Abstract
The fragile X mental retardation gene, FMR1, contains a polymorphic CGG repeat in the 5′-untranslated region of exon 1. Once unstable, this repeat is capable of expansion across generations. Women who carry a premutation allele (55–199 repeats) are at risk of passing on a full mutation allele (>200 repeats) to their offspring. A full mutation leads to the most common form of inherited intellectual disability, fragile X syndrome (FXS). Mounting evidence suggests that premutation carriers may be vulnerable to symptoms of anxiety and depression. The goal of this study was to test the hypothesis that among women who carry a premutation, the stress of raising a child with FXS would be moderated by genetic factors influencing endogenous cortisol responses, which could in turn modulate anxiety and depression symptoms. To this end, we genotyped single nucleotide polymorphisms (SNPs) at the corticotrophin releasing hormone receptor 1 locus (CRHR1) in 460 women. Participants completed self-report questionnaires assessing symptoms of depression [Centers for Epidemiological Studies Depression Scale (CESD)], anxiety [State-Trait Anxiety Inventory (STAI) and Social Phobia and Anxiety Inventory (SPAI)], and mood [Positive and Negative Affect Schedule (PANAS)]. Results indicate a statistically significant interaction between CRHR1 genotype and the status of raising a child with FXS to predict social anxiety symptoms reported on the SPAI (rs7209436, P = 0.0001). Our data suggest that genetic variants in CRHR1 that associate with differential cortisol activation may also modulate levels of anxiety related to the stress of raising a child with FXS among women who carry an FMR1 premutation.
Keywords: FMR1 premutation, fragile X syndrome, hypothalamic-pituitary-adrenal axis, cortisol, gene–environment interaction
INTRODUCTION
The X-linked fragile X mental retardation gene (FMR1) contains a highly polymorphic CGG repeat in the 5′-untranslated region of exon 1. Most common alleles contain roughly 30 repeats and are stable from one generation to the next [Snow et al., 1993]. Alleles with greater than 200 repeats, termed full mutations, are associated with hypermethylation and transcriptional silencing of the gene [Sutcliffe et al., 1992]. The subsequent loss of the protein product, FMRP, is responsible for the neurodevelopmental disorder, fragile X syndrome (FXS) [Pieretti et al., 1991; Ashley et al., 1993]. FXS is the most common cause of inherited intellectual disability and is associated with a range of cognitive and behavioral deficits, including autism spectrum disorders (ASDs).
Alleles with repeats in the range of 55–199, termed premutation alleles, are associated with a spectrum of phenotypes. First, roughly 30% of male carriers of premutation alleles develop a late-onset neurodegenerative disorder, fragile X-associated tremor/ataxia syndrome (FXTAS) [Hagerman et al., 2001; Jacquemont et al., 2004]. FXTAS among female carriers is less frequent, about 10–16% [Coffey et al., 2008; Rodriguez-Revenga et al., 2009], most likely due to the masking effect of the other X-chromosome allele. Second, female carriers of premutation alleles are at risk of fragile-X primary ovarian insufficiency (FXPOI) [Allen et al., 2007]. Lastly, premutation alleles are capable of expanding to a full mutation in the next generation with maternal transmission [Snow et al., 1993; Nolin et al., 1996]. In other words, females who carry a premutation allele are at risk of having a child with FXS.
The presence of additional neuropsychological and behavioral impairment among carriers of premutation alleles is unclear (for a review, see Hunter et al., 2009). Several studies point to a potential vulnerability among carriers of premutation alleles for increased symptoms of depression and anxiety [Johnston et al., 2001; Bailey et al., 2008a; Roberts et al., 2009]. Concurrently, additional studies point to the significant impact that stress has on mothers of children with FXS [Abbeduto et al., 2004; Lewis et al., 2006; Bailey et al., 2008b; Hartley et al., 2011; Seltzer et al., 2011]. Bailey et al. [2008b] assessed 95 mothers of children with FXS who also carried a premutation allele and reported that the severity of behavior problems in the child significantly impacted not only maternal stress, but also depressive symptoms, anxiety, anger, and quality of life. Most recently, Seltzer et al. [2011] analyzed 82 premutation women who had a child with FXS and found that the length of the premutation allele interacted with the severity of life stress to predict the severity of symptoms associated with depression and anxiety and daily cortisol levels. Specifically, mothers with midrange premutation alleles with the highest number of life stressors had higher levels of symptoms associated with depression and anxiety and blunted cortisol compared to mothers with low- and high-range premutation alleles. However, this group of mid-range premutation alleles, in the absence of stressors, had lower levels of depression and anxiety and typical cortisol responses than other premutation carriers.
In previously published studies of women who carry a premutation allele, we detected slightly elevated symptoms of depression and anxiety and an increased risk of meeting diagnostic criteria for depressive [15% non-carriers (NC) vs. 25% premutation carriers] and anxiety disorders (6.4% NC and 13% premutation carriers), though results did not reach statistical significance [Hunter et al., 2008a, 2010]. However, together with independent reports of elevated rates of depression and anxiety [Bailey et al., 2008a; Roberts et al., 2009], these results suggested that although not all women who carry a premutation allele are affected, perhaps a subgroup are more vulnerable to elevated symptoms of depression and anxiety.
It is clear that depression and anxiety are complex disorders, with multiple genetic and environmental factors influencing onset and severity. The hypothalamic-pituitary-adrenal (HPA) axis is responsible for the endogenous stress response [Romeo and McEwen, 2006]. Abundant evidence suggests that the HPA axis plays important roles in modulating the impact of stress or trauma on symptoms of depression and anxiety [Heim et al., 2008]. Recent studies suggest that genetic variation in the corticotrophin-releasing hormone type 1 receptor (CRHR1) gene, the product of which mediates regulation of expression and release of adrenocorticotrophin (ACTH) from the anterior pituitary which in turn stimulates release of cortisol from the adrenal cortex, can interact with environmental stress or trauma to influence mood and anxiety-related outcomes [Bradley et al., 2008; Polanczyk et al., 2009; Deyoung et al., 2011]. Bradley et al. [2008] and Polanczyk et al. [2009] were the first to show that a haplotype formed from three single nucleotide polymorphisms (SNPs) of intron 1 of corticotrophin releasing hormone receptor 1 locus (CRHR1; rs7209436, rs110402, and rs242924) significantly interacted with a history of childhood trauma to modify the risk for adult depression: an increasing number of copies of the less common haplotype, TAT, associated with less severe adult depressive symptoms in the individuals with histories of childhood trauma. Those observations suggested that the TAT haplotype exerted a protective effect in persons exposed to childhood trauma, a known risk factor for development of depression.
Prior research indicates that raising children with FXS is a stressor that impacts mothers at least as severely as raising children with other intellectual disabilities, such as Down syndrome [Abbeduto et al., 2004; Lewis et al., 2006]. The goal of this study was to test the hypothesis that variation at CRHR1 associates differentially with severity of depression and anxiety symptoms among women carrying premutation FMR1 alleles who have been exposed to a defined chronic stress by virtue of having raised children with FXS, as compared to women carrying the premutation who have not raised children with FXS. We genotyped 460 women, including 169 women with a premutation who were mothers of a child with FXS and 117 women with a premutation who did not have a child with FXS, at 5 CRHR1 SNPs to assess this hypothesis.
