Abstract
Objectives
Biliary atresia is a rapidly progressive form of biliary fibrosis affecting neonates. We previously reported that primary cilia on the intrahepatic cholangiocytes of patients with both syndromic and non-syndromic biliary atresia were structurally abnormal. Our objective was to determine whether extrahepatic cholangiocytes in human biliary atesia, intrahepatic and extrahepatic cholangiocytes of rhesus rotavirus (RRV)-infected neonatal mice, and RRV-infected primary neonatal extrahepatic cholangiocytes also demonstrate ciliary abnormalities.
Methods
The livers of neonatal BALB/c mice injected with RRV that developed jaundice, human extra-hepatic bile duct samples obtained at time of hepatoportoenterostomy, and RRV-infected primary neonatal cholangiocytes were stained with antibodies against acetylated alpha tubulin to identify primary cilia.
Results
Extrahepatic cholangiocytes from RRV-treated mice demonstrated minimal loss of primary cilia at day 3 but almost complete loss at day 8 and partial loss at day 12. No changes were seen in mouse intrahepatic bile ducts at any of the time points. In the human biliary atresia samples, primary cilia were almost completely absent from extrahepatic duct cholangiocytes. There were, however, abundant cilia in the peri-biliary glands adjacent to extrahepatic ducts in the biliary atresia sample. Cilia in RRV-infected primary neonatal cholangiocytes were significantly decreased compared to control.
Conclusions
Primary cilia are selectively lost from neonatal extrahepatic but not intrahepatic cholangiocytes after RRV infection in BALB/c mice. The cilia are also decreased in rhesus rotavirus-infected primary cholangiocytes and the extrahepatic ducts from human biliary atresia patients. This suggests that ciliary abnormalities are part of the pathophysiology of biliary atresia.
Keywords: Rhesus rotavirus, primary cilia, extra-hepatic bile ducts, peribiliary glands
Introduction
Biliary atresia (BA) is a rapidly progressive fibro-inflammatory and obliterative disease of the extrahepatic bile ducts that occurs in 1 in 8,000 to 15,000 patients (1). The etiology is unknown although likely involves an environmental insult such as infection or toxic exposure combined with a genetic predisposition (3). Injection of BALB/c mice with rhesus rotavirus (RRV) within 24 hours of birth results in a BA-like syndrome, lending support to an infectious cause (4). Approximately 20% of patients with BA have a variant called syndromic BA characterized by laterality defects (also known as embryonic BA or BA with splenic malformation syndrome) (2, 5–7). There are case reports of patients with syndromic BA having abnormal cilia, suggesting that cilia might be involved in the pathogenesis of the disease (5–7).
Primary cilia are non-motile organelles that serve as mechano- and chemo-sensors (8–10). They are found on the apical surfaces of most cells, including cholangiocytes, but are notably absent on hepatocytes (11). In addition, they have roles in cell proliferation, cell differentiation, organogenesis, axis orientation during development, and tissue remodeling (8). Ciliary abnormalities have been implicated in many human diseases such as polycystic kidney disease, CNS malformations, retinal degeneration, situs defects, polydactyly, and skeletal and gonadal malformations (8,9,12–14). We recently showed that the intrahepatic cholangiocytes of patients with both syndromic and non-syndromic BA have primary cilia that are decreased in number and are morphologically abnormal (2). In addition, Hartley et al. recently found decreased staining for the ciliary protein fibrocystin/polyductin in the livers of children with non-syndromic BA (15).
We hypothesized that, if cilia abnormalities were part of the pathophysiology of BA, these defects would be prominent in the extrahepatic biliary ducts of human BA livers and would appear early after infection in the mouse RRV model. We therefore examined RRV infected cholangiocytes in culture, RRV-infected neonatal BALB/c mouse livers at various stages after infection, and human BA extrahepatic biliary remnants for the presence of abnormal cilia.
