Abstract
Background
Carriers of the FMR1 premutation allele are at a significantly increased risk for a late-onset neurodegenerative disorder, fragile X-associated tremor/ataxia syndrome (FXTAS). This disorder is distinct from fragile X syndrome (FXS) in its molecular aetiology and clinical presentation. The primary features of FXTAS are late-onset intention tremor and gait ataxia. Associated features include parkinsonism, neuropsychological dysfunction, autonomic dysfunction and peripheral neuropathy.
Aim
To investigate the usefulness of a quantitative neurological test battery implemented through the CATSYS instrument to identify preclinical symptoms of FXTAS.
Methods
Both premutation carriers with 70–199 repeats (62 men) and their low-repeat allele carrier siblings (27 men), identified through families with an individual affected with FXS, were tested.
Results
As expected, because of its sensitivity, use of the instrument allowed identification of tremor in 23% of men who had not self-reported tremor, and ataxia in 30% of men who had not self-reported ataxia. Among subjects with self-reported tremor and ataxia, we found significant concordance between measures of the CATSYS system and the self-report.
Conclusion
Rates of these traits among premutation carriers and low-repeat allele carrier siblings could be identified, and are presented in this paper, along with the minimum estimates of age-related prevalence.
Carriers of the FMR1 premutation allele (55–199 repeats as defined by the American College of Medical Genetics1) are at increased risk for two disorders distinct from the phenotype, fragile X syndrome (FXS), seen in full mutation carriers. First, female premutation carriers are at a 16-fold increased risk for premature ovarian failure compared with noncarriers.2 In addition, older premutation men and possibly some women have a significantly increased risk for a late-onset neurodegenerative disorder, fragile X-associated tremor/ ataxia syndrome (FXTAS).3,4 The primary features of FXTAS are intention tremor and gait ataxia. Associated features include parkinsonism, neuropsychological dysfunction, autonomic dysfunction, and peripheral neuropathy in the lower extremities.3 Cognitive changes are highly variable and include global impairments, specific executive cognitive dysfunction, and impairments in working and declarative memory.5,6 The pattern of cognitive performance is thought to be similar to that observed in the frontal variant of frontotemporal dementia and several of the spinocerebellar ataxias, but is different from the impairments observed in dementia of the Alzheimer type.6 Preliminary reports suggest that psychiatric symptoms may be present, including anxiety, agitation, disinhibition and depression.7
Neuroimaging features of FXTAS include white-matter disease in the periventricular white matter, subcortical white matter, and middle cerebellar peduncles (MCP) on T2-weighted MRI.8 The increased signal intensities of the middle cerebellar peduncles have been incorporated in the diagnostic criteria for “definite” FXTAS diagnosis3 (see below), as they are distinctive and occur in about 60% of individuals with FXTAS.9 Other changes include global cerebral, cerebellar and cerebral cortex atrophy.3,8,10–12
The most significant neuropathological features of FXTAS are: (1) cerebral and cerebellar white matter disease, (2) astrocytic pathology most evident in cerebral white matter and 3) the presence of eosinophilic, ubiquitin-positive intra-nuclear inclusions in the neurons and astrocytes throughout the central nervous system.13,14
Diagnostic criteria were established early in the understanding of FXTAS and were based on the assessment of patients identified through families with FXS.3 These have helped to standardise FXTAS diagnoses across sites. FXTAS is classified as “definite” (a premutation carrier with the MCP sign or neuropathological confirmation along with intention tremor or gait ataxia); “probable” (a carrier with the MCP sign and either Parkinsonism, at least moderate short-term memory dysfunction or an executive dysfunction deficit, or has both tremor and ataxia in the absence of the MCP sign); or “possible” (a carrier with either tremor or ataxia and either white matter lesions in the cerebrum or at least moderate generalised cerebral atrophy).
A recent retrospective study using abstracted medical records of patients diagnosed with FXTAS provides a general picture of the disorder.15 Based on 55 FXTAS patients, tremor was usually the first major motor sign of the disorder, with a median onset at around 60 years of age. Ataxia followed about 2 years later, onset of falls about 6 years later, dependence on a walking aid about 15 years later and death about 21 years later. Although these preliminary retrospective data provide a sketch of the course of FXTAS for diagnosed patients, they cannot replace unbiased data collected prospectively, as emphasised by Leehey et al.15
Importantly, information on penetrance of FXTAS among adult premutation carriers is incomplete. To date, only one study has been carried out to estimate penetrance of FXTAS-related motor symptoms, which is based on 40 premutation carrier and 59 non-carrier men ascertained through families with known FXS.10 This survey indicated that about one-third of male carriers >50 years old had both symptoms of intention tremor and gait ataxia. Age-related penetrance estimates indicated that >50% of premutation men aged >70 years have these motor symptoms.
Our primary goal in this work was to investigate the usefulness of a quantitative neurological test battery, implemented using the CATSYS computer-based testing system (www.catsys.dk) to identify preclinical symptoms of FXTAS in premutation carrier men. We used these data to test the association of symptoms with repeat size and to establish an age-related prevalence of symptoms for premutation carrier men and our control population. Our overall goal in this project is to determine the natural history and prevalence of symptoms of FXTAS in premutation carriers, and in so doing, potentially identify treatment regimens to ameliorate FXTAS-associated symptoms. Our current study population consists of both premutation carrier men with 70–199 repeats (n = 62) and their low-repeat allele carrier male siblings (n = 27) identified through families with a member affected with FXS.