METHODS
Study Population
The participants were recruited as part of an ongoing study to investigate neuropsychological phenotypes associated with FMR1 premutation alleles [Allen et al., 2005; Hunter et al., 2008a,b]. Participants were identified from the general population (primarily NC) as well as from families with a known history of FXS (both NC and premutation carriers). Participants ranged in age from 18 to 50 years and spoke English as their primary language. The study population included 460 women from 309 pedigrees (221 pedigrees with a single participant, 56 pedigrees with two related participants, 17 pedigrees with 3 related participants, 5 pedigrees with 4 related participants, 6 pedigrees with 5 related participants, 3 pedigrees with 6 related participants, and 1 pedigree with 8 related participants). All participants were asked if they raised a child with FXS. The protocols and consent forms for ascertainment were approved by the Institutional Review Board at Emory University.
Measurement of Depression and Anxiety Symptoms
Study participants completed a medical history questionnaire and a 4-hr neuropsychological test battery that included measures of symptoms associated with depression and anxiety. Tests were administered by a trained psychometrist blind to the subject’s FMR1 genotype as well as family history of FXS.
Center for Epidemiologic Studies Depression Scale (CES-D)
The Center for Epidemiologic Studies Depression Scale (CES-D) is a 20-item self-report questionnaire that measures the frequency of depression symptoms experienced in the past week [Radloff, 1977]. Scores range from 0 to 60, with higher scores indicative of higher levels of depression symptomology. CES-D scores were missing for three participants.
The State-Trait Anxiety Inventory (STAI)
The severities of current anxiety (state anxiety) and general anxiety susceptibility (trait anxiety) were measured with the State-Trait Anxiety Inventory (STAI) [Spielberger, 1983]. The STAI is a 40-item self-report questionnaire, with 20 items each for state and trait anxiety. Subscale scores ranged from 20 to 80, with higher scores indicative of higher levels of anxiety. The STAI was not included in the initial test battery and was added later in the study. Thus state anxiety scores were missing for 122 participants and trait anxiety scores were missing for 121 participants. It is important to note that missing STAI scores were associated with repeat classes (see Statistical Analysis Section below for repeat class definitions), where 46.4% of NC, 61.0% of intermediate, 11.4% of low premutation, 7.1% of mid premutation, and 15.3% of high premutation allele carriers were missing scores for STAI state anxiety ( , P < 0.0001). Thus rates of missing STAI scores were lowest in the premutation groups. This is due to the change in recruitment strategy of our study where recruitment from the general population was highest at the beginning of the study before the STAI was introduced into the test battery. Thus many NC from the general population recruited during this time are missing these scores.
Social Phobia and Anxiety Inventory (SPAI)
Symptoms associated with social phobia and agoraphobia were measured with the Social Phobia and Anxiety Inventory (SPAI) which measures the severity of distress experienced in various social situations [Turner et al., 1996]. The total social phobia score is based on 32 items and rates the frequency of social phobia experienced in various social situations. The agoraphobia score is based on 13 items and rates social distress due to panic and agoraphobia symptoms. The difference score, calculated by subtracting the agoraphobia score from the social phobia score, is indicative of “pure” social phobia symptoms without panic symptoms, and is the social phobia score we use in this study. SPAI scores were missing for two participants.
The Positive and Negative Affect Schedule (PANAS)
Affective states were measured with the 60-item Positive and Negative Affect Schedule (PANAS) [Watson and Clark, 1994]. Positive affect and negative affect were measured by 10 items each to assess the psychological well-being of the past year. Positive affect and negative affect scores range from 10 to 50 with higher scores indicative increased pleasurable experience and increased feelings for upset and unpleasant arousal, respectively. PANAS scores were missing for one participant.
Molecular Analysis
All participants provided a biological sample for molecular analysis. DNA was extracted using the Qiagen QiAmp DNA Blood Mini Kit.
FMR1 genotyping
FMR1 CGG repeat lengths were measured using a fluorescent-sequencing method using an ABI Prism 377 DNA Sequencer or ABI 3100 Genetic Analyzer [Meadows et al., 1996]. In the event only a single band was present upon genotyping, a second PCR-based method that detects high repeat alleles was used [Brown et al., 1993]. For participants who were heterozygous, the CGG repeat length from the larger repeat allele was used in statistical analyses.
CRHR1 genotyping and haplotype analysis
Bradley et al. [2008] analyzed gene × environment interactions at 10 SNPs that span CRHR1, with rs7209436, rs110402, rs242924, rs242940, and rs173365 showing the strongest interaction. Therefore, these five SNPs were chosen for analysis in the current study. All participants were genotyped using TaqMan allelic discrimination assays as described by Bradley et al. [2008] or the Sequenom MassArray platform [Ehrich et al., 2005; Gabriel et al., 2009]. The SNPs did not deviate from Hardy–Weinberg equilibrium (rs7209436: , P = 0.7877; rs110402: , P = 0.3342; rs242924: , P = 0.4092; rs242940: , P = 0.7157; rs173365: , P = 0.5121).
Statistical Analysis
CRHR haplotype analyses
The haplotype block consisting of three SNPs analyzed here (rs7209436, rs110402, and rs242924) had previously been shown to significantly interact with life stress to predict depression outcomes [Bradley et al., 2008; Polanczyk et al., 2009; Deyoung et al., 2011]. Thus we estimated the haplotype frequencies of our study population using PHASE 2.1 [Stephens et al., 2001; Stephens and Donnelly, 2003]. The number of copies of the less common TAT genotype of this haplotype was determined. If any participant was missing genotype data at any of the SNPs of the haplotype, their haplotype data were also considered missing.
Linear modeling of depression and anxiety symptoms
Prior to modeling, chi-squared analyses were used to test for independence between CRHR1 genotypes and having a child with FXS as well as between CRHR1 and FMR1 repeat groups to ensure a lack of gene–environment and gene–gene correlations, respectively. In addition, all depression and anxiety symptom scores were tested for normality and homoskedasticity and transformed if necessary (CES, STAI, and PANAS negative affect scores were natural log-transformed; SPAI agoraphobia score was square-root transformed; and PANAS positive affect and SPAI social phobia scores required no transformation).