Material and Methods
Tissue Samples
Formalin-fixed, paraffin-embedded extra-hepatic bile duct remnants from hepatoportoenterostomies of BA patients were obtained with IRB approval from the Children’s Hospital of Philadelphia. A sample of normal extrahepatic bile duct from an autopsy sample was obtained as a control. For the mouse samples, neonatal mice were injected with RRV and saline within 24 hours of birth, and livers were removed at days 3, 7–8, and 12–14 (16,17). All of the day 7–12 mice were jaundiced, which is associated with bile duct obstruction and the development of BA in more than 90% of cases (18). Blocks including the right lobe of the liver, extra hepatic ducts, and a small piece of bowel were dissected, fixed with 10% formalin, arranged in a similar orientation, and paraffin embedded. A liver from a mouse approximately 12 hrs after birth was similarly fixed and embedded. Bile duct ligation was carried out in adult mice as described (19); mice were euthanized at 14 days after surgery and livers removed and processed for immunofluorescence. Animal experiments were carried out with local Institutional Animal Care and Use Committee approval.
Primary cholangiocytes isolation and RRV infection
Neonatal extrahepatic bile ducts were isolated from day 0–3 day old pups. Ducts were carefully dissected away from stroma and surrounding cells, then imbedded and cultured in a thick collagen gel (2 mg/ml) for 3–4 weeks with cholangiocyte media (20). The cells were then split and cultured on thin collagen (1 mg/ml) coated dishes. Cells were used for experiments after two weeks. Neonatal cholangiocytes were infected with RRV at an MOI of 5 for 90 minutes, and then incubated in serum free media for 12 hrs before fixation and staining (21,22).
Immunofluorescence
Tissue samples and cells were stained with antibodies against the cholangiocyte marker K19 (1:10, Developmental Studies Hybridoma Bank, University of Iowa), the primary cilia marker acetylated alpha tubulin (1:5000; Sigma, St. Louis, MO), and with the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI). RRV-infected cells in culture were also stained with anti-rotavirus antibody (1:1000; Abcam, Cambridge, MA). Confocal pictures were obtained using a Zeiss LSM 710 microscope and Zen software. Image composites and z-stacks were made using FIJI software.
Statistical Analysis
For all the samples (tissue and cells in culture), cilia and nuclei were manually counted. For mouse liver analyses, 3–5 mice were evaluated for each condition and 2–9 high-power-fields were assessed per liver, depending on the number of bile ducts visible. Cell data are from three independent experiments. Data represent the mean +/- standard deviation. Normality tests were performed and the results were subjected to two tailed, paired (for cell culture studies) or unpaired (for tissue samples), parametric testing using GraphPad InStat software (La Jolla, CA). A p value of <0.05 was considered statistically significant.
Results
Analysis of cilia in the RRV model
Primary cilia are found on the apical surfaces of cholangiocytes even in newborn mouse livers, as shown by acetylated alpha tubulin staining of a 12 hr old mouse liver (Fig. 1A, B). In mice injected with RRV within 24 hours of birth, a model of BA, cilia are present and appear normal on intrahepatic cholangiocytes even up to days 12–14 (Fig. 1C–J). Minor decreases in the ratio of cilia to nuclei were not statistically significant (Fig. 1E, H, K). Note that all day 7–12 samples were from jaundiced mice, indicating the development of bile duct obstruction and BA.
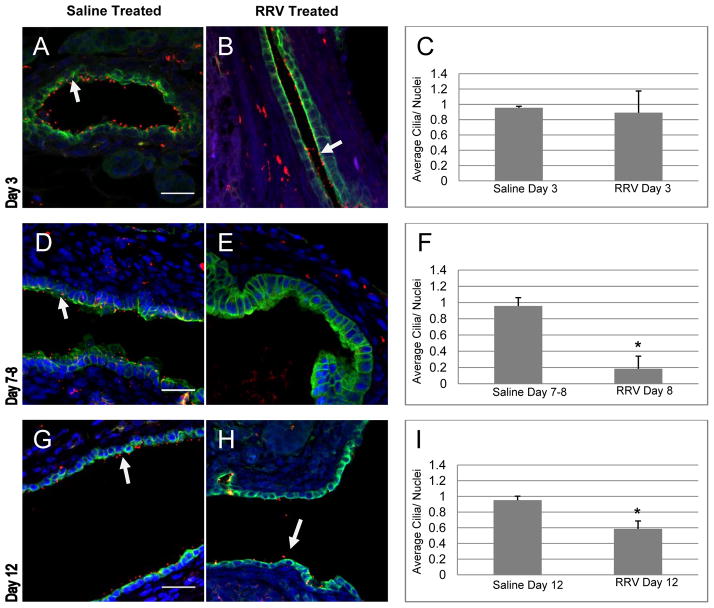
Figure 1.
