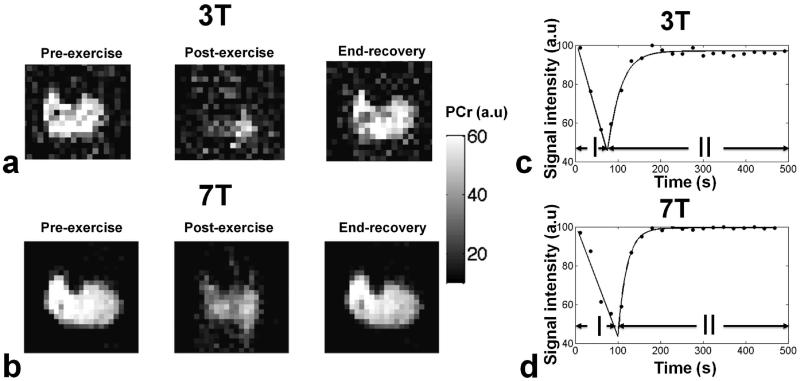
MR spectra from a volunteer pre- and post- exercise, as well as at the end of the recovery period both at 3T and 7T can be seen in Fig.3.a-b. At 3T, the FWHM of the PCr resonance peak was 13.67 ± 5.8 Hz (mean ± SD) across all subjects and the SNR was 35.80 ± 10.14. At 7T, we observed an almost three-fold increase of SNR at 108.91 ± 21.5, while the FWHM was 24.5 ± 8.35 Hz. Figure 4.a shows a cross-section of the lower of the same volunteer, at 3T before the beginning, and at the end of the exercise, as well as at the end of the recovery period. Images acquired at 7T can be seen in Fig.4.b. The SNR in the imaging experiments across all subjects pre-exercise was 6.5 ± 0.3 while at 7T it was 8.2 ± 1.1. We present a quality comparison between the two methods (i.e. MRS and MRI) in Table 1.
Fig.3.
MR spectra of the same volunteer pre- and post-exercise, and at the end of the recovery period a) at 3T and b) at 7T. We have normalized the amplitude of all post-exercise spectra to the pre-exercise ones. We observed an almost threefold increase of SNR at 7T relative to 3T. Evolution of PCr MRS signal intensity during the execution of the exercise (I) and the recovery period (II) from the same volunteer. PCr depletion rates were estimated by fitting a linear function to phase I, while the PCr recovery kinetics were characterized by fitting a mono-exponential growth function to phase II of the exercise. (c) At 3T, the PCr depletion rate is 0.35 s−1 (estimated from the slope of the fitted line, r = 0.997) and the recovery rate constant is 22.4 s (r = 0.981). (d) At 7T the PCr depletion rate is 0.49 s−1 (r = 0.945) and the recovery rate constant is 23.89 s (r = 0.998).
Fig.4.
Cross-sectional slice of the lower leg muscles of the same subject before the beginning and at the end of the exercise, as well as at the end of the recovery period a) at 3T and b) at 7T c) Evolution of the mean signal intensity of all voxels of the muscle. At 3T, the depletion rate (I) is 0.61 s−1 (r = 0.999) and recovery rate constant (II) is 22.4 s (r = 0.974) d) At 7T, the depletion rate is 0.62 s−1 (r = 0.976) and the recovery rate constant is 22.4 s (r = 0.998).
Table 1. Quality comparison between the MRS and MRI method.