Each depression and anxiety score was assessed independently as a continuous outcome. We used linear mixed-effects models to allow us to model correlated outcomes among participants from the same pedigree due to shared environmental and genetic factors. The main predictors were having a child with FXS (0 = participant did not have a child with FXS; 1 = participant did have a child with FXS) and CRHR1 genotype (coded under an additive model with the less common allele modeled as the “risk” allele). Models were also adjusted for FMR1 repeat length group: NC (<40 repeats), intermediate allele carriers (IM; 40–60 repeats), low premutation allele carriers (low PM; 61–80 repeats), mid premutation allele carriers (mid PM; 81–100 repeats), and high premutation allele carriers (high PM; 100–199 repeats). Cutoff points for repeat length groups have been recommended by the American College of Medical Genetics [Sherman et al., 2005] and are based on the probability of instability and risk of expansion to a full mutation. However, the repeat length ranges have not been established for the risk of depression and anxiety outcomes or other premutation-associated disorders. Thus we based our cutoff points on previously published studies that showed a nonlinear effect of FMR1 repeat length in the premutation range on FXPOI [Sullivan et al., 2005; Allen et al., 2007] and to be consistent with previously published studies [Hunter et al., 2008a,b]. Indicator variables were created for each repeat group using the NC group as the reference to allow for a potential nonlinear association with FMR1. Additional covariates included age at testing, self-reported race/ethnicity, level of education, household income, method of ascertainment (general population vs. families with FXS), and use of anxiety and/or depression medication at the time of testing.
Two interaction terms were tested to detect potential significant interactions between predictors. First, to address the main hypothesis that genotypes at the CRHR1 gene moderate the effect of a major life stressor (in this instance, raising a child with FXS), on anxiety and depression symptoms, we tested the interaction between each CRHR1 genotype and the variable for having a child with FXS. Second, given a previously published study that reported the moderation of FMR1 repeat length by life stress to predict cortisol levels and anxiety and depression outcomes [Seltzer et al., 2011], we also tested for a potential interaction between FMR1 repeat length group and having a child with FXS.
Sensitivity analyses were performed to ensure the results above were robust to potential confounding. First, given that NC and IM cannot expand to a full mutation in the next generation, women in these FMR1 repeat length groups do not have a child with FXS (Table I). Thus we ran a set of models that only included the three premutation allele groups, as these groups included all the participants who had raised a child with FXS. These models accounted for the potential of a reporting bias due to carrier status: that is, women without a risk for having a child with FXS may report the severity of symptoms differently than those with the premutation who also do not have a child with FXS. Second, to account for a potential different distribution of CRHR1 genotype alleles across racial groups, we fit models using only participants who reported as being Caucasian. We attempted also to fit separate models using self-reporting African-Americans, but, due to small sample size, such models failed to converge and could not be run. Third, as repeat length group definitions are somewhat arbitrary with respect to premutation-associated phenotypes (see above), we ran models using repeat length as a continuous variable to determine whether the results were robust to the coding strategy for FMR1 repeat length. And lastly, we ran models using only the women who had at least one child irrespective of FXS status, to account for the stress associated with raising a child in general, with or without special needs.
TABLE I.
Demographic Data and Background Characteristics of the 460 Females in the Study Population, Stratified by FMR1 Repeat Group
| FMR1 repeat group | NC | IM | Low PM | Mid PM | High PM | All |
|---|---|---|---|---|---|---|
| N | 97 | 77 | 63 | 152 | 71 | 460 |
| Mean age (SD)a | 33.6 (10.1) | 32.6 (10.8) | 39.6 (7.4) | 37.8 (8.0) | 36.0 (9.2) | 36.0 (9.4) |
| Self-reported racea,b | ||||||
| % Caucasian/White | 69.1 | 59.7 | 90.5 | 85.9 | 95.8 | 80.1 |
| % African-American/other | 30.9 | 40.3 | 9.5 | 14.1 | 4.2 | 19.9 |
| Household incomec | ||||||
| % <$10,000 | 3.1 | 9.2 | 1.6 | 2.7 | 2.9 | 3.8 |
| % $10,000–$25,000 | 11.3 | 11.8 | 3.3 | 5.4 | 8.8 | 8.0 |
| % $25,000–$50,000 | 24.7 | 29.0 | 24.6 | 23.0 | 17.7 | 23.8 |
| % $50,000–$75,000 | 29.9 | 21.1 | 16.4 | 22.3 | 25.0 | 23.3 |
| % $75,000–$100,000 | 13.4 | 10.5 | 23.0 | 25.0 | 25.0 | 19.8 |
| % >$100,000 | 17.5 | 18.4 | 31.2 | 21.6 | 20.6 | 21.3 |
| Education | ||||||
| % Some high school | 0.0 | 0.0 | 1.6 | 0.0 | 1.4 | 0.4 |
| % High school graduate or GED | 7.2 | 11.7 | 6.4 | 15.1 | 14.1 | 11.5 |
| % Trade or vocational school | 2.1 | 5.2 | 6.4 | 4.6 | 1.4 | 3.9 |
| % Some college | 39.2 | 46.8 | 27.0 | 27.6 | 28.2 | 33.3 |
| % College graduate | 34.0 | 29.9 | 34.9 | 36.2 | 39.4 | 35.0 |
| % Some graduate school | 17.5 | 6.5 | 23.8 | 16.5 | 15.5 | 15.9 |
| Method of ascertainmenta | ||||||
| % General population | 54.6 | 84.4 | 4.8 | 0.0 | 0.0 | 26.3 |
| % Families with history of FXS | 45.4 | 15.6 | 95.2 | 100.0 | 100.0 | 73.7 |
| Anxiety/depression medication usea | ||||||
| % No | 89.7 | 88.3 | 69.8 | 75.0 | 76.1 | 79.8 |
| % Yes | 10.3 | 11.7 | 30.2 | 25.0 | 23.9 | 20.2 |
| Has child with FXSa | ||||||
| % No | 100.0 | 100.0 | 55.6 | 38.2 | 33.8 | 63.3 |
| % Yes | 0.0 | 0.0 | 44.4 | 61.8 | 66.2 | 36.7 |
NC, non-carrier; IM, intermediate; PM, premutation; SD, standard deviation; GED, General Equivalency Diploma; FXS, fragile X syndrome.
Repeat groups differed for demographic variable at P < 0.05 significance level.
Three participants had missing data for race/ethnicity.
Ten participants had missing data for household income.
To account for the testing of multiple correlated SNPs [due to linkage disequilibrium (LD)] across multiple correlated phenotypes, we adjusted our significance level using the Cheverud–Nyholt approach [Cheverud, 2001; Nyholt, 2004]. Applying this method, we identified 5.6 effectively independent trait outcomes and 2.4 effectively independent SNPs. These analyses were conducted using a reduced set of independent (unrelated) subjects from our sample to ensure that the correlation among outcomes and SNPs was not due to relatedness. The product of these numbers was then used as the denominator in a Bonferroni correction, yielding a significance threshold of 0.0037.
In a follow-up analysis, we analyzed the potential clinical relevance of significant outcomes from the analyses above using threshold values that indicate the probable presence of a clinical disorder (e.g., an SPAI difference score of 80 or higher is indicative of probable social phobia) [Turner et al., 1996]. Logistic regression models were run using the dichotomous outcome for the presence/absence of a clinical disorder and adjusted for all covariates as the models above. We report odds ratios (ORs) and 95% confidence intervals (CIs). These models included a random effect to account for correlation among individuals from the same pedigree.
All models were run using PROC MIXED and PROC GENMOD in SAS version 9.2.