Rhesus rotavirus (RRV) infection does not affect numbers of intrahepatic cholangiocyte cilia. Livers from (A,B) 12 hour old mouse, (C,F,I) mice injected with saline 24 hours after birth, and (D,G,J) mice injected with RRV 24 hours after birth, were stained with antibodies against acetylated alpha tubulin for primary cilia (red), with K19 for cholangiocytes (green), and with the nuclear stain DAPI (blue). Livers from saline- and RRV-injected animals were harvested after 3 (C,D), 8 (F,G), and 12 (I,J) days. Representative confocal micrographs shown. The ratios of cilia to nuclei were calculated (E, H, K) differences between saline and RRV were not statistically significant for any of the time points: day 3 (p = 0.07), 7–8 (p = 0.55), and 12–14 (p = 0.27). White arrows point to cilia, bars=25 μM.
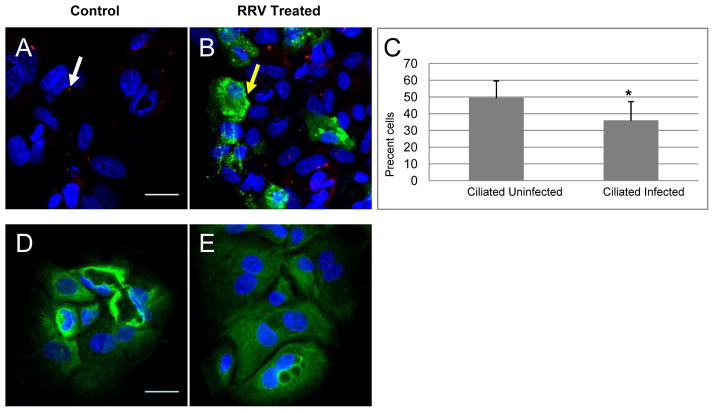
In comparison, analysis of the extrahepatic ducts from mice injected with RRV demonstrated no change in primary cilia 3 days after injection with RRV, but did show a marked decrease at days 8 (p ≤ 0.0001) and 12 (p = 0.0084) when compared with saline-injected animals (Fig. 2). Interestingly, there appeared to be a partial recovery of cilia at day 12, with a statistically significant increase compared to day 8 (p = 0.005). To determine whether RRV infection could cause a direct loss of cilia, primary neonatal cholangiocytes were infected with RRV. RRV-infected neonatal cholangiocytes showed a statistically significant reduction in cilia (Fig 3A–C) when compared to non-infected cells (p=0.0484) 12 hours after infection.
Figure 2.
Rhesus rotavirus (RRV) infection results in decreased cilia on extrahepatic cholangiocytes. Livers from mice injected with saline (A, D, G) and RRV (B, E, H) at 24 hours of age were removed at days 3 (A, B, C), 7–8 (D, E, F), and 12 (G, H, I), and stained with antibodies against acetylated alpha tubulin (red) and K19 (green), and with DAPI (blue). Analysis of the ratio of cilia to nuclei (C, F, I) demonstrated a statistically significant decrease in cilia in RRV-injected animals at days 7–8 (*, p ≤ 0.0001) and day 12 (*, p = 0.0084). White arrows point to cilia. Representative confocal micrographs, bars=25 μM.
Figure 3.
Neonatal extrahepatic cholangiocytes infected with rhesus rotavirus (RRV) demonstrate a significant decrease in cilia. Primary neonatal cholangiocytes, either control (A, D) or RRV-infected (B, E) were stained (A, B) with antibodies against acetylated alpha tubulin (red), with the nuclear stain DAPI (blue), and with antibody against rotavirus (green); or with antibodies (D, E) against K19 (green). (C) There was a 27.5% decrease in ciliation in the infected cells versus the uninfected cells (p=0.048). Average percentage of cells infected in 3 independent experiments was 15% (range 12.3–19.5%). White arrows point to cilia, and yellow arrows point to RRV infected cells. Representative confocal images, bars=25 μM.
Loss of cilia may be a non-specific response to obstruction. We examined the large ducts of adult mice 14 days post bile duct ligation, and found no change in cilia (online-only figure, http://links.lww.com/MPG/A225). This suggests that obstruction in and of itself does not lead to cilia damage, although it does not rule out such an effect in the neonatal bile duct.