| 3T | 7T | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNR | PCr line width | Voxel size | Tem poral | SNR | PCr line width | Voxel size | Temporal | ||||||||
| (mean ± SD, a.u) | (Hz) | (ml) | resolution (s) | (mean ± SD, a.u) | (Hz) | (ml) | resolution (s) | ||||||||
| MRS | MRI | MRS | MRI | MRS | MRI | MRS | MRI | MRS | MRI | MRS | MRI | MRS | MRI | MRS | MRI |
| 35.8±10.1 | 6.5±0.3 | 13.7±5.8 | - | - | 4.2 | 6 | 24 | 108.9±21.1 | 8.2±1.1 | 24.5±8.3 | - | - | 1.6 | 6 | 24 |
The evolution of PCr signal during exercise and during the recovery period measured with the MRS method can be seen in Fig.3.c-d, while Fig.4.c-d shows the signal evolution measured with the MRI experiment. During exercise (phase I) decrease of the PCr levels was observed in all volunteers. We measured the working amplitude for each subject by estimating the rate of PCr depletion from the fits of a linear function to the data. After the end of the exercise (phase II), PCr levels increased following exponential growth kinetics. We estimated the recovery rate constant by fitting Eq.1 to the data during the recovery phase. We summarized the results from each subject at both field strengths with the two methods in Table 2. PCr depletion levels ranged between 20.2 and 55.5 % and were not locally exhaustive. At 3T the PCr recovery rate constants across all subjects were 25.4 ± 3.7 s using the MRS method and 25.6 ± 4.4 s using the MRI method. A paired t-test between the measurements showed no statistically significant differences between the two types of measurement (significance level of 0.05). At 7T the measured rate constants were 26.4 ± 3.2 s and 26.2 ± 4.7 s for MRS and MRI, respectively, again with no statistically significant differences between the two measurements (paired t-test with significance level of 0.05). R.2.3 the results not exhaustive
Table 2. Measurement of PCr depletion rates and recovery time constant with MRS and MRI.
| 3T | 7T | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRS | MRI | MRS | MRI | |||||||||||
| Subject | Gender | Age | ΔPCr | dPCr/dt | k (s) | ΔPCr | dPCr/dt | k (s) | ΔPCr | dPCr/dt | k (s) | ΔPCr | dPCr/dt | k (s) |
| (%) | (s−1) | (%) | (s−1) | (%) | (s−1) | (%) | (s−1) | |||||||
| 1 | F | 32 | 28.4 | 0.35 | 22.4 | 50.2 | 0.61 | 20.2 | 39.2 | 0.49 | 23.9 | 55.5 | 0.62 | 20.3 |
| 2 | M | 34 | 28.0 | 0.25 | 24.2 | 22.1 | 0.35 | 24.9 | 35.7 | 0.31 | 27.6 | 35.2 | 0.42 | 27.5 |
| 3 | F | 25 | 30.2 | 0.22 | 30.8 | 24.8 | 0.18 | 30.9 | 31.2 | 0.25 | 30.5 | 20.2 | 0.15 | 31.7 |
| 4 | M | 28 | 27.6 | 0.21 | 24.1 | 22.1 | 0.15 | 26.6 | 23.5 | 0.28 | 23.6 | 25.1 | 0.20 | 25.3 |
Figure 5.a. shows a cross-section of the lower leg muscles from a 3D anatomical 1H-GRE image. After manually segmenting the voxels of each muscle, we fitted Eq.1 to measure the local recovery rate constant (shown in Fig.5.b). The results for the local PCr measurements for all subjects are summarized in Table 3. In some cases, there was little PCr depletion (typically less than 8%) and fitting of the data to Eq.1 was not possible (r < 0.900).
Fig.5.
a) Cross-section of the lower leg muscles from anatomical 1H-GRE image acquired at 7T for subject 1. The different muscle groups are identified with labels. b) Spatially localized depletion and recovery rate constants from different muscles of subject 1 at 7T. The correlation coefficients for the fits are: TA: r = 0.987, TP: r = 0.992, S: r = 0.985, P: r = 0.998, GL: r = 0.998, GM: r = 0.992.
Abbreviations: tibialis anterior (TA), tibialis posterior (TP), peroneus (P), soleus (S), gastrocnemius lateral (GL) and gastrocnemius medial (GM).
Table 3. PCr recovery k (s) in the muscles of the lower leg.