RESULTS
Neurobehavioral data and FMR1 and CRHR1 genotyping data from 460 women were included in these analyses. Demographic data stratified by FMR1 repeat group are shown in Table I. Groups differed in mean age (P < 0.0001), self-reported race (P < 0.0001), household income (P = 0.0213), ascertainment (P < 0.0001), the use of anxiety or depression medication at the time of testing (P = 0.0036), and having a child with FXS (P < 0.0001).
Before testing our primary hypothesis, we verified that there was no gametic disequilibrium between repeat length at FMR1 and genotypes at CRHR1 by confirming that CRHR1 genotype frequencies were independent of having a child with FXS (Table II), as well as independent of FMR1 repeat group status (rs7209436: P = 0.33; rs110402: P = 0.50; rs242924: P = 0.55; rs242940: P = 0.60; rs173365: P = 0.70; TAT: P = 0.44).
TABLE II.
Independence of CRHR1 Genotype Frequencies and Having a Child With FXS
| CRHR1 SNPs | Genotypes | Genotype frequencies
|
Chi-square test, P-value | |
|---|---|---|---|---|
| No FXS child (N = 291) | FXS child (N = 169) | |||
| rs7209436 | CC | 110 (38.2%) | 66 (39.3%) | 0.11, 0.95 |
| CT | 141 (49.0%) | 82 (48.8%) | ||
| TT | 37 (12.9%) | 30 (11.9%) | ||
| rs110402 | GG | 97 (33.6%) | 59 (35.1%) | 0.27, 0.87 |
| AG | 150 (51.9%) | 83 (49.4%) | ||
| AA | 42 (14.5%) | 26 (15.5%) | ||
| rs242924 | GG | 101 (35.1%) | 61 (36.5%) | 0.15, 0.93 |
| GT | 145 (50.4%) | 81 (48.5%) | ||
| TT | 42 (14.6%) | 25 (15.0%) | ||
| rs242940 | AA | 96 (33.6%) | 58 (34.7%) | 0.32, 0.85 |
| AG | 136 (47.6%) | 81 (48.5%) | ||
| GG | 54 (18.9%) | 28 (16.8%) | ||
| rs173365 | AA | 81 (28.0%) | 37 (22.2%) | 1.96, 0.38 |
| AG | 135 (46.7%) | 86 (51.5%) | ||
| GG | 73 (25.3%) | 44 (26.4%) | ||
| TAT genotype | 0 Copies | 110 (38.6%) | 68 (41.2%) | 0.30, 0.86 |
| 1 Copy | 141 (48.5%) | 78 (47.3%) | ||
| 2 Copies | 34 (11.9%) | 19 (11.5%) | ||
FXS, fragile X syndrome.
Initial analyses focused on linear models with only the main effect variables for CRHR1 SNP genotype and raising a child with FXS and also included FMR1 repeat group indicator variables and all covariates, such age and race, but no interaction terms. Overall, neither of the main effects, CRHR1 genotype or raising a child with FXS, were a significant predictor of any outcome measure.
We then tested our primary hypothesis that polymorphisms of CRHR1 interact with raising a child with FXS to impact depression and anxiety symptoms. These models included all main effect variables for CRHR1 SNP genotype and having a child with FXS as well as FMR1 repeat group indicator variables and all covariates. These models also included two interaction terms: CRHR1 × -having a child with FXS and FMR1 × having a child with FXS. Interactions between FMR1 repeat groups and having a child with FXS were not significant for any model (data not shown), thus these terms were removed from the final models.
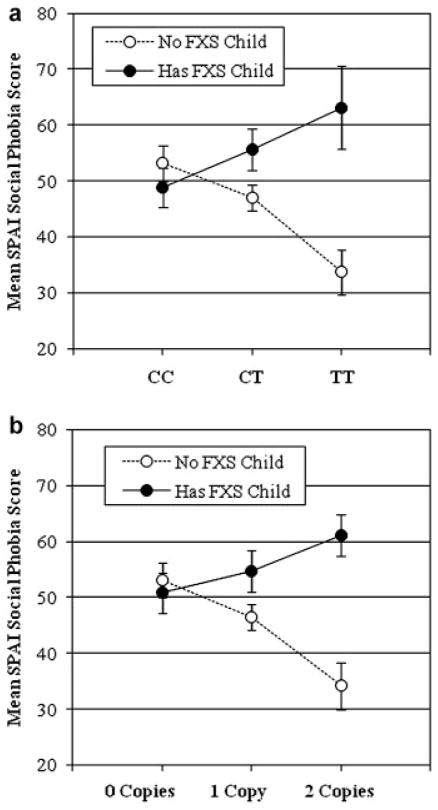
A significant interaction between having a child with FXS and CRHR1 genotype was detected for the SPAI social phobia scores for most SNPs (rs7209436, P = 0.0001; rs110402, P = 0.0129; rs242924, P = 0.0138; and rs242940, P = 0.0237) as well as the TAT haplotype (P = 0.0004; Table III). However, after adjustment for multiple testing, only rs7209436 and the TAT haplotype significantly moderated the effect of having a child with FXS on the SPAI social phobia scores (Table III). Among individuals who had a child with FXS, the less common T allele of rs7409436 associated in an allele- and dose-dependent manner with higher mean SPAI social phobia scores while among individuals who did not have a child with FXS, increasing copies of the T allele of rs7209436 were associated with increasingly lower mean SPAI social phobia scores (Fig. 1). In analyses of the three-SNP haplotype, similar patterns were noted: among participants who had a child with FXS, increasing numbers of copies of the TAT haplotype were associated with increasing SPAI social phobia scores (Fig. 1).
TABLE III.