Analysis of cilia in extrahepatic ducts from BA patients
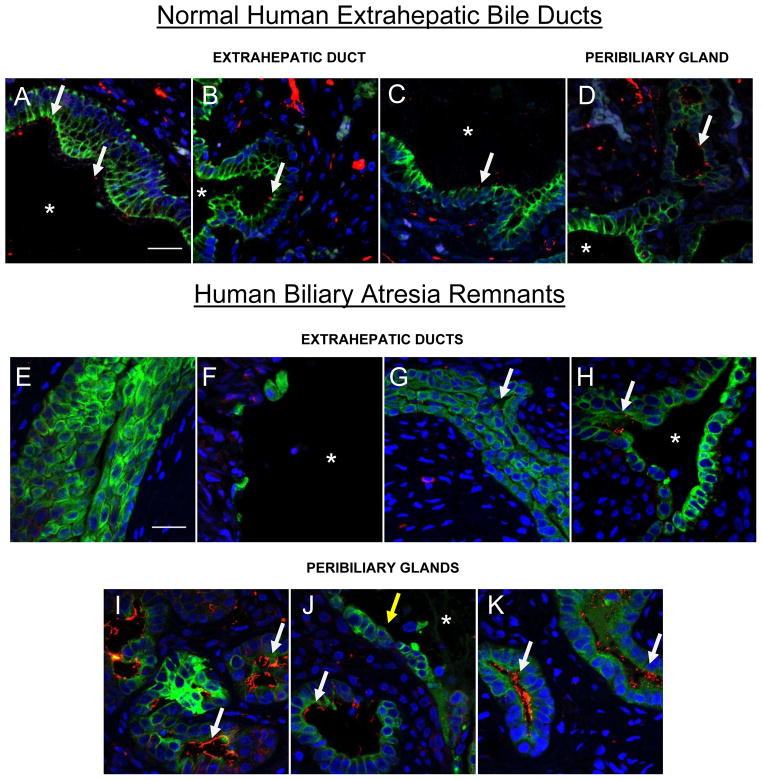
We previously showed that the intrahepatic bile ducts of BA patients have decreased and morphologically abnormal cilia (2). Given the specific loss of cilia from extrahepatic ducts in the RRV model, we examined the extra-hepatic ducts from 4 BA patients for cholangiocyte ciliation (Fig. 4). Markedly decreased cilia were found in the larger ducts; however, adjacent clusters of peribiliary glands showed numerous cilia (Fig. 4D, I–K). Two of the samples showed ducts with patent lumens (Fig 4, F, H), while the other two (Fig 4E, G) showed obliterated lumens. A dense inflammatory infiltrate and expanded fibrotic matrix surrounded all ducts (not shown). For comparison, a normal extrahepatic bile duct from an infant, taken at autopsy, demonstrated abundant cilia (Fig. 4A–C).
Figure 4.
Cilia are decreased on extrahepatic cholangioctyes in human biliary atresia (BA). Extrahepatic bile duct remnants from a normal human liver (A-D) and four BA livers (E-K) were stained with antibodies against acetylated alpha tubulin (red) and K19 (green), and with DAPI (blue). (A–C, E–H) Extrahepatic bile ducts from patients with BA all show reduction or absence of cilia compared to the ducts from a normal liver. (D and I-K) Peribiliary glands adjacent to the large external bile ducts of both normal (D) and BA (I-K) samples demonstrate abundant cilia. White arrows point to cilia. Yellow arrow indicates cholangiocytes of the extrahepatic duct lacking cilia. Asterisks represent patent lumens (A-D, F, H, J). Representative confocal micrographs, bars=25 μM.
Discussion
We report here that extrahepatic cholangiocytes demonstrate a loss of cilia in both human BA and in the mouse RRV model of the disease. BA is thought to result from an initial insult to the extrahepatic bile ducts. Our current and previous observations are consistent with this hypothesis (2), although it is not clear whether ciliary resorption is a secondary phenomenon (reflecting general cellular disruption) or is a key part of the mechanism of BA. A recent paper demonstrating the development of BA in Sox17 haploinsufficient mice found no ciliary loss, suggesting that this is not necessary for the development of BA (23). It is interesting, however, that RRV infection of primary cholangiocytes rapidly alters ciliation. Hartley et al. have shown that levels of fibrocystin/polyductin are decreased in BA (15). This ciliary protein is mutated in the disorder autosomal recessive polycystic kidney disease, which is associated with bile duct dilation and peribiliary fibrosis. Our data (in particular the specific loss of cilia from extrahepatic cholangiocytes) in combination with this previous work from Hartley et al. raise the intriguing (although speculative) possibility that BA is an acquired ciliopathy.