| 3T | ||||||
|---|---|---|---|---|---|---|
| Subject | TA | TP | S | P | GL | GM |
| 1 | 31.8 | 25.2 | 38.8 | 31.5 | 35.3 | 23.1 |
| 2 | - | - | 32.6 | 29.0 | - | 30.3 |
| 3 | 35.7 | - | - | 32.2 | 32.9 | 29.0 |
| 4 | - | - | 35.2 | 19.1 | 24.3 | 22.3 |
| 7T | ||||||
| 1 | 30.6 | 22.2 | 25.0 | 25.3 | 17.1 | 24.0 |
| 2 | 23.0 | 27.0 | 30.9 | 26.8 | 29.8 | 28.9 |
| 3 | 32.7 | 36.3 | - | 28.2 | 33.9 | - |
| 4 | 20.4 | 25.6 | 37.7 | 18.9 | 24.8 | 19.0 |
The results obtained from the recruited diabetic patient can be seen in Fig.6. Figure 6.a shows 31P spectra pre-exercise (left), at the end of the exercise (middle), and at the end of the recovery period (right). Figure 6.b shows the PCr recovery together with the fit to Eq.1. The patient repeated the exercise in order for us to collect imaging data shown in Fig.6.c. The fit of the mean signal of the entire volume of the calf muscle to Eq.1 can be seen in Fig.6.d. We measured local resynthesis rates by manually segmenting the different muscles. In this particular case, the recovery of P appears to be faster (29.5 s), compared to the recovery of the GL (53.9 s) and GM (51.9 s) muscles respectively. Figure 6.e shows that there was no sufficient PCr depletion for the TA, TP and S muscles in order to fit Eq.1 to the data.
Fig.6.
MRS and MRI data obtained from the diabetic patient. a) 31P spectra pre-exercise (left), at the end of the exercise (middle), and at the end of the recovery period (right). b) PCr levels post-exercise together with the fits to Eq.1 (r = 0.997). c) Anatomical cross-sectional image of the lower leg and corresponding PCr images pre and post exercise and after recovery. d) Fitting of the mean voxel value of the entire calf muscle to Eq.1 (r = 0.988). e) Resynthesis rate constants measurements from segmentations of individual muscles. GL (r = 0.989) and GM (r = 0.968) exhibit similar recovery rate constants, while P muscle recovers significantly faster (r = 0.969). There was very little depletion in the TA, TP and S muscles, and the data were not fitted to Eq.1.
DISCUSSION
The main aim of this preliminary study was the development and implementation of a quantitative 3D imaging technique to map PCr resynthesis rates in several muscles of the lower leg, both at 3T and 7T, following a single exercise experiment. The sequence was tested on four healthy volunteers and gave results in close agreement to those obtained with the more established MRS method. The measurement of PCr recovery rates in several muscles using existing methods requires repetition of the exercise protocol within the same examination (17). In our implementation, the use of volume coils provide full 3D coverage of the muscle and maps local PCr resynthesis rate constants in a single execution of the exercise protocol, which is the main innovation of this imaging method.
Measurements of PCr resynthesis rates after exercise have been mostly performed with the use of unlocalized MRS methods. More recently Meyerspeer and co-workers (33) performed localized single-voxel experiments (with voxel sizes between 24 and 57 ml) acquiring measurements from a single muscle of the lower leg. From the results of their study, they concluded that unlocalized measurements, or measurements that contain signals from weaker exercising muscles, can lead to erroneous estimation of the PCr resynthesis rates, which can appear slower compared to the rates estimated from signals arising from muscles involved in the exercise. These findings suggest that more accurate analysis of cellular metabolism processes can be performed with localized measurements or measurements that allow for differentiation of the active from the passive muscle groups. Our method yields significantly improved spatial resolution compared to existing MRS methods, with voxel sizes of 4.2 ml and 1.6 ml at 3T and 7T respectively, which allows for accurate segmentation of the different muscles of the lower leg. Also, full coverage of all muscle groups does not require any prior knowledge or assumptions about the muscles involved during the exercise. Local depletion and recovery rates can be measured in several muscles as shown in Fig.4,5 and 6, following one execution of the exercise. Three dimensional MRS methods, such as 31P chemical shift imaging (19,20), require very long acquisition times compared to the dynamics of muscle recovery. However, such approaches can be implemented for partial coverage of several muscles by using gated exercise protocols (18). Forbes and co-workers (17) used this approach to measure the dynamic recovery of muscles from a cross-section of the lower leg (GL, GM and S muscles). For the measurement of the anterior muscles of the lower leg (TA, TP, P) they repositioned the surface coil and repeated the gated exercise protocol. However, gated exercises require long examination times, and therefore cannot be easily combined with additional measurements during the same session, such as magnetization transfer experiments (34), that can give additional information on the metabolic function of patients. Spectroscopic methods, on the other hand, compared to spectrally-selective imaging sequences, have the advantage of providing full 31P spectral information that enables estimation of changes in the levels of ATP, Mg2+ and phosphomonoesters (PME), together with information about the intracellular pH, which can be estimated by the chemical shift of the Pi peak (35). The use of low impact exercise protocols avoids acidosis in healthy subjects and certain patients [i.e. type 2 diabetes (36)]. However, in certain pathologies knowledge of pH is essential in order to compare data between healthy controls and patients (37), which is a limitation of our method as implemented currently. The use of dual-band irradiation pulses that excite both the PCr and Pi peaks (38,39) can potentially solve this issue by reconstructing phase images of Pi that are sensitive to the changes of its chemical shift, a method that is currently being used in 1H MR thermometry (40,41).