Overall, we found significant concordance between measures of the CATSYS system and self-report for tremor and ataxia. We identified tremor in 23% of men who did not self-report having tremor. In addition, we identified ataxia using the CATSYS system in 30% of subjects who not did report having ataxia. Comparison of rates of these traits among high-repeat (≥70 repeats) and low-repeat (<70 repeats) allele carriers are presented, along with the minimum estimates of age-related prevalence in men.
METHODS
The protocols and consent forms were approved by the institutional review board at Emory University, and informed consent was obtained from all subjects.
Study population
Our goal was to ascertain a sample of premutation carriers and their low-repeat allele carrier siblings to investigate the association of reported FXTAS phenotypes with repeat size. To accomplish this, we tested relatives in families with individuals affected with FXS for premutation carrier status. If a premutation man was identified in a sibship, he and all of his premutation and non-carrier siblings, blinded to the symptoms of FXTAS, were recruited. The only eligibility criterion was that subjects had to be >50 years of age. We identified and contacted 121 eligible men and enrolled 89 (73.5%). We also recruited female siblings, but because of the small numbers their results are not included here.
Normative population
Normative data for age-appropriate measures obtained from the CATSYS system were obtained from several studies (table 1). The unexposed groups from a study of mercury exposure by Frumkin et al16 and a study on arsenic exposure from Gerr et al17 were used. For intention tremor, we recruited participants from the general population who were >50 years. For the pronation/ supination task of coordination on the CATSYS, norms from the CATSYS manual were used.
Table 1.
Summary of normative data for CATSYS and grooved pegboard
| Variable tested (units) | Reference | Mean (SD) age (years) | n | Mean (SD) score | |
|---|---|---|---|---|---|
| Ataxia (eyes open) | Sway velocity (mm/s) | Frumkin et al16 | 48.6 (12.3) | 236 | 16.788 (3.673) |
| Gerr et al17 | 44.5 (14.9) | ||||
| Ataxia (eyes closed) | Sway velocity (mm/s) | Frumkin et al16 | 48.6 (12.3) | 236 | 21.375 (6.837) |
| Gerr et al17 | 44.5 (14.9) | ||||
| Postural tremor I | Tremor intensity (m/s2) | Frumkin et al16 | 48.6 (12.3) | 236 | −1.02 (0.14)* (LH) |
| Gerr et al17 | 44.5 (14.9) | −1.02 (0.15)* (RH) | |||
| Postural tremor II | Tremor intensity (m/s2) | Frumkin et al16 | 48.6 (12.3) | 236 | −0.52 (0.14)* (LH) |
| Gerr et al17 | 44.5 (14.9) | −0.52 (0.15)* (RH) | |||
| Intention tremor | Tremor intensity (m/s2) | Current paper | 62.1 ( 8.8) | 43 | −0.52 (0.23)* (LH) |
| −0.53 (0.27)* (RH) | |||||
| Pronation/ supination | Standard deviation | CATSYS manual18 | 43.7 ( 2.7) | 30 | 0.04 (0.03) (LH) |
| 0.03 (0.02 ) (RH) | |||||
| Grooved pegboard | Time (s) | Frumkin et al16 | 48.6 (12.3 ) | 96 | 77.6 (15.1) (LH) |
| 73.6 (14.5) (RH) |
LH, left hand; RH, right hand.
Log-transformed value of tremor acceleration.
Data collection
An initial medical history interview was used to screen participants for eligibility and to ascertain self-reported evidence of the major symptoms of FXTAS, namely tremor and ataxia. This initial screening was performed by the project coordinator, who was aware of the family’s FXS history. All eligible participants were then asked to provide a biological sample for testing or confirmation of carrier status, complete the quantitative neurological test battery and, if eligible, complete a neuropsychological test battery. Testing was completed at the Emory site or at the home of the participant. The focus of this paper is the evaluation of the quantitative neurological testing results.
Neurological test battery
The CATSYS 2000 (www.catsys.dk), a portable testing system, was used to measure coordination ability, reaction time, tremor and postural stability. Output variables used for analysis were selected based on their correlation with a neurologist’s clinical rating.19
Ataxia was assessed using a force-plate attachment to the CATSYS system. Subjects were asked to stand on the platform with their eyes open (two trials) or closed (two trials). Results were recorded for 30 seconds per trial. The variable used to assess ataxia was sway velocity (mm/s). A mean score was calculated for the eyes-open trials and the eyes-closed trials. The comparison data described in table 1 were used to establish the mean (SD) for the “general population”. Subjects who scored >2SD above the mean were scored as having ataxia. Participants who were unable to stand on the platform due to postural instability, as noted by the test administrator, were also scored as positive for the eyes-open and eyes-closed trials and, thereby, scored as having ataxia (n = 4).
For tremor, three types of measurements were obtained using the CATSYS system. Each measurement was obtained from two trials on each hand, using a stylus that contained an accelerometer that measured movement in two dimensions. The variable analysed was the tremor intensity, defined as the root mean square of acceleration, measured in m/s2.
The first task (postural tremor I) measured postural tremor. Subjects were asked to hold the stylus so that it was parallel with the floor and parallel with their body with their little finger in front of their navel. They were instructed to relax their arm as much as they could without letting it touch their body. The second task (postural tremor II) was also a measure of postural tremor. Subjects were asked to stand with their arms extended perpendicular to their body. A stylus was affixed to the middle finger to detect any movement. The last task was a measurement of intention tremor. Subjects were asked to hold the stylus as they would a pencil, and track the movement of a bar across the monitor of a computer without actually touching the screen. Results were recorded for 8.2 seconds of every 10-second trial for all three measurements of tremor. For each subject, a mean for each hand was calculated for each of the tremor measurements. Subjects who scored >2SD above the mean obtained from the comparison group (table 1) for either hand were scored as having tremor. An overall mean for each type of tremor was also calculated (an average of the trials for left and right hands) for each subject.