Testing this hypothesis will require determining the consequences of cilia loss, particularly as it relates to the loss of cholangiocyte polarity and epithelial integrity – and thereby to the potential for bile duct obstruction. It will be particularly critical to make use of 3-D culture systems in which cholangiocytes form ducts, and to determine whether the artificial loss of cilia in these systems leads to obstruction or other damage to the ducts
Regardless of whether cilia damage and loss is a primary or secondary event in BA, it may offer insight into the pathophysiology of the disease. There is compelling evidence in both human BA and the RRV model that duct obstruction is necessary for the disease to develop: the RRV model requires obstruction, even in the setting of autoimmune defects (24); bile duct ligation in neonatal rats (but not adult rats or adult mice) causes a syndrome that appears identical to BA (25); and it has been appreciated since the pre-Kasai era that human BA is also invariably associated with ductal obstruction (26). Although we cannot rule out that ciliary damage is secondary to obstruction of the neonatal bile duct, it is also possible that understanding the cause and functional ramifications of cilia damage may suggest a mechanism for loss of bile duct epithelial integrity and obstruction.
Interestingly, we observed a partial return of cilia on the extrahepatic cholangiocytes of neonatal mice 12 days after RRV infection. This may be related to why icteric RRV mice occasionally recover fully (4). It is conceivable that cells from the peribiliary glands, which are thought to be a stem cell niche (27,28), participate in this recovery of ciliated cells. Our data suggest that in BA these glands are relatively resistant (at least in terms of cilia resorption) to damage, raising the possibility that they serve as a reservoir for cholangiocyte renewal. A limitation of our study is that only a small number of human samples were examined. Our data suggest, however, that understanding the relevance of cilia restoration will require a better understanding of the relationship between cilia loss and obstruction.
In summary, we have demonstrated in an animal model of BA as well as in human tissue that there is significant loss of cilia from extrahepatic cholangiocytes. Future work will need to focus on the functional ramifications of this cilia loss, and on the mechanism whereby this occurs after RRV infection and potentially other cholangiocyte insults.
Supplementary Material
Acknowledgments
We are grateful to the Molecular Pathology and Imaging Core of the UPenn NIDDK Center for Molecular Studies in Digestive and Liver Diseases (P30 DK50306) and to the UPenn Cell & Developmental Biology Microscopy Core for assistance with imaging.
Sources of Support: This work was supported by grants from the Fred and Suzanne Biesecker Pediatric Liver Center (to RGW and JRF) and by a fellowship from the Childhood Liver Disease Research and Education Network (to SK). JRF was supported by R01-DK079881.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
The authors report no conflicts of interest.
References
- 1.Haber BA, Russo P. Biliary atresia. Gastroenterol Clin N Am. 2003;32:891–911. doi: 10.1016/s0889-8553(03)00049-9. [DOI] [PubMed] [Google Scholar]
- 2.Chu AS, Russo PA, Wells RG. Cholangiocyte cilia are abnormal in syndromic and non-syndromic biliary atresia. Mod Pathol. 2012;25:751–757. doi: 10.1038/modpathol.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezerra JA. Potential etiologies of biliary atresia. Pediatr Transplant. 2005;9:646–651. doi: 10.1111/j.1399-3046.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 4.Riepenhoff-Talty M, Schaekel K, Clark HF, et al. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res. 1993;33:394–399. doi: 10.1203/00006450-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 5.de Carvalho E, Ivantes CA, Bezerra JA. Extrahepatic biliary atresia: Current concepts and future directions. J Pediatr. 2007;83:105–120. doi: 10.2223/JPED.1608. [DOI] [PubMed] [Google Scholar]
- 6.Teichberg S, Markowitz J, Silverberg M, et al. Abnormal cilia in a child with the polysplenia syndrome and extrahepatic biliary atresia. J Pediatr. 1982;100:399–401. doi: 10.1016/s0022-3476(82)80438-1. [DOI] [PubMed] [Google Scholar]