Dynamic studies of PCr resynthesis after exercise have shown up to a 50% difference in resynthesis rates between healthy subjects and diabetic patients (36). The muscles of diabetic patients exhibit alternation of the spatial distribution of the fibre composition and the blood flow that can impact the muscular oxygen energy metabolism (42,16). Greenman et al. (43) have shown that using 31P-MRI abnormalities can be detected in resting diabetic foot muscles. Therefore, exercise may reveal abnormalities in skeletal muscle oxidative capacity that are not detectable under steady conditions. In this study, we acquired data from a single diabetic patient (Fig.6), and therefore no conclusive results can be drawn. However, the ability of our method to measure local PCr resynthesis in metabolic altered disease states can potentially be a more powerful approach, compared to single voxel MRS methods, for the study of such diseased populations.
Accurate measurement of the PCr kinetics, require selective excitation of the PCr resonance, without contamination from other metabolite resonances. In Fig.2, the selectivity of the Gaussian excitation pulse is shown. By placing the transmission frequency at the γ-ATP, PCr and Pi peaks respectively, we acquired images of each metabolite with the signal from the other metabolites being on the noise level. Movement of the leg inside the coil during the course of the exercise can lead to broadening of the 31P resonance peaks, as it can be seen in Fig.3.a-b. It could therefore be possible that the side-lobes of the Gaussian pulse (shown in Fig.2a) can excite other unwanted resonances (such as γ-ATP and PME and PDE) that can contaminate the PCr signal measurement. For the case of γ-ATP, Chao and co-workers (22) demonstrated that the ATP signals, that modulate due to J-coupling between the three resonances of the ATP molecule, can be eliminated by setting the echo-spacing to 26 ms, this has also been validated by Greenman (25) in a separate study. The low concentration of PME, which typically is between 1 and 2 mM in the muscles of the lower leg (44), compared to the concentration of PCr [typically close to 30 mM at rest (45)], if excited by the sidelobes of the Gaussian pulse employed in this study, can introduce small errors (estimated at less than 1%) to the measurement of the PCr signals. However, in certain pathologies (46) the increased PDE concentration together with its small peak separation from the PCr peak can cause higher contamination of the signal especially at high PCr depletion levels (typically on the order of ~5-10%).
Most 31P metabolites, including PCr, suffer from long T1 relaxation rates. At increased field, T1 values decrease, as a result of chemical shift anisotropy (47), enabling more efficient MRS and MRI acquisitions. Typical values for T1 of PCr at 3T are 6.7 s that decrease by 40% at 7T to 4.0 s as reported elsewhere (48,49) and as measured in our previous work (31). This enabled us to increase spatial resolution by a factor of 2.6 at 7T relative to 3T and still improve the SNR by 30% (8.3 at 7T compared to 6.4 at 3T as shown in Table 1). Phosphorus metabolites, and PCr in particular, exhibit long T2 values, hence a multi-echo imaging sequence (such as the TSE developed in this study) can be used efficiently for their imaging. Typical T2 values of PCr in the muscles of the lower leg are 350 ms at 3T and 200 ms at 7T (31,50). In the centric implementation of our sequence, at the effective echo time (26 ms), assuming single exponential decay of the signal, we expect 7% decay of the signal at 3T, and 12% decay at 7T.