Standardized β Coefficients From Linear Mixed Models Testing for Interaction Between Having a Child With FXS and CRHR1 Genotypes to Impact Severity of Symptoms Associated With Anxiety and Depression
| CRHR1 SNP | Measure
|
Standardized β
|
|||
|---|---|---|---|---|---|
| FXS child | CRHR1 | FXS child × CRHR1 | |||
| rs7209436 | CES | Depression | −0.05 | −0.02 | 0.07 |
| STAI | State anxiety | −0.05 | 0.08 | <0.01 | |
| Trait anxiety | −0.07 | <0.01 | 0.06 | ||
| PANAS | Negative affect | −0.09 | <0.03 | 0.09 | |
| Positive affect | 0.01 | 0.01 | −0.02 | ||
| SPAI | Social phobia | −0.14 | −0.18** | 0.30** | |
| Agoraphobia | 0.02 | 0.05 | −0.03 | ||
| rs110402 | CES | Depression | −0.03 | −0.03 | 0.04 |
| STAI | State anxiety | −0.02 | −0.05 | −0.04 | |
| Trait anxiety | −0.04 | <0.01 | 0.02 | ||
| PANAS | Negative affect | −0.08 | −0.01 | 0.09 | |
| Positive affect | 0.03 | 0.03 | −0.06 | ||
| SPAI | Social phobia | −0.09 | −0.15* | 0.25* | |
| Agoraphobia | <0.01 | 0.04 | <0.01 | ||
| rs242924 | CES | Depression | −0.03 | −0.03 | 0.04 |
| STAI | State anxiety | −0.03 | 0.05 | −0.03 | |
| Trait anxiety | −0.05 | −0.01 | 0.02 | ||
| PANAS | Negative affect | −0.09 | −0.02 | 0.09 | |
| Positive affect | 0.03 | 0.02 | −0.04 | ||
| SPAI | Social phobia | −0.08 | −0.13* | 0.20* | |
| Agoraphobia | −0.02 | 0.03 | 0.03 | ||
| rs242940 | CES | Depression | 0.01 | −0.03 | −0.02 |
| STAI | State anxiety | −0.04 | 0.01 | −0.02 | |
| Trait anxiety | −0.05 | −0.03 | 0.03 | ||
| PANAS | Negative affect | −0.07 | −0.05 | 0.06 | |
| Positive affect | 0.04 | 0.04 | −0.06 | ||
| SPAI | Social phobia | −0.07 | −0.18* | 0.18* | |
| Agoraphobia | 0.01 | 0.04 | −0.01 | ||
| rs173365 | CES | Depression | 0.02 | −0.08 | −0.03 |
| STAI | State anxiety | −0.03 | 0.02 | −0.03 | |
| Trait anxiety | −0.09 | −0.08 | 0.08 | ||
| PANAS | Negative affect | −0.07 | −0.07 | 0.06 | |
| Positive affect | 0.02 | 0.03 | −0.04 | ||
| SPAI | Social phobia | −0.07 | −0.17** | 0.17 | |
| Agoraphobia | 0.06 | 0.07 | −0.09 | ||
| TAT haplotype | CES | Depression | −0.04 | −0.02 | 0.06 |
| STAI | State anxiety | −0.03 | 0.10 | −0.02 | |
| Trait anxiety | −0.07 | <0.01 | 0.06 | ||
| PANAS | Negative affect | −0.08 | −0.02 | 0.07 | |
| Positive affect | 0.01 | 0.01 | −0.03 | ||
| SPAI | Social phobia | −0.13 | −0.18** | 0.27** | |
| Agoraphobia | 0.01 | 0.04 | −0.02 | ||
FXS, fragile X syndrome; CES, Center for Epidemiologic Studies Depression Scale; STAI, State-Trait Anxiety Inventory; PANAS, Positive and Negative Affect Schedule; SPAI, Social Phobia and Anxiety Inventory.
P < 0.05.
P < 0.0037.
FIG. 1.
Having a child with FXS moderates the effect of CRHR1 genotype to impact the SPAI social phobia score for (a) rs7209436 SNP genotype (P =0.0001) and (b) TAT genotype (P =0.0004).
Sensitivity analyses were performed to ensure that the results for the significant interactions between having a child with FXS and CRHR1 genotypes were robust. One set of models analyzed participants from the three premutation groups only, since the possibility of having a child with FXS is limited to these groups. A second set of models analyzed only Caucasian participants, in the event that CRHR1 genotype frequencies differed across races and could confound results. None of the results of the follow-up models changed the conclusions above (data not shown). Modeling the data using repeat length as a continuous variable yielded similar results as the models using repeat length group: raising a child with FXS significantly interacted with the genotypes at rs7209436 and the TAT haplotype to predict SPAI social phobia scores (P < 0.0001 and P = 0.0003, respectively). Lastly, analyses among only those women who were mothers yielded similar results, where having a child with FXS significantly interacted with rs7209436 and the TAT haplotype to predict SPAI social phobia scores across all repeat classes (P = 0.0014 and 0.0039, respectively) and among premutation carriers only (P = 0.0017 and P = 0.0017, respectively). In all of the follow-up models, having a child with FXS did not significantly interact with any other SNP genotype to predict other outcome scores (e.g., depression or anxiety, data not shown) and did not interact with FMR1 genotype to predict any outcome (data not shown).
The results of models presented above indicate that having a child with FXS moderates the impact of CRHR1 SNP genotypes on the severity of symptoms associated with social phobia, as measured by the SPAI social phobia score. However, it is not clear whether this moderation impacts the presence or absence of a clinical diagnosis of social phobia. In order to determine the potential clinical relevance of these findings, we created a new variable that coded for the presence or absence of probable social phobia, indicated by an SPAI social phobia score of 80 or greater [Turner et al., 1996], and performed logistic regression analyses including the interaction term between CRHR1 SNP genotype and having a child with FXS. A significant interaction between having a child with FXS and CRHR1 genotype was detected for all SNPs (rs7209436, P = 0.0002; rs110402, P = 0.0390; rs242924, P = 0.0238; and rs242940, P = 0.0382; rs173365, P = 0.0052) as well as the TAT genotype (P = 0.0016; Table IV). However, after adjustment for multiple testing, only rs7209436 and the TAT genotype significantly moderated the effect of having a child with FXS to predict the presence or absence of probable social phobia (Table IV).
TABLE IV.
Results From Logistic Regression Models Analyzing Interactions Between Having a Child With FXS and CRHR1 SNP Genotypes to Predict the Presence of Probable Social Phobia, as Measured by an SPAI Social Phobia Score of ≥80
| CRHR1 SNP | Genotype | No FXS child
|
Has FXS child
|
Interaction P-value | ||
|---|---|---|---|---|---|---|
| N | % With SP | N | % With SP | |||
| rs7209436 | CC | 110 | 21.8 | 66 | 15.2 | 0.0002 |
| CT | 140 | 11.4 | 82 | 24.4 | ||
| TT | 37 | 2.7 | 20 | 30.0 | ||
| rs110402 | GG | 97 | 19.6 | 59 | 18.6 | 0.0390 |
| AG | 150 | 12.7 | 83 | 22.9 | ||
| AA | 41 | 9.8 | 26 | 23.1 | ||
| rs242924 | GG | 101 | 18.8 | 61 | 16.4 | 0.0238 |
| GT | 145 | 12.4 | 81 | 23.5 | ||
| TT | 41 | 9.8 | 25 | 24.0 | ||
| rs242940 | AA | 96 | 19.8 | 58 | 17.2 | 0.0382 |
| AG | 136 | 12.5 | 81 | 24.7 | ||
| GG | 53 | 7.5 | 28 | 17.9 | ||
| rs173365 | AA | 80 | 26.3 | 37 | 18.9 | 0.0052 |
| AG | 135 | 11.1 | 86 | 20.9 | ||
| GG | 73 | 6.8 | 44 | 22.7 | ||
| TAT genotype | 0 Copies | 110 | 20.9 | 68 | 17.6 | 0.0016 |
| 1 Copy | 140 | 11.4 | 78 | 23.1 | ||
| 2 Copies | 34 | 2.9 | 19 | 26.3 | ||
FXS, fragile X syndrome.