- 7.Gershoni-Baruch R, Gottfried E, Pery M, et al. Immotile cilia syndrome including polysplenia, situs inversus, and extrahepatic biliary atresia. Am J Med Genet. 1989;33:390–393. doi: 10.1002/ajmg.1320330320. [DOI] [PubMed] [Google Scholar]
- 8.Irigoin F, Badano JL. Keeping the balance between proliferation and differentiation: The primary cilium. Curr Genomics. 2011;12:285–297. doi: 10.2174/138920211795860134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh EC, Katsanis N. Cilia in vertebrate development and disease. Development. 2012;139:443–448. doi: 10.1242/dev.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berbari NF, O’Connor AK, Haycraft CJ, et al. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang BQ, Masyuk TV, Muff MA, et al. Isolation and characterization of cholangiocyte primary cilia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G500–G509. doi: 10.1152/ajpgi.00064.2006. [DOI] [PubMed] [Google Scholar]
- 12.Masyuk AI, Gradilone SA, Banales JM. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol. 2008;295:G725–G734. doi: 10.1152/ajpgi.90265.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banales JM, Masyuk TV, Gradilone SA, et al. The cAMP effectors epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2009;49:160–174. doi: 10.1002/hep.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waters AM, Beales PL. Ciliopathies: An expanding disease spectrum. Pediatr Nephrol. 2011;26:1039–1056. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartley JL, O’Callaghan C, Rossetti S, et al. Investigation of primary cilia in the pathogenesis of biliary atresia. J Pediatr Gastroenterol Nutr. 2011;52:485–488. doi: 10.1097/MPG.0b013e318200eb6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen SR, Jafri M, Donnelly B, et al. Effect of rotavirus strain on the murine model of biliary atresia. J Virol. 2007;81:1671–1679. doi: 10.1128/JVI.02094-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hand NJ, Horner AM, Master ZR, et al. MicroRNA profiling identifies miR-29 as a regulator of disease-associated pathways in experimental biliary atresia. J Pediatr Gastroenterol Nutr. 2012;54:186–192. doi: 10.1097/MPG.0b013e318244148b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shivakumar P, Campbell KM, Sabla GE, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-γ in experimental biliary atresia. J Clin Invest. 2004;114:322–329. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen AL, Sackey BK, Marcinkiewicz C, et al. Fibronectin extra domain-A promotes hepatic stellate cell motility but not differentiation into myofibroblasts. Gastroenterology. 2012;142:928–937. doi: 10.1053/j.gastro.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vroman B, LaRusso NF. Development and characterization of polarized primary cultures of rat intrahepatic bile duct epithelial cells. Lab Invest. 1996;74:303–313. [PubMed] [Google Scholar]
- 21.Arnold M, Patton JT, McDonald SM. Culturing, storage, and quantification of rotaviruses. Curr Protoc Microbiol. 2009;15:15C.3. doi: 10.1002/9780471729259.mc15c03s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes BH, Tucker RM, Wehrmann F, et al. Cholangiocytes as immune modulators in rotavirus-induced murine biliary atresia. Liver Int. 2009;29:1253–1261. doi: 10.1111/j.1478-3231.2008.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uemura M, Ozawa A, Nagata T, et al. Sox17 haploinsufficiency results in perinatal biliary atresia and hepatitis in C57BL/6 background mice. Development. 2013;140:639–648. doi: 10.1242/dev.086702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu BR, Brindley SM, Tucker RM, et al. alpha-enolase autoantibodies cross-reactive to viral proteins in a mouse model of biliary atresia. Gastro. 2010;139:1753–61. doi: 10.1053/j.gastro.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibelli NE, Tannuri U, de Mello ES, et al. Bile duct ligation in neonatal rats: is it a valid experimental model for biliary atresia studies? Pediatr Transplant. 2009;13:81–7. doi: 10.1111/j.1399-3046.2008.00947.x. [DOI] [PubMed] [Google Scholar]
- 26.Thompson J. On congenital obliteration of the bile ducts. Edinburgh Med J. 1892;37:523. [PMC free article] [PubMed] [Google Scholar]
- 27.Cardinale V, Wang Y, Carpino G, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- 28.Nakanuma Y, Hoso M, Sanzen T, et al. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc Res Tech. 1997;38:552–570. doi: 10.1002/(SICI)1097-0029(19970915)38:6<552::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.