There are two main limitations in this feasibility study. First, the inability to control the intensity of physical exercise that each subject performed makes it difficult to compare results between subjects. For this work, we used the PCr depletion rate as a quantitative marker of physical activity of the subjects. In future studies, we will use an MRI compatible ergometer to ensure reproducibility of the physical exercise. Second, while we acquired MRS data with a temporal resolution of 6 s, the temporal resolution in the MRI experiments was 24 s. Especially for the case of the regularly physically active young subjects, this temporal resolution may not be sufficient for accurate measurement of the PCr resynthesis rate. Furthermore, it is possible that during the same image acquisition both the last phase of exercise and the first phase of recovery occur, a fact that can complicate data analysis. To overcome this, we estimated the first point of the PCr recovery by extrapolating the fitted line of the depletion phase to the end time of the exercise. Acquiring data at increased temporal resolution could avoid this additional post-processing step. Accelerated image acquisition can be achieved by using compressed sensing (51) and by taking advantage of the temporal sparsity of the images during the recovery period that can significantly improve temporal resolution. However, for diabetic patients and older populations, accurate measurements are possible with the current setup and temporal resolution. Lastly, it should be noted that the limited number of recruited subjects in this preliminary study suggest the feasibility of this novel imaging method. However, further validation of the method’s ability to distinguish spatial differences in muscle recovery kinetics both in healthy and diseased subjects will be performed in future studies.
In summary, we have shown that mapping of PCr recovery rates in the human calf muscle is feasible both at 3T and 7T within a single exercise experiment; this is a result that cannot be obtained with current localized or unlocalized MRS methods. At 7T, we were able to acquire dynamic images with significantly higher spatial resolution compared to 3T and compared to existing localized MRS studies at 7T. Using imaging methods that simultaneously measure the dynamic response of several muscles can prove to be insightful for the understanding of muscle function both for healthy and diseased cases.
Acknowledgments
Grant sponsor: The authors would like to acknowledge the support by research grants RO1 AR053133, RO1 AR056260, and RO1 AR060238 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Health (NIH).
LIST OF ABBREVIATIONS
- ATP
Adenosine triphosphate
- GL
Gastrocnemius lateral muscle
- GM
Gastrocnemius medial muscle
- P
Peroneus muscle
- PCr
Phosphocreatine
- Pi
Inorganic phosphate
- r
Pearson’s correlation coefficient
- S
Soleus muscle
- SD
Standard deviation
- PSF
Point spread function
- TA
Tibialis anterior muscle
- TP
Tibialis posterior muscle
- TSE
Turbo spin echo
REFERENCES
- 1.Chance B, Eleff S, Leigh JS. Non-invasive, non-destructive approaches to cell bioenergetics. Proc Natl Acad Sci USA. 1980;77(12):7430–7434. doi: 10.1073/pnas.77.12.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chance B, Eleff S, Leigh JS, Sokolow D, Sapega A. Mitochondrial regulation of phosphocreatine inorganic-phosphate ratios in exercising human-muscle-a gated 31P NMR study. Proc Natl Acad Sci USA. 1981;78(11):6714–6718. doi: 10.1073/pnas.78.11.6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman RJ, Bore PJ, Chan L, Gadian DG, Styles P, Taylor D, Radda GK. Nuclear magnetic-resonance studies of forearm muscle in Duchenne dystrophy. Brit Med J. 1982;284(6322):1072–1074. doi: 10.1136/bmj.284.6322.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bottomley PA, Charles HC, Roemer PB, Flamig D, Engeseth H, Edelstein WA, Mueller OM. Human invivo phosphate metabolite imaging with 31P NMR. Magn Reson Med. 1988;7(3):319–336. doi: 10.1002/mrm.1910070309. [DOI] [PubMed] [Google Scholar]