Manual coordination was also measured using the CATSYS system. Subjects were asked to tap on a drum in an alternating hand pronation–supination movement as close to a constant metronome beat as possible. The variable used to assess coordination was the standard deviation(s) of offsets from the metronome sound. For each hand, there were two trials of a slow rhythm and of a fast rhythm. An age-matched control population was used to establish the mean and standard deviation for the general population (table 1). Subjects who scored >2SD above the mean were scored as having decreased fine motor skills.
Coordination was also measured using the Lafayette grooved pegboard test. Subjects were asked to place directional (or grooved) pegs into a pegboard as quickly as possible. The time and the number of dropped pegs were recorded. A total mean time was calculated for the dominant and non-dominant hands. Subjects who scored >2SD above the mean time of the comparison group were scored as having decreased fine motor skills (table 1).
Laboratory methods
DNA was extracted from buccal samples or blood (Qiagen Inc QiAmp DNA Blood Mini Kit; Qiagen, Valencia, California, USA). FMR1 CGG repeat sizes were determined by a fluorescent-sequencer method, as described preveiously,20 using an automated DNA sequencer (ABI Prism 377; Applied Biosystems, Foster City, California, USA). For male samples that did not amplify or for female samples that had only one allele, a second PCR-based hybridisation technique21 was used to identify a possible high repeat size band. The PCR reaction consisted of 1× PCR buffer, 10% dimethyl sulphoxide, 370 μmol/l deazaG, 500 μmol/l d(ACT), 0.3 μmol/l of each primer, 15 ng T4 gene 32, and 1.05 U Taq polymerase (Expand Long Taq; Roche Applied Biosciences, Indianapolis, Indiana, USA). Primers for the FMR1 repeat region were C: 5′-Cy5GCTCAGCTCCGTTTCGGTTTCACTTCCGGT-3′ and F: 5′-AGCCCCGCACTTCCACCAGCTCCTCCA-3′.22 If a ladder of bands was seen in the PCR result for a subject, indicating mosaicism, a Southern blot was also performed to ensure that there were no full mutation-sized bands.23 Any subject with full mutation alleles was not included in the analysis.
Statistical analyses
The CATSYS 2000 is a sensitive electronic system with delicate attachments, particularly the sway platform, which can be damaged in transport. At each session, the field tester noted any indication of equipment failure or unusual circumstances that might cause abnormal CATSYS results. Before analysis, all results were manually checked for any indication of equipment malfunction (eg outliers or unusual patterns), and any questionable data were deleted from the final dataset. Thus, owing to this equipment malfunction, sample sizes varied for each analysis and are noted in the tables.
Frequencies of presence or absence of ataxia and tremor as defined above were compared by repeat size group using either χ2 analysis or Fisher exact test depending on sample size. These outcome measures were also tested in a logistic regression model, adjusting for age. Based on the findings of Jacquemont et al,24 we defined high-repeat premutation carriers as those subjects with “at risk” alleles with≥70 repeats. These subjects were compared with those with “low risk” alleles (<70 repeats). The “low risk” allele carriers included 10 men with 55–69 repeats. In preliminary analyses, these were not found to differ from non-carriers, which is similar to the finding of Jacquemont et al, and thus, they were combined with the group of non-carrier men.
Analyses were also performed using the scores on the different tasks as continuous variables. First, the means for each repeat-size group were compared using Student t test. We also used repeat size and the measurement of interest as continuous variables in a linear regression model to determine if the risk for ataxia, tremor or decreased manual coordination increased linearly with repeat size. Age at testing was used as a covariate in these models.
In all regression models, we assessed the effects of repeat size and the phenotypes of interest using the generalised estimating equation (GEE) method,25 which accounts for the dependent nature of measures among family relatives; the corresponding significance p values from the GEE analyses are reported.
Table 2 shows the total number of participants tested by repeat size category and other basic demographic features. Although there was no significant difference in mean age between repeat groups (table 2), our a priori hypothesis was that “at-risk” premutation carriers will show age-related symptoms earlier than their low-repeat allele carrier siblings, thus we included age at testing in all statistical models. Interestingly, an interaction term of repeat size group and age was tested in all regression models, but was not found to be significant (data not shown). Table 2 also shows the median and range of repeat sizes for each of the repeat size groups.
Table 2.
Age at evaluation and repeat size of male participants by repeat size group
| Low-repeat allele carriers (<70 repeats) | High-repeat allele carriers (70–199 repeats) | |
|---|---|---|
| Sample size | 27 | 62 |
| Age at test | ||
| Mean (SD) | 65.6 (8.6) | 65.1 (7.7) |
| Range | 52 to 84 | 51 to 84 |
| CGG repeat number | ||
| Median | 35 | 91 |
| Range | 20 to 68 | 70 to 180 |
We also estimated age-related prevalence of symptoms in 5-year intervals starting at 55 years of age. For these estimates, the numerator was defined as the number of participants who were positive for tremor (any of the three measures) and/or ataxia at that age or earlier. The denominator included those same individuals plus those who had reached that age without developing tremor or ataxia. The age-related prevalence of ataxia or tremor was calculated for both repeat size groups at each age interval. For these estimates of prevalence, we did not include the single participant who was ascertained because he had diagnosis of FXTAS.
