Abstract
Amyloid-β peptide (Aβ) is considered a key protein in the pathogenesis of Alzheimer’s disease (AD) because of its neurotoxicity and capacity to form characteristic insoluble deposits known as senile plaques. Aβ derives from amyloid-β protein precursor (AβPP), whose proteolytic processing generates several fragments including Aβ peptides of various lengths. The normal function of AβPP and its fragments remains poorly understood. While some fragments has been suggested to have a function in normal physiological cellular processes, Aβ has been widely considered as a “garbage” fragment that becomes toxic when it accumulates in the brain, resulting in impaired synaptic function and memory. Aβ is produced and released physiologically in the healthy brain during neuronal activity. In the last 10 years, we have been investigating whether Aβ plays a physiological role in the brain. We first demonstrated that picomolar concentrations of a human Aβ42 preparation enhanced synaptic plasticity and memory in mice. Next, we investigated the role of endogenous Aβ in healthy murine brains and found that treatment with a specific antirodent Aβ antibody and an siRNA against murine AβPP impaired synaptic plasticity and memory. The concurrent addition of human Aβ42 rescued these deficits, suggesting that in the healthy brain, physiological Aβ concentrations are necessary for normal synaptic plasticity and memory to occur. Furthermore, the effect of both exogenous and endogenous Aβ was seen to be mediated by modulation of neurotransmitter release and α7-nicotinic receptors. These findings need to be taken into consideration when designing novel therapeutic strategies for AD.
Keywords: Amyloid-β peptide, hippocampus, memory, nicotinic receptor, synaptic plasticity
GREAT IS THE POWER OF MEMORY
Great is the power of memory, a fearful thing, O my God, a deep and boundless manifoldness; and this thing is the mind, and this am I myself. […] So great is the force of memory, so great the force of life, even in the mortal life of man.
from “The Confessions of St. Augustine”
Memory has a central role in life: our experiences contribute to making us who we are and give us an identity, a history, a culture. What would we be without our past, without a story to tell, without people to remember? Our existence would be like a leaky bucket that, though filled with new memories every day, lets them flow away, erasing the past and threatening the future. It is this intriguing, perfectly designed and harmonically constructed mechanism that we have set out to explore: to clarify how memory works helps avoid the dreadful notion of forgetfulness. A greater understanding of the physiological basis of memory formation is therefore required if we are to gain deeper insights into the impairment of cognitive functions related to neurodegenerative disorders such as Alzheimer’s disease (AD).
THE AMYLOID HYPOTHESIS OF ALZHEIMER’S DISEASE
Amyloid-β peptide (Aβ), a protein found in large amounts in AD brains, has been the focus of AD research for the last 30 years. We owe to Glenner and Wong in 1984 the “initial report of the purification and characterization of a novel cerebrovascular amyloid protein” associated with AD [1]. Subsequent discoveries led to an explosion of studies on the toxic effects of Aβ as the main pathogenic factor in AD. Milestones in Aβ-AD research have been the demonstration that: i) the characteristic senile plaques in AD brains consist of Aβ aggregates [2]; ii) amyloid-β protein precursor (AβPP) is located on chromosome 21 (21q21.2-3) [3, 4], the same chromosome is involved in Down syndrome, which is characterized by Aβ deposition and AD-like neurodegeneration [5]; and iii) AβPP genetic mutations are involved in familial AD [6–10].
AβPP and its processing have been intensively investigated. AβPP is a type-1 transmembrane glycoprotein expressed in several cells (e.g., neurons, glia, endothelial cells, fibroblasts) that undergoes a complex cleavage process by secretases. AβPP is initially cleaved into α- and β-fragments, generating two soluble extracellular domains (sAβPPα and sAβPPβ) that differ only by a 17AA at the COOH terminus. The remaining AβPP portion, the carboxy-terminal fragment (CTF), contains 83AA (C83) after cleavage by α-secretase, or 99AA (C99) after cleavage by β-secretase. Then, γ-secretase generates a p3 fragment and a 57-59AA CTF from C83 and generally a 40 to 42 AA fragment called Aβ40, or 42 together with AβPP intracellular domain (AICD) fragment from C99. Aβ generation thus requires the action of β- and γ-secretase on AβPP. Based on the knowledge of AβPP processing, a number of therapeutic strategies aimed at reducing Aβ production in the AD brain have been developed. At the same time, several genetically-modified animals have been generated carrying AβPP or secretase mutations [11]. In particular, amyloid-depositing mice overexpressing human AβPP or proteins belonging to the γ-secretase complex, known as presenilins (PS1 and PS2), have been widely used to study AD features. For example, AβPP/PS1 mice [12] show impaired long-term potentiation (LTP)—a form of synaptic plasticity underlying memory [13]—as early as 3 months of age and a decline of reference memory at approximately 6 months of age, in parallel to increased Aβ production and deposition [14]. Synaptic plasticity and memory are also impaired after administration of high Aβ concentrations [15–29]. A large body of data suggests that, at least in the early stages of AD, synaptic disorders underlying memory impairment could be due to raised Aβ levels [30, 31].
Aβ accumulation would lead to oligomerization followed by peptide deposition in senile plaques, resulting in irreversible structural damage. Based on these findings, several therapeutic approaches to AD have been developed using strategies such as specific anti-Aβ antibodies, drugs aimed to shift AβPP processing, block Aβ accumulation, and/or act on Aβ downstream pathways. However, none of the approaches aimed at reducing amyloid load has been successful so far because, even when these drugs effectively clear the brain from Aβ deposits, they do not improve cognition and have several side effects [32–37]. Indeed, whereas a large number of findings support the amyloid hypothesis, there are important aspects that need to be clarified. First of all, a direct correlation between amyloid deposits and dementia severity has not been demonstrated, since some patients without amyloid deposition show severe memory deficits while other patients with cortical Aβ deposits have no dementia symptoms. Second, it is very difficult to study the pathophysiological role of each Aβ species and their aggregation status (e.g., Aβ42/Aβ40 and monomers/oligomers/fibrils), because the very