DISCUSSION
It is clear that depression and anxiety are complex disorders, with multiple genetic and environmental factors influencing onset and severity. The HPA axis is responsible for the endogenous stress response [Romeo and McEwen, 2006]. Abundant evidence suggests that the HPA axis plays important roles in modulating the impact of stress or trauma on depressive and anxiety symptoms [Heim et al., 2008]. Recent studies suggest that genetic variation in the CRHR1 gene, the product of which mediates regulation of expression and release of ACTH from the anterior pituitary which in turn stimulates release of cortisol from the adrenal cortex, can interact with environmental stress or trauma to influence mood- and anxiety-related outcomes [Bradley et al., 2008; Polanczyk et al., 2009; Deyoung et al., 2011]. Bradley et al. [2008] and Polanczyk et al. [2009] were the first to show that a haplotype formed from three SNPs of intron 1 of CRHR1 (rs7209436, rs110402, and rs242924) significantly interacted with a history of childhood trauma to modify the risk for adult depression: an increasing number of copies of the less common haplotype, TAT, associated with less severe adult depressive symptoms in the individuals with histories of childhood trauma. Those observations suggested that the TAT haplotype exerted a protective effect in persons exposed to childhood trauma, a known risk factor for development of depression.
Our results indicate that genetic variance within CRHR1 moderates the stress of raising a child with FXS to impact the severity of symptoms associated with social phobia among women who carry an FMR1 premutation allele. We tested the hypothesis that polymorphisms of the CRHR1 gene, which associate with differences in HPA axis function, would interact with having a child with FXS, a surrogate for emotional stress/trauma, to predict the severity of symptoms associated with anxiety and depression. This hypothesis was based on prior research that indicated that genetic variation within CRHR1 can interact with environmental stress or trauma to influence mood- and anxiety-related outcomes [Bradley et al., 2008; Polanczyk et al., 2009; Deyoung et al., 2011].
The observed interaction was detected at a single SNP within intron 1 of CRHR1 (rs7209436) as well as at a haplotype of three SNPs spanning intron 1 (rs7209436, rs110402, and rs242924). Specifically, among women who had a child with FXS, the less common T allele at rs7209436 and the three-SNP TAT haplotype were associated in a dose-dependent manner with increasing social phobia symptom scores from the STAI. In contrast, among women who did not have a child with FXS, increasing copies of the T allele at rs7209436 and increasing copies of the TAT haplotype were associated with decreasing social phobia symptom scores. The results suggest that in the presence of a chronic life stressor, in this case having a child with FXS, the T allele of rs7209436 and the TAT haplotype are risk alleles for symptoms of social phobia in women who carry FMR1 premutations.
Bradley et al. [2008] had previously reported an interaction between CRHR1 polymorphisms and a history of childhood trauma to impact significantly adult depression. In contrast to the results of this study, they detected an apparent “protective” effect of the rs7209436 T allele and of the TAT haplotype. Further, DeYoung et al. [2011] also reported a significant interaction between CRHR1 polymorphisms and childhood maltreatment to predict childhood neuroticism. The TAT haplotype showed different patterns of interaction depending on the severity and type of maltreatment, where increasing copies of the TAT haplotype were associated with higher levels of neuroticism among maltreated children who had experienced 1 or 2 types of maltreatment and lower levels in those who had experienced 3 or 4 types. In addition, increasing copies of the TAT haplotype were associated with higher levels of neuroticism in those who had experienced physical abuse and lower levels in those who had experienced sexual abuse. This “flip-flop” association with apparent “reversal” of the risk allele could indicate a complex relationship between life stressors and neurobehavioral outcomes that depends on the developmental timing of the stressor (childhood vs. adulthood), how recently the stressor occurred (past vs. current), the chronicity of the stressor (e.g., raising a disabled child is a chronic source of current stress [Abbeduto et al., 2004; Lewis et al., 2006]), and the types and severity of the stressor experienced. Alternatively, this “flip-flop” association could be a statistical phenomenon common among association studies that arises due to population variation in interlocus interaction [Lin et al., 2007].
Interestingly, though we were able to show that women who carry a premutation and have a child with FXS are at risk for clinical social phobia (as indicated by STAI scores), and that CRHR1 modifies that risk, we did not detect a direct association between the FMR1 premutation allele and any neurobehavioral outcome. Published studies investigating neurobehavioral outcomes among FMR1 premutation carriers have produced mixed results [Hunter et al., 2009]. Several studies have not detected an increased neurobehavioral pathology associated with the premutation [Reiss et al., 1993; Sobesky et al., 1994; Riddle et al., 1998; Kogan et al., 2008], while others have reported increased incidences of depression [Johnston et al., 2001; Bailey et al., 2008a; Roberts et al., 2009] and anxiety [Hessl et al., 2005; Bailey et al., 2008a; Bourgeois et al., 2011]. Some studies have controlled for the potential stress of having a child with FXS by either comparing these mothers to other mothers of children with intellectual and developmental disabilities (IDD) or comparing them to premutation carriers who do not have children with FXS [Franke et al., 1998; Rodriguez-Revenga et al., 2008]. Franke et al. [1998] reported an increased incidence of anxiety, specifically social phobia, among premutation carriers who were mothers of children with FXS as compared to their siblings who also carried a premutation but did not have a child with FXS as well as to mothers of children with autism. In addition, premutation mothers had an increased incidence of major depressive episodes compared to their premutation siblings. More recently, Rodriguez-Revenga et al. [2008] reported that mothers with a premutation who have a child with FXS and mothers of children with non-FXS IDD did not differ on any measure of neurobehavior, while both groups differed from the control group (i.e., those without IDD) for several factors, including depression. While Roberts et al. [2009] reported that increasing behavioral problems among children with FXS predicted anxiety disorders among premutation carrier mothers. Altogether, these studies indicate that neurobehavioral phenotypes detected among premutation carriers could, at least in part, be due to the stress of raising a child with FXS.
CRHR1 encodes a receptor that plays a central role in the regulation of HPA axis function in the endogenous stress response. Recent studies have reported altered diurnal cortisol rhythms among parents of children with special needs [Seltzer et al., 2009] and ASDs [Seltzer et al., 2010], supporting the conclusion that raising a child with special needs alters HPA axis function. We did not detect a significant interaction between raising a child with FXS and FMR1 repeat status to predict anxiety or depression outcomes, either modeling FMR1 repeat length as a group variable or as a continuous variable. These results indicate that the moderation of the impact of raising a child with FXS on neurobehavioral outcomes did not depend on CGG repeat size and appears to be limited to the influence of CRHR1 and perhaps other genes. This is in contrast to a recent study by Seltzer et al. [2011], where the severity of life stress among mothers of children with FXS was moderated by the premutation size to predict anxiety and depression levels and diurnal cortisol levels. However, the two studies differed in the measurement of the stressor (having a child with FXS vs. negative life events in the previous year) and the modeling of HPA axis function (genotypes for CRHR1 vs. diurnal cortisol levels). Further, the current study tested the interaction between CRHR1 genotypes and stress to predict anxiety and depression self-report scores, while Seltzer et al. [2011] tested the interaction between repeat length and negative life events to predict cortisol levels and depression and anxiety as the outcomes. These differing methodological approaches could explain the differing conclusions.