RESULTS
CATSYS as a screening tool for symptoms of FXTAS
We used the CATSYS instrument to determine if we could identify “preclinical” symptoms of FXTAS, primarily tremor and/or ataxia. Preclinical was defined as symptoms that had not reached a level of impairment that was acknowledged by the person. Thus, to determine the effectiveness of this instrument as a screening tool for symptoms of FXTAS, we compared the ability of the CATSYS instrument to detect tremor or ataxia (as defined above) with self-report (table 3). A subject was positive for CATSYS tremor if they were positive for any of the three tremor measurements. For ataxia, a subject was scored as positive if they were positive for the eyes closed trial. Participants were given the opportunity to self-report any problems with tremor or ataxia on a medical history questionnaire before their testing session. There was close to 70% concordance between the CATSYS and self-report for ataxia (table 3). Of the 50 men that did not self-report having ataxia, 15 were positive for ataxia as measured by the CATSYS system (30%). Similarly, for any tremor, there was almost 80% concordance between the two definitions. Of the 62 men that did not self-report tremor, 14 were positive on the CATSYS system for tremor (23%). Only a few men who self-reported ataxia or tremor were not found to be positive by CATSYS (table 3).
Table 3.
Comparison of CATSYS results and self-report for presence of ataxia and tremor
| Method of diagnosis
|
<70 repeats
|
70–199 repeats
|
|||
|---|---|---|---|---|---|
| CATSYS | Self-report | Ataxia | Tremor | Ataxia | Tremor |
| + | + | 1 (5) | 1 (3.85) | 8 (18) | 15 (27) |
| + | − | 2 (10.5) | 6 (23.1) | 13 (30) | 8 (14) |
| − | + | 2 (10.5) | 1 (3.85) | 2 (4) | 3 (5) |
| − | − | 14 (74) | 18 (69.2) | 21 (48) | 30 (54) |
Positive; −, negative.
Data are n (%).
Evaluation of ataxia among high-repeat and low-repeat carriers
Using a binary variable defined at 2SD above the mean of the comparison group, approximately 25% of all high-repeat carrier men and no low-repeat allele carrier men were scored as positive for ataxia on the eyes-open trials (Fisher exact test, p = 0.01). When the data were stratified by age group, there was a significant difference in the mean sway velocity for the young and middle-aged groups (table 4). In a linear regression model adjusted for age at testing, there was a significant relationship between the sway velocity and repeat size (p = 0.001; partial r2 = 0.16). Age at testing was also a significant covariate (p = 0.0004; partial r2 = 0.18). A logistic regression model, using presence or absence of ataxia as the outcome variable, could not be tested because there were no low-repeat allele carriers who scored positive for the eyes-open trial.
Table 4.
Measures of tremor and ataxia stratified by age group
| Measure and carrier status (no. of repeats) | Age group (years)
|
OR (95 CI)* | p Value | ||
|---|---|---|---|---|---|
| 51–59 | 60–69 | ≥70 | |||
| Sway (eyes open) | N/A | ||||
| <70 | |||||
| n | 7 | 7 | 5 | ||
| % | 0 | 0 | 0 | ||
| Mean (SD) | 8.5 (3.5) | 13.0 (0.8) | 15.8 (3.5) | ||
| 70–199 | |||||
| n | 10 | 20 | 14 | ||
| % | 0 | 25.0 | 42.9 | ||
| Mean (SD) | 14.8 (4.2)† | 18.0 (9.3)† | 23.2 (14.3) | ||
| Sway (eyes closed) | 4.88 (1.79 to 13.31) | 0.002 | |||
| <70 | |||||
| n | 7 | 7 | 5 | ||
| % | 0 | 0 | 60.0 | ||
| Mean (SD) | 12.6 (5.4) | 24.4 (5.0) | 40.2 (13.1) | ||
| 70–199 | |||||
| n | 10 | 20 | 14 | ||
| % | 40.0 | 50‡ | 50.0 | ||
| Mean (SD) | 35.7 (16.6)† | 40.5 (24.9)† | 37.5 (20.3) | ||
| Postural tremor I | 2.06 (0.73 to 5.80) | 0.17 | |||
| <70 | |||||
| n | 9 | 7 | 8 | ||
| % | 10.0 | 14.3 | 50.0 | ||
| Mean (SD) | 0.1 (0.1) | 0.1 (0.1) | 0.2 (0.1) | ||
| 70–199 | |||||
| n | 14 | 26 | 15 | ||
| % | 35.7 | 34.6 | 46.7 | ||
| Mean (SD) | 0.2 (0.1) | 0.2 (0.2) | 0.2 (0.3) | ||
| Postural tremor II | 0.84 (0.19 to 3.65) | 0.82 | |||
| <70 | |||||
| n | 10 | 8 | 8 | ||
| % | 10.0 | 12.5 | 25.0 | ||
| Mean (SD) | 0.3 (0.1) | 0.3 (0.3) | 0.5 (0.2) | ||
| 70–199 | |||||
| n | 14 | 26 | 15 | ||
| % | 0 | 19.2 | 20.0 | ||
| Mean (SD) | 0.3 (0.1) | 0.4 (0.2) | 0.4 (0.2) | ||
| Intention tremor | 3.28 (0.84 to 12.76) | 0.09 | |||
| <70 | |||||
| n | 10 | 6 | 8 | ||
| % | 0 | 0 | 12.5 | ||
| Mean (SD) | 0.2 (0.1) | 0.3 (0.1) | 0.4 (0.4) | ||
| 70–199 | |||||
| n | 14 | 27 | 15 | ||
| % | 7.1 | 25.9 | 6.7 | ||
| Mean (SD) | 0.3 (0.4) | 0.7 (1.0) | 0.4 (0.2) | ||
Data are sample size (n), percentage of patients who scored >2SD above the mean, and overall mean and SD of intensity measure.