- 5.Venkatasubramanian PN, Mafee MF, Barany M. Quantitation of phosphate metabolites in human leg invivo. Magn Reson Med. 1988;6(3):359–363. doi: 10.1002/mrm.1910060314. [DOI] [PubMed] [Google Scholar]
- 6.Bottomley PA, Hardy CJ, Roemer PB. Phosphate metabolite imaging and concentration measurements in human heart by nuclear magnetic resonance. Magn Reson Med. 1990;14(3):425–434. doi: 10.1002/mrm.1910140302. [DOI] [PubMed] [Google Scholar]
- 7.Dunn JF, Kemp GJ, Radda GK. Depth selective quantification of phosphorus metabolites in human calf muscle. NMR Biomed. 1992;5(3):154–160. doi: 10.1002/nbm.1940050309. [DOI] [PubMed] [Google Scholar]
- 8.Arnold DL, Matthews PM, Radda GK. Metabolic recovery after exercise and the assessment of mitochondrial function invivo in human skeletal-muscle by means of 31P NMR. Magn Reson Med. 1984;1(3):307–315. doi: 10.1002/mrm.1910010303. [DOI] [PubMed] [Google Scholar]
- 9.Kemp GJ, Taylor DJ, Radda GK. Control of phosphocreatine resynthesis during recovery from exercise in human skeletal-muscle. NMR Biomed. 1993;6(1):66–72. doi: 10.1002/nbm.1940060111. [DOI] [PubMed] [Google Scholar]
- 10.Prompers JJ, Jeneson JAL, Drost MR, Oomens CCW, Strijkers GJ, Nicolay K. Dynamic MRS and MRI of skeletal muscle function and biomechanics. NMR Biomed. 2006;19(7):927–953. doi: 10.1002/nbm.1095. [DOI] [PubMed] [Google Scholar]
- 11.Keller U, Oberhansli R, Huber P, Widmer LK, Aue WP, Hassink RI, Muller S, Seelig J. Phosphocreatine content and intracellular pH of calf muscle measured by phosporus NMR spectroscopy in occlusive arterial disease if the legs. Eur J Clin Invest. 1985;15(6):382–388. doi: 10.1111/j.1365-2362.1985.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 12.Crowther GJ, Milstein JM, Jubrias SA, Kushmerick MJ, Gronka RK, Conley KE. Altered energetic properties in skeletal muscle of men with well-controlled insulin-dependent (type 1) diabetes. Am J Physiol Endocrinol Metab. 2003;284(4):E655–E662. doi: 10.1152/ajpendo.00343.2002. [DOI] [PubMed] [Google Scholar]
- 13.Isbell DC, Berr SS, Toledano AY, Epstein FH, Meyer CH, Rogers WJ, Harthun NL, Hagspiel KD, Weltman A, Kramer CM. Delayed calf muscle phosphocreatine recovery after exercise identifies peripheral arterial disease. J Am Coll Cardiol. 2006;47(11):2289–2295. doi: 10.1016/j.jacc.2005.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khushu S, Rana P, Sekhri T, Sripathy G, Tripathi RP. Bio-energetic impairment in human calf muscle in thyroid disorders: a P-31 MRS study. Magn Reson Imaging. 2010;28(5):683–689. doi: 10.1016/j.mri.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Bottinelli R, Reggiani C. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol. 2000;73(2-4):195–262. doi: 10.1016/s0079-6107(00)00006-7. [DOI] [PubMed] [Google Scholar]
- 16.Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, Schon MR, Bluher M, Punkt K. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care. 2006;29(4):895–900. doi: 10.2337/diacare.29.04.06.dc05-1854. [DOI] [PubMed] [Google Scholar]
- 17.Forbes SC, Slade JM, Francis RM, Meyer RA. Comparison of oxidative capacity among leg muscles in humans using gated (31)P 2-D chemical shift imaging. NMR Biomed. 2009;22(10):1063–1071. doi: 10.1002/nbm.1413. [DOI] [PubMed] [Google Scholar]
- 18.Slade JM, Towse TF, DeLano MC, Wiseman RW, Meyer RA. A gated P-31 NMR method for the estimation of phosphocreatine recovery time and contractile ATP cost in human muscle. NMR Biomed. 2006;19(5):573–580. doi: 10.1002/nbm.1037. [DOI] [PubMed] [Google Scholar]