A limitation of this study is that these results are based self-reported measures and the interpreted diagnostic categories are based on the terms provided by the instrument. In other words, the study participants were not administered a structured diagnostic evaluation that would be necessary to determine formal psychiatric diagnoses. Thus, it would be inappropriate to attempt to relate the gene × environment associations observed in this study to specific psychiatric diagnoses. Rather, we suspect that we are observing an interaction between genotypes at CRHR1 and the challenges inherent in raising a child with FXS, and a latent stress-related phenotype, that was most efficiently captured by the “social phobia” responses of the SPAI in this particular study. We did not observe similar interactions impacting “depression” and “anxiety” as defined by the self-report measures we used. Future studies with in-depth formal psychiatric assessments should be performed to determine what psychiatric diagnostic domains, if any, are associated with the interactions detected here.
An additional limitation to the analyses presented in this study is that we assessed only whether or not a woman was a mother of a child with FXS, a clearly simplified representation of maternal stress levels given the wide variation in behavior problems among children with FXS, particularly between those with and without an additional diagnosis of autism [Smith et al., 2012]. Future studies should capture the severity of stress related to raising a child with FXS including traits, such as the severity of behavior problems of their child(ren), the number of children with FXS in the family, the age of the child(ren) at the time of testing, or the gender of their child(ren). The severity of stress, rather than simply the presence or absence of this particular stressor, would likely be a better predictor of neurobehavioral outcomes and should be analyzed more closely in future studies.
Overall, the results of this study are intriguing. We have provided a potential predictor of resilience and vulnerability for increasing symptoms of social phobia in mothers of children with FXS. Why the gene × environment interaction detected in this study is specific to social phobia scores, and not to scores for depression or general anxiety, is unclear and warrants further investigation. However, there is little information on gene × environment interactions for social phobia symptoms, so the results of this study can potentially provide understanding on the etiology of social phobia outside the context of the FMR1 premutation [Gregory et al., 2008]. In addition, these results provide evidence for a vulnerability to neurobehavioral disorders not directly associated with the FMR1 premutation.
Acknowledgments
We would like to thank Weiya He, Maneesha Yadav-Shah, Ashima Amin, and Lareesa Woodard for their laboratory analyses. We would also like thank the study participants who made this work possible. This work was supported by the National Institutes of Health grants R01 HD29909 and P30 HD24064. The authors do not have any conflicting interest associated with the publication of this manuscript; however, we disclose that Dr. Binder has received grant support from PharmaNeuroBoost.
Grant sponsor: National Institutes of Health; Grant numbers: R01 HD29909, P30 HD24064; Grant sponsor: PharmaNeuroBoost.
References
- Abbeduto L, Seltzer MM, Shattuck P, Krauss MW, Orsmond G, Murphy MM. Psychological well-being and coping in mothers of youths with autism, Down syndrome, or fragile X syndrome. Am J Ment Retard. 2004;109(3):237–254. doi: 10.1352/0895-8017(2004)109<237:PWACIM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Allen EG, Sherman S, Abramowitz A, Leslie M, Novak G, Rusin M, Scott E, Letz R. Examination of the effect of the polymorphic CGG repeat in the FMR1 gene on cognitive performance. Behav Genet. 2005;35(4):435–445. doi: 10.1007/s10519-005-2792-4. [DOI] [PubMed] [Google Scholar]
- Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, Charen K, He W, Taylor KC, Sherman SL. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007;22(8):2142–2152. doi: 10.1093/humrep/dem148. [DOI] [PubMed] [Google Scholar]
- Ashley CT, Jr, Wilkinson KD, Reines D, Warren ST. FMR1 protein: Conserved RNP family domains and selective RNA binding. Science. 1993;262(5133):563–566. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: Findings from a National Parent Survey. Am J Med Genet Part A. 2008a;146A(16):2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Sideris J, Roberts J, Hatton D. Child and genetic variables associated with maternal adaptation to fragile X syndrome: A multidimensional analysis. Am J Med Genet Part A. 2008b;146A(6):720–729. doi: 10.1002/ajmg.a.32240. [DOI] [PubMed] [Google Scholar]
- Bourgeois JA, Seritan AL, Casillas EM, Hessl D, Schneider A, Yang Y, Kaur I, Cogswell JB, Nguyen DV, Hagerman RJ. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry. 2011;72(2):175–182. doi: 10.4088/JCP.09m05407blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WT, Houck GE, Jr, Jeziorowska A, Levinson FN, Ding X, Dobkin C, Zhong N, Henderson J, Brooks SS, Jenkins EC. Rapid fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test. JAMA. 1993;270(13):1569–1575. [PubMed] [Google Scholar]
- Cheverud JM. A simple correction for multiple comparisons in interval mapping genome scans. Heredity. 2001;87(Pt 1):52–58. doi: 10.1046/j.1365-2540.2001.00901.x. [DOI] [PubMed] [Google Scholar]
- Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, Bronsky HE, Yuhas J, Borodyanskaya M, Grigsby J, Doerflinger M, Hagerman PJ, Hagerman RJ. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet Part A. 2008;146A(8):1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyoung CG, Cicchetti D, Rogosch FA. Moderation of the association between childhood maltreatment and neuroticism by the corticotropin-releasing hormone receptor 1 gene. J Child Psychol Psychiatry. 2011;52(8):898–906. doi: 10.1111/j.1469-7610.2011.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich M, Bocker S, van den Boom D. Multiplexed discovery of sequence polymorphisms using base-specific cleavage and MALDI-TOF MS. Nucleic Acids Res. 2005;33(4):e38. doi: 10.1093/nar/gni038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke P, Leboyer M, Gansicke M, Weiffenbach O, Biancalana V, Cornillet-Lefebre P, Croquette MF, Froster U, Schwab SG, Poustka F, Hautzinger M, Maier W. Genotype–phenotype relationship in female carriers of the premutation and full mutation of FMR-1. Psychiatry Res. 1998;80(2):113–127. doi: 10.1016/s0165-1781(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Gabriel S, Ziaugra L, Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet. 2009;Chapter 2(Unit 2):12. doi: 10.1002/0471142905.hg0212s60. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Lau JY, Eley TC. Finding gene–environment interactions for phobias. Eur Arch Psychiatry Clin Neurosci. 2008;258(2):76–81. doi: 10.1007/s00406-007-0786-3. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57(1):127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- Hartley SL, Seltzer MM, Hong J, Greenburg JS, Smith L, Almeida D, Coe C, Abbeduto L. Cortisol response to behavior problems in FMR1 premutation mothers of adolescents and adults with fragile X syndrome: A diathesis-stress model. Int J Beh Dev. 2011;36(1):53–61. doi: 10.1177/0165025411406857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey MA, Gane LW, Barbato I, Rice C, Gould E, Hall DA, Grigsby J, Wegelin JA, Harris S, Lewin F, Weinberg D, Hagerman PJ, Hagerman RJ. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet Part B. 2005;139B(1):115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- Hunter JE, Allen EG, Abramowitz A, Rusin M, Leslie M, Novak G, Hamilton D, Shubeck L, Charen K, Sherman SL. Investigation of phenotypes associated with mood and anxiety among male and female fragile X premutation carriers. Behav Genet. 2008a;38(5):493–502. doi: 10.1007/s10519-008-9214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Allen EG, Abramowitz A, Rusin M, Leslie M, Novak G, Hamilton D, Shubeck L, Charen K, Sherman SL. No evidence for a difference in neuropsychological profile among carriers and non-carriers of the FMR1 premutation in adults under the age of 50. Am J Hum Genet. 2008b;83(6):692–702. doi: 10.1016/j.ajhg.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Abramowitz A, Rusin M, Sherman SL. Is there evidence for neuropsychological and neurobehavioral phenotypes among adults without FXTAS who carry the FMR1 premutation? A review of current literature. Genet Med. 2009;11(2):79–89. doi: 10.1097/GIM.0b013e31818de6ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Rohr JK, Sherman SL. Co-occurring diagnoses among FMR1 premutation allele carriers. Clin Genet. 2010;77(4):374–381. doi: 10.1111/j.1399-0004.2009.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, Zhang L, Jardini T, Gane LW, Harris SW, Herman K, Grigsby J, Greco CM, Berry-Kravis E, Tassone F, Hagerman PJ. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291(4):460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- Johnston C, Eliez S, Dyer-Friedman J, Hessl D, Glaser B, Blasey C, Taylor A, Reiss A. Neurobehavioral phenotype in carriers of the fragile X premutation. Am J Med Genet. 2001;103(4):314–319. [PubMed] [Google Scholar]
- Kogan CS, Turk J, Hagerman RJ, Cornish KM. Impact of the fragile X mental retardation 1 (FMR1) gene premutation on neuropsychiatric functioning in adult males without fragile X-associated tremor/ataxia syndrome: A controlled study. Am J Med Genet Part B. 2008;147B(6):859–872. doi: 10.1002/ajmg.b.30685. [DOI] [PubMed] [Google Scholar]
- Lewis P, Abbeduto L, Murphy M, Richmond E, Giles N, Bruno L, Schroeder S, Anderson J, Orsmond G. Psychological well-being of mothers of youth with fragile X syndrome: Syndrome specificity and within-syndrome variability. J Intellect Disabil Res. 2006;50(Pt 12):894–904. doi: 10.1111/j.1365-2788.2006.00907.x. [DOI] [PubMed] [Google Scholar]
- Lin PI, Vance JM, Pericak-Vance MA, Martin ER. No gene is an island: The flip-flop phenomenon. Am J Hum Genet. 2007;80(3):531–538. doi: 10.1086/512133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows KL, Pettay D, Newman J, Hersey J, Ashley AE, Sherman SL. Survey of the fragile X syndrome and the fragile X E syndrome in a special education needs population. Am J Med Genet. 1996;64(2):428–433. doi: 10.1002/(SICI)1096-8628(19960809)64:2<428::AID-AJMG39>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Nolin SL, Lewis FA, III, Ye LL, Houck GE, Jr, Glicksman AE, Limprasert P, Li SY, Zhong N, Ashley AE, Feingold E, Sherman SL, Brown WT. Familial transmission of the FMR1 CGG repeat. Am J Hum Genet. 1996;59(6):1252–1261. [PMC free article] [PubMed] [Google Scholar]
- Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74(4):765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: Replication and extension. Arch Gen Psychiatry. 2009;66(9):978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- Reiss AL, Freund L, Abrams MT, Boehm C, Kazazian H. Neurobehavioral effects of the fragile X premutation in adult women: A controlled study. Am J Hum Genet. 1993;52(5):884–894. [PMC free article] [PubMed] [Google Scholar]
- Riddle JE, Cheema A, Sobesky WE, Gardner SC, Taylor AK, Pennington BF, Hagerman RJ. Phenotypic involvement in females with the FMR1 gene mutation. Am J Ment Retard. 1998;102(6):590–601. doi: 10.1352/0895-8017(1998)102<0590:piifwt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Bailey DB, Jr, Mankowski J, Ford A, Sideris J, Weisenfeld LA, Heath TM, Golden RN. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet Part B. 2009;150B(1):130–139. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Revenga L, Madrigal I, Alegret M, Santos M, Mila M. Evidence of depressive symptoms in fragile-X syndrome premutated females. Psychiatr Genet. 2008;18(4):153–155. doi: 10.1097/YPG.0b013e3282f97e0b. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, Xuncla M, Badenas C, Kulisevsky J, Gomez B, Mila M. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet. 2009;17(10):1359–1362. doi: 10.1038/ejhg.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Almeida DM, Greenberg JS, Savla J, Stawski RS, Hong J, Taylor JL. Psychosocial and biological markers of daily lives of midlife parents of children with disabilities. J Health Soc Behav. 2009;50(1):1–15. doi: 10.1177/002214650905000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Greenberg JS, Hong J, Smith LE, Almeida DM, Coe C, Stawski RS. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord. 2010;40(4):457–469. doi: 10.1007/s10803-009-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Barker ET, Greenberg JS, Hong J, Coe C, Almeida D. Differential sensitivity to life stress in FMR1 premutation carrier mothers of children with fragile X syndrome. Health Psychol. 2011 doi: 10.1037/a0026528. [Epub 12 Dec] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: Diagnostic and carrier testing. Genet Med. 2005;7(8):584–587. doi: 10.1097/01.GIM.0000182468.22666.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Barker ET, Seltzer MM, Abbeduto L, Greenberg JS. Behavioral phenotype of fragile X syndrome in adolescence and adulthood. Am J Intellect Dev Disabil. 2012;117(1):1–17. doi: 10.1352/1944-7558-117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow K, Doud LK, Hagerman R, Pergolizzi RG, Erster SH, Thibodeau SN. Analysis of a CGG sequence at the FMR-1 locus in fragile X families and in the general population. Am J Hum Genet. 1993;53(6):1217–1228. [PMC free article] [PubMed] [Google Scholar]
- Sobesky WE, Pennington BF, Porter D, Hull CE, Hagerman RJ. Emotional and neurocognitive deficits in fragile X. Am J Med Genet. 1994;51(4):378–385. doi: 10.1002/ajmg.1320510416. [DOI] [PubMed] [Google Scholar]
- Spielberger C. Manual for the state-trait anxiety inventory for adults (Form Y) Redwood City, CA: Mind Garden; 1983. [Google Scholar]
- Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73(5):1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68(4):978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20(2):402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxe D, Warren ST. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1(6):397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- Turner SM, Beidel DC, Dancu CV. Social phobia and anxiety inventory: Manual. Toronto, ON: Multi-Health Systems, Inc; 1996. [Google Scholar]
- Watson D, Clark LA. Manual for the positive and negative affect schedule (Expanded form) Iowa City, IA: University of Iowa; 1994. [Google Scholar]