Adjusted for age at testing.
The t test (comparing two repeat-size groups within an age group), significant at p<0.05.
Fisher exact test (comparing two repeat-size groups within an age group) significant at p<0.05.
For the eyes-closed trials for ataxia, 48% of high-repeat allele carriers and 16% of low-repeat allele carrier men were positive (Fisher’s exact test, p value = 0.01). Similar to the eyes-open trials, there was a significant difference in the mean sway velocity for the young and middle-aged groups, but not for the older group (table 4). There was also a significant linear relationship between the mean sway velocity and repeat size (p = 0.0003, partial r2 = 0.14) when adjusted for age at testing. Age at testing was also a significant covariate in the linear model (p = 0.03; partial r2 = 0.09). Using a logistic model, we regressed presence or absence of ataxia as measured by the eyes-closed trials on to the variables of repeat size group and age at testing. Repeat size group was a significant predictor although age at testing was not (table 4).
Evaluation of tremor among high-repeat and low-repeat carriers
Among men, there was an increased frequency of postural tremor I between high-repeat and low-repeat allele carriers in the young and middle-aged groups (table 4). There was no significant linear relationship between the log-transformed average tremor intensity and repeat size (p = 0.15) in a model adjusted for age at testing. Using a logistic regression model adjusted for age at testing, we found an increased risk for postural tremor I among high-repeat allele carriers (OR = 2.06; 95% CI 0.73 to 5.80; table 4). Age at testing was not a significant covariate in either the linear or logistic models.
For postural tremor II, no differences were found between high-repeat carriers and low-repeat carriers (14.5% compared with 15.4%, p>0.10). No significant differences were found when stratified by age group, using linear regression analysis (data not shown), or using logistic regression analysis (table 4).
Although there were more high-repeat allele carrier men that exceeded the threshold indicating intention tremor, this increase was not significant (16.1% of high repeat allele carriers compared with 4.1% of low-repeat allele carriers). There was no significant difference observed when stratified by age group and the linear (data not shown) and logistic regression (table 4) models were non-significant.
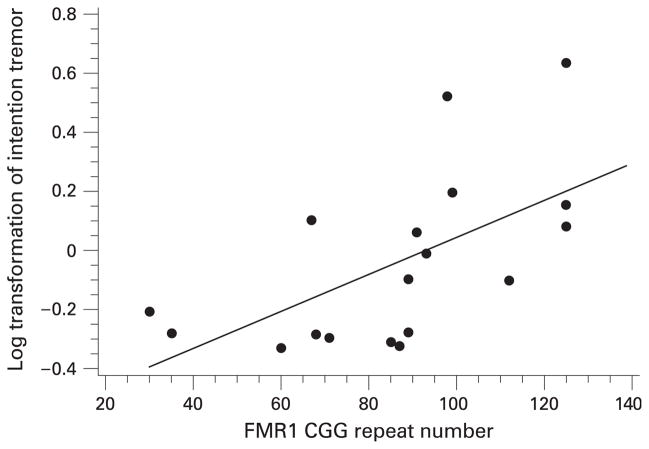
The lack of association between repeat size and intention tremor was surprising based on the initial report by Hagerman et al.4 As a post hoc analysis, we evaluated only participants who scored positive for intention tremor in a linear regression model. For this analysis, we needed to redefine “positive” to include men who had results >1SD instead of 2SD above the normative data, because of small sample sizes. For these men, there was a positive correlation with tremor intensity and repeat size, adjusting for age at testing (p<0.0001; partial r2 = 0.37). Age at testing was not a significant covariate in this model. The scatterplot of the age-unadjusted data is shown in fig 1.
Figure 1.
Association of intention tremor intensity (log transformed) and repeat size among men who were positive for intention tremor.
Evaluation of manual coordination among high-repeat and low-repeat carriers
The first measure of manual coordination was obtained from the pronation/supination task on the CATSYS system. For the slow trials, 1.8% of high-repeat carrier men were scored as having decreased fine motor skills compared with 0% among low-repeat carriers. For the fast trials, 61.8% of high-repeat and 56.0% of low-repeat carriers were scored as having decreased fine motor skills. When stratified by age, there was a significant difference between the repeat size groups only in the oldest age group (table 5). There were no significant effects in the linear regression model, but the logistic regression model showed an increased point estimate.
Table 5.