- 19.Brown TR, Kincaid BM, Ugurbil K. NMR chemical-shift imaging in 3 dimensions. Proc Natl Acad Sci. 1982;79(11):3523–3526. doi: 10.1073/pnas.79.11.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki E, Kashiwagi A, Hidaka H, Maegawa H, Nishio Y, Kojima H, Haneda M, Yasuda H, Morikawa S, Inubushi T, Kikkawa R. H-1- and P-31-magnetic resonance spectroscopy and imaging as a new diagnostic tool to evaluate neuropathic foot ulcers in Type II diabetic patients. Diabetologia. 2000;43(2):165–172. doi: 10.1007/s001250050025. [DOI] [PubMed] [Google Scholar]
- 21.Chao H, Bowers JL, Holtzman D, Mulkern RV. Multi-echo 31P spectroscopic imaging of ATP: A scan time reduction strategy. J Magn Reson Im. 1997;7(2):425–433. doi: 10.1002/jmri.1880070229. [DOI] [PubMed] [Google Scholar]
- 22.Chao H, Bowers JL, Holtzman D, Mulkern RV. RARE imaging of PCr in human forearm muscles. J Magn Reson Im. 1997;7(6):1048–1055. doi: 10.1002/jmri.1880070617. [DOI] [PubMed] [Google Scholar]
- 23.Greenman RL, Elliott MA, Vandenborne K, Schnall MD, Lenkinski RE. Fast imaging of phosphocreatine using a RARE pulse sequence. Magn Reson Med. 1998;39(5):851–854. doi: 10.1002/mrm.1910390523. [DOI] [PubMed] [Google Scholar]
- 24.Greenman RL, Axel L, Ferrari VA, Lenkinski RE. Fast imaging of phosphocreatine in the normal human myocardium using a three-dimensional RARE pulse sequence at 4 tesla. J Magn Reson Im. 2002;15(4):467–472. doi: 10.1002/jmri.10081. [DOI] [PubMed] [Google Scholar]
- 25.Greenman RL. Quantification of the P-31 metabolite concentration in human skeletal muscle from RARE image intensity. Magn Reson Med. 2004;52(5):1036–1042. doi: 10.1002/mrm.20258. [DOI] [PubMed] [Google Scholar]
- 26.Parasoglou P, Xia D, Regatte RR. Spectrally selective 3D TSE imaging of phosphocreatine in the human calf muscle at 3 T. Magn Reson Med. 2012 doi: 10.1002/mrm.24288. DOI: 10.1002/mrm.24288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenman RL, Smithline HA. The feasibility of measuring phosphocreatine recovery kinetics in muscle using a single-shot (31)P RARE MRI sequence. Acad Radiol. 2011;18(7):917–923. doi: 10.1016/j.acra.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenman RL, Wang X, Smithline HA. Simultaneous acquisition of phosphocreatine and inorganic phosphate images for Pi:PCr ratio mapping using a RARE sequence with chemically selective interleaving. Magn Reson Imaging. 2011;29(8):1138–1144. doi: 10.1016/j.mri.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hennig J, Nauerth A, Friedburg H. RARE imaging-a fast imaging method for clinical MR. Magn Reson Med. 1986;3(6):823–833. doi: 10.1002/mrm.1910030602. [DOI] [PubMed] [Google Scholar]
- 30.Poon CS, Henkelman RM. Practical T2 quantitation for clinical-applications. J Magn Reson Imaging. 1992;2(5):541–553. doi: 10.1002/jmri.1880020512. [DOI] [PubMed] [Google Scholar]
- 31.Parasoglou P, Xia D, Chang G, Regatte RR. Spectrally selective 3D imaging of phosphocreatine in the human calf muscle at 3T and 7T. Proc ISMRM; Melbourne, Australia. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blumler P. The MATLAB NMR-library. Available from: http://www.econmr.org/bluemler/software/
- 33.Meyerspeer M, Robinson S, Nabuurs C, Scheene T, Schoisengeier A, Unger E, Kemp GJ, Moser E. Comparing localized and nonlocalized dynamic 31P magnetic resonance spectroscopy in exercising muscle at 7T. Magn Reson Med. 2012 doi: 10.1002/mrm.24205. DOI: 10.1002/mrm.24205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid AI, Schrauwen-Hinderling VB, Andreas M, Wolzt M, Moser E, Roden M. Comparison of Measuring Energy Metabolism by Different P-31-Magnetic Resonance Spectroscopy Techniques in Resting, Ischemic, and Exercising Muscle. Magn Reson Med. 2012;67(4):898–905. doi: 10.1002/mrm.23095. [DOI] [PubMed] [Google Scholar]