Measures of coordination stratified by age group
| Measure and carrier status (no. of repeats) | Age group (years)
|
OR (95 CI)* | p Value | ||
|---|---|---|---|---|---|
| 51–59 | 60–69 | ≥70 | |||
| Pronation/supination (fast) | 1.42 (0.50–4.03) | 0.51 | |||
| <70 | |||||
| n | 9 | 8 | 8 | ||
| % | 66.7 | 62.5 | 37.5 | ||
| Mean (SD) | 0.07 (0.03) | 0.08 (0.04) | 0.08 (0.02) | ||
| 70–199 | |||||
| n | 13 | 27 | 15 | ||
| % | 46.1 | 55.6 | 86.7† | ||
| Mean (SD) | 0.07 (0.04) | 0.08 (0.02) | 0.09 (0.02) | ||
| Grooved pegboard | 3.14 (0.99–9.98) | 0.05 | |||
| <70 | |||||
| n | 8 | 9 | 8 | ||
| % | 12.5 | 22.2 | 37.5 | ||
| Mean (SD) | 82.2 (13.8) | 101.7 (27.8) | 107.1 (19.6) | ||
| 70–199 | |||||
| n | 14 | 25 | 15 | ||
| % | 14.3 | 48.0 | 66.7 | ||
| Mean (SD) | 89.8 (14.1) | 112.1 (31.0) | 132.8 (30.2)‡ | ||
Data are sample size (n), percentage of patients who scored >2SD above the mean, and overall mean and SD of intensity measure.
Adjusted for age at testing.
Fisher exact test (comparing 2 repeat size groups within an age group) significant at p<0.05.
The t test (comparing noncarriers and carriers within an age group) significant at p<0.05.
The second measure of coordination was the grooved pegboard. For this measure, 44.4% of high-repeat and 24.0% of low-repeat carrier men were scored as having decreased fine motor skills (χ21 = 3.03; p = 0.08). When this comparison was stratified by age, there were no significant differences by repeat group (table 5). The linear regression model using the average time for completion as a continuous variable was significant, with both the repeat size variable (p = 0.006; partial r2 = 0.10) and the age at testing (p<0.0001; partial r2 = 0.29) contributing significant effects to the model. The logistic regression model, adjusted for age at testing, showed an increased risk for high-repeat carriers compared with low repeat carriers (OR = 3.14; 95% CI 0.99 to 9.98; table 5). Age at testing was also a significant covariate in this model.
Age-related prevalence of ataxia and tremor
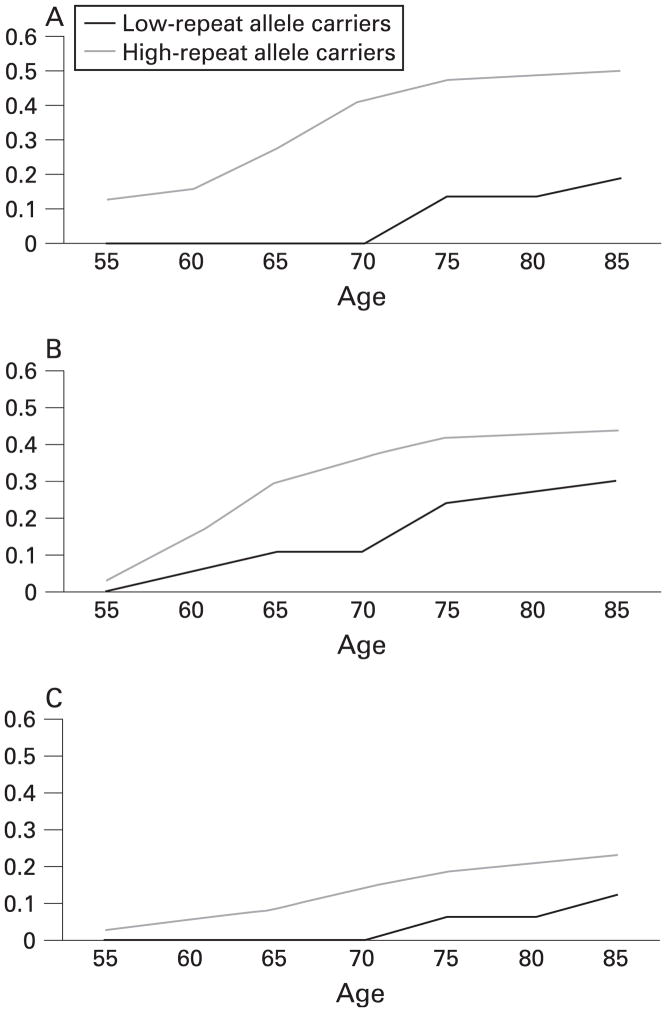
Using the measures of tremor and ataxia as detected by the CATSYS system, we calculated the age-related prevalence of ataxia as measured by the eyes-closed trials (fig 2A), any tremor (fig 2B), and having both tremor and ataxia (fig 2C) for each repeat size group. These estimates are based on the presence or absence of such traits at the time of evaluation. They do not incorporate age of onset, thus the prevalence rates in the younger age groups are the minimum estimates. These results differ from the frequencies shown above, as these represent a cumulative frequency for tremor and ataxia—that is, they include individuals who were positive for tremor and/or ataxia at that age or earlier (for more detailed description, see Methods). These figures further confirm the lack of interaction between carrier status and age for each trait—that is, for both groups, the prevalence of the trait increases at approximately the same rate. Overall, the estimate of the lifetime prevalence of ataxia is about 50% among high repeat carriers compared with 20% in the other group (fig 2A), and the lifetime prevalence of tremor is about 45% among high-repeat allele carriers compared with 30% among those with <70 repeats (fig 2B).
Figure 2.
Age-related prevalence of (A) tremor, (B ataxia), and (C) both tremor and ataxia among high-repeat (70–199) and low-repeat (<70) allele carriers.
DISCUSSION
The overall goal of our research is to determine the natural history and prevalence of symptoms of FXTAS among premutation carriers and identify associated risk factors. The initial step to achieve this goal was to identify potential subjects who are positive for FXTAS-associated symptoms. To identify these subjects, we implemented a protocol using the CATSYS as a screening tool for tremor, ataxia, and decreased coordination. Using this portable system, we are able to send trained testers to subjects’ homes to obtain quantitative measures of these symptoms. The next step in the research protocol is to invite these individuals with symptoms of FXTAS for full neurological investigation with a neurologist. These subjects are then classified as having definite, probable, possible, or no FXTAS based on the diagnostic criteria.3 These studies are ongoing. The ultimate goal, of course, is to identify treatment regimens to ameliorate FXTAS-associated symptoms among carriers. We have focused on results from men only, as those from women were too limited to draw worthwhile conclusions (data not shown).
Based on the CATSYS measures, we found that premutation carrier men aged >50 years have an increased risk for ataxia, tremor (specifically postural tremor I and intention tremor) and decreased manual coordination. The largest differences in the proportion of high-repeat and of low-repeat carriers scoring positive for these phenotypes usually occurred in the younger age groups, although these differences were not always significant. With the exception of the eyes-open sway measure and measures of manual coordination, there were only small or no differences between repeat groups in frequencies in the oldest age group. This is not surprising, as such traits occur at high frequency in the general aged population and require a larger sample size to detect differences by carrier status. Small sample sizes among age groups in this study (tables 4 and 5) show that our conclusions are limited.
All of the above conclusions are based on the definition of “affected” as being 2SD above the mean of the normative sample. As shown in tables 1 and 2, the mean age of the normative sample was younger on most measures than our participants. Although a limitation of the study, it is important to note that our focus was on the differences between low and high repeat carriers, both of which were compared with the normative data. Thus, the differences between groups are meaningful. Ideally, the use of a better age-matched comparison group will help define the lowest repeat group that has an increased risk for symptoms of FXTAS.
Overall, the CATSYS system seems to be a useful screening tool for symptoms of FXTAS. It enables researchers to travel to the subjects’ homes for testing, which increases our total sample size. It is not only more convenient for subjects, but for those subjects with ataxia and/or tremor, it is not always feasible to have them attend a clinic for testing. One of the limitations of the CATSYS system is the sensitivity of the instruments. Travel and shipping occasionally cause some components to become broken or uncalibrated.
Our results also indicate that measures from the CATSYS may be helpful in identifying “preclinical” signs of the disorder: a significant portion of the men in our study did not report having tremor (23%) or ataxia (30%), but were scored as positive by the CATSYS system. There were a few subjects that did report tremor or ataxia, but were not scored as positive based on the CATSYS screen. For tremor, this is not surprising, as we only tested a subset of various tremor types using CATSYS but did not have participants provide any details of their self-reported tremor. For example, we did not test for resting tremor in our protocol, as it was not indicated as an early symptom of FXTAS in previous reports. In addition, medications taken for tremor may have reduced the ability of the CATSYS system to identify tremor; however, we obtained a full list of patients’ current medication and would have noted this possibility. Ataxia may not be reported by an individual until it interferes with their life (eg, successive falls). It is interesting that a greater percentage of high-repeat premutation carriers were unaware of problems with ataxia, compared with low-repeat allele carriers (30% compared with 10.5%, table 3), perhaps indicating poor insight or denial on the part of high-repeat allele carriers.
A full clinical examination will provide insight into these specific issues. We are currently conducting full clinical examinations on all individuals with tremor or ataxia diagnosed by CATSYS results and/or by self-report to determine sensitivity and specificity rates. It is important to note that the CATSYS variables we used have been shown to correlate well with clinical evaluation by a neurologist.19
We also estimated the age-related prevalence estimates for ataxia and/or tremor in our study population (fig 2A–C), which were very similar to the previous results from Jacquemont et al.10 However, we need to note several limitations of our estimates. We only included subjects who were able to complete the testing. For those who were incapable of completing the neurological tests due to severe illness, we had to exclude them from this analysis. We obtained information from family members through a medical history screening tool and through medical records, but these data were not incorporated in the analyses reported in this paper. In addition, we did not incorporate age of onset into this analysis, but instead used age at examination. Thus, both limitations will underestimate the prevalence of symptoms among all participants.
In conclusion, we have developed an informative, quantitative screening battery for symptoms of FXTAS. We are able to identify individuals as having symptoms of tremor or ataxia, sometimes before the individual recognises them. This early screening potential will be invaluable in longitudinal studies and in clinical trials as “presymptomatic” drug treatments become available. The CATSYS system may also be clinically important in the early stages of FXTAS. For example, this system has the potential to identify early symptoms of ataxia. This early identification may be helpful to prevent early related falls. Clearly, multisite longitudinal studies are the next step to better define the natural history of FXTAS and to identify associated risk factors for onset and severity of symptoms.
Acknowledgments
We would like to thank W He and M Yadav-Shah for laboratory assistance. We dedicate this work to Dr R Letz, who initiated this study using the CATSYS system. This project could not have been accomplished without his significant input. Finally, we thank the study subjects, whose participation made this work possible. This work was supported by NIH grants R01 HD29909 and P30 HD24064.
Footnotes
Competing interests: None declared.