- 35.de Graaf RA. Front Matter. In Vivo NMR Spectroscopy. John Wiley & Sons, Ltd; 2007. [Google Scholar]
- 36.Schrauwen-Hinderling VB, Kooi ME, Hesselink MKC, Jeneson JAL, Backes WH, van Echteld CJA, van Engelshoven JMA, Mensink M, Schrauwen P. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetoliogia. 2007;50(1):113–120. doi: 10.1007/s00125-006-0475-1. [DOI] [PubMed] [Google Scholar]
- 37.van den Broek NMA, De Feyter HMML, de Graaf L, Nicolay K, Prompers JJ. Intersubject differences in the effect of acidosis on phosphocreatine recovery kinetics in muscle after exercise are due to differences in proton efflux rates. Am J Physiol - Cell Physiol. 2007;293(1):C228–C237. doi: 10.1152/ajpcell.00023.2007. [DOI] [PubMed] [Google Scholar]
- 38.Steinseifer IK, Wijnen JP, Hamans BC, Heerschap A, TW S. Fast 31P metabolic imaging of human muscle.Proc ISMRM; Stockholm, Sweden. 2010. [Google Scholar]
- 39.Lu A, Atkinson IC, Zhou XJ, Thulborn KR. PCr/ATP ratio mapping of the human head by simultaneously imaging of multiple spectral Peaks with interleaved excitations and flexible twisted projection imaging readout trajectories at 9.4 T. Magn Reson Med. 2012 doi: 10.1002/mrm.24281. DOI: 10.1002/mrm.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diakite M, Hadley R, Parker D. Modified Turbo Spin Echo sequence for PRF based thermometry.Proc ISMRM. Montreal, Quebec. 2011. [Google Scholar]
- 41.Vogel MW, Pattynama PMT, Lethimonnier FL, Le Roux P. Use of fast spin echo for phase shift magnetic resonance thermometry. Magn Reson Imaging. 2003;18(4):507–512. doi: 10.1002/jmri.10393. [DOI] [PubMed] [Google Scholar]
- 42.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 43.Greenman RL, Panasyuk S, Wang X, Lyons TE, Dinh T, Longoria L, Giurini JM, Freeman J, Khaodhiar L, Veves A. Early changes in the skin microcirculation and muscle metabolism of the diabetic foot. Lancet. 2005;366(9498):1711–1717. doi: 10.1016/S0140-6736(05)67696-9. [DOI] [PubMed] [Google Scholar]
- 44.Vandenborne K, McCully K, Kakihira H, Prammer M, Bolinger L, Detre JA, Demeirleir K, Walter G, Chance B, Leigh JS. Metabolic heterogeneity in human calf muscle during maximal exercise. Proc Natl Acad Sci U S A. 1991;88(13):5714–5718. doi: 10.1073/pnas.88.13.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kemp GJ, Meyerspeer M, Moser E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by P-31 MRS: a quantitative review. NMR Biomed. 2007;20(6):555–565. doi: 10.1002/nbm.1192. [DOI] [PubMed] [Google Scholar]
- 46.Taylor DJ, Kemp GJ, Woods CG, Edwards JH, Radda GK. Skeletal muscle bioenergetics in myotonic-dystrophy. J Neurol Sci. 1993;116(2):193–200. doi: 10.1016/0022-510x(93)90325-s. [DOI] [PubMed] [Google Scholar]
- 47.Evelhoch JL, Ewy CS, Siegfried BA, Ackerman JJH, Rice DW, Briggs RW. P-31 spin-lattice relaxation-times and resonance linewidths of rat-tissue invivo - dependence upon the static magnetic-field strength. Magn Reson Med. 1985;2(4):410–417. doi: 10.1002/mrm.1910020409. [DOI] [PubMed] [Google Scholar]
- 48.Andrew ER, Gaspar R. Mechanisms of 31P relaxation in phosphorus metabolites. Magn Reson Mater Phys Biol Med. 1994;2(3):421–423. [Google Scholar]
- 49.Bogner W, Chmelik M, Schmid AI, Moser E, Trattnig S, Gruber S. Assessment of (31)P Relaxation Times in the Human Calf Muscle: A Comparison between 3 T and 7 T In Vivo. Magn Reson Med. 2009;62(3):574–582. doi: 10.1002/mrm.22057. [DOI] [PubMed] [Google Scholar]
- 50.Bogner W, Chmelik M, Andronesi OC, Sorensen AG, Trattnig S, Gruber S. In Vivo (31)P Spectroscopy by Fully Adiabatic Extended Image Selected In Vivo Spectroscopy: A Comparison Between 3 T and 7 T. Magn Reson Med. 2011;66(4):923–930. doi: 10.1002/mrm.22897. [DOI] [PubMed] [Google Scholar]
- 51.Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182–1195. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]