References
- 1.Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med. 2005;7:584–7. doi: 10.1097/01.GIM.0000182468.22666.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherman SL, Taylor K, Allen EG. FMR1 premutation: a leading cause of inherited ovarian dysfunction. In: Arrieta I, Penagarikano O, Telez M, editors. Fragile sites: new discoveries and changing perspectives. New York: Nova Science Publishers; 2007. [Google Scholar]
- 3.Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, Berry-Kravis E, Brown WT, Schaeffer S, Kissel J, Tassone F, Hagerman PJ. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–78. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–30. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 5.Grigsby J, Brega AG, Jacquemont S, Loesch DZ, Leehey MA, Goodrich GK, Hagerman RJ, Epstein J, Wilson R, Cogswell JB, Jardini T, Tassone F, Hagerman PJ. Impairment in the cognitive functioning of men with fragile X-associated tremor/ ataxia syndrome (FXTAS) J Neurol Sci. 2006;248:227–33. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Grigsby J, Brega AG, Leehey MA, Goodrich GK, Jacquemont S, Loesch DZ, Cogswell JB, Epstein J, Wilson R, Jardini T, Gould E, Bennett RE, Hessl D, Cohen S, Cook K, Tassone F, Hagerman PJ, Hagerman RJ. Impairment of executive cognitive functioning in males with fragile X-associated tremor/ataxia syndrome. Mov Disord. 2007;22:645–50. doi: 10.1002/mds.21359. [DOI] [PubMed] [Google Scholar]
- 7.Bacalman S, Farzin F, Bourgeois JA, Cogswell J, Goodlin-Jones BL, Gane LW, Grigsby J, Leehey MA, Tassone F, Hagerman RJ. Psychiatric phenotype of the fragile X-associated tremor/ataxia syndrome (FXTAS) in males: newly described frontosubcortical dementia. J Clin Psychiatry. 2006;67:87–94. doi: 10.4088/jcp.v67n0112. [DOI] [PubMed] [Google Scholar]
- 8.Brunberg JA, Jacquemont S, Hagerman RJ, Berry-Kravis EM, Grigsby J, Leehey MA, Tassone F, Brown WT, Greco CM, Hagerman PJ. Fragile X premutation carriers: characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. AJNR Am J Neuroradiol. 2002;23:1757–66. [PMC free article] [PubMed] [Google Scholar]
- 9.Hagerman PJ, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome—an older face of the fragile X gene. Nat Clin Pract Neurol. 2007;3:107–12. doi: 10.1038/ncpneuro0373. [DOI] [PubMed] [Google Scholar]
- 10.Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, Zhang L, Jardini T, Gane LW, Harris SW, Herman K, Grigsby J, Greco CM, Berry-Kravis E, Tassone F, Hagerman PJ. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291:460–9. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 11.Loesch DZ, Litewka L, Brotchie P, Huggins RM, Tassone F, Cook M. Magnetic resonance imaging study in older fragile X premutation male carriers. Ann Neurol. 2005;58:326–30. doi: 10.1002/ana.20542. [DOI] [PubMed] [Google Scholar]
- 12.Loesch DZ, Litewka L, Churchyard A, Gould E, Tassone F, Cook M. Tremor/ataxia syndrome and fragile X premutation: diagnostic caveats. J Clin Neurosci. 2007;14:245–8. doi: 10.1016/j.jocn.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, Hessl D, Becker EJ, Papazian J, Leehey MA, Hagerman RJ, Hagerman PJ. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–55. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 14.Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–71. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- 15.Leehey MA, Berry-Kravis E, Min SJ, Hall DA, Rice CD, Zhang L, Grigsby J, Greco CM, Reynolds A, Lara R, Cogswell J, Jacquemont S, Hessl DR, Tassone F, Hagerman R, Hagerman PJ. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord. 2007;22:203–6. doi: 10.1002/mds.21252. [DOI] [PubMed] [Google Scholar]
- 16.Frumkin H, Letz R, Williams PL, Gerr F, Pierce M, Sanders A, Elon L, Manning CC, Woods JS, Hertzberg VS, Mueller P, Taylor BB. Health effects of long-term mercury exposure among chloralkali plant workers. Am J Ind Med. 2001;39:1–18. doi: 10.1002/1097-0274(200101)39:1<1::aid-ajim1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Gerr F, Letz R, Ryan PB, Green RC. Neurological effects of environmental exposure to arsenic in dust and soil among humans. Neurotoxicology. 2000;21:475–87. [PubMed] [Google Scholar]
- 18.CATSYS. User’s Manual. Danish Product Development Ltd; 2000. [Google Scholar]
- 19.Gerr F, Letz R, Green RC. Relationships between quantitative measures and neurologist’s clinical rating of tremor and standing steadiness in two epidemiological studies. Neurotoxicology. 2000;21:753–60. [PubMed] [Google Scholar]
- 20.Meadows KL, Pettay D, Newman J, Hersey J, Ashley AE, Sherman SL. Survey of the fragile X syndrome and the fragile X E syndrome in a special education needs population. Am J Med Genet. 1996;64:428–33. doi: 10.1002/(SICI)1096-8628(19960809)64:2<428::AID-AJMG39>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Brown WT, Houck GE, Jr, Jeziorowska A, Levinson FN, Ding X, Dobkin C, Zhong N, Henderson J, Brooks SS, Jenkins EC. Rapid fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test. Jama. 1993;270:1569–75. [PubMed] [Google Scholar]
- 22.Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Jr, Warren ST, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–58. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 23.Rousseau F, Heitz D, Biancalana V, Blumenfeld S, Kretz C, Boue J, Tommerup N, Van Der Hagen C, DeLozier-Blanchet C, Croquette MF, et al. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med. 1991;325:1673–81. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- 24.Jacquemont S, Leehey MA, Hagerman RJ, Beckett LA, Hagerman PJ. Size bias of fragile X premutation alleles in late-onset movement disorders. J Med Genet. 2006;43:804–9. doi: 10.1136/jmg.2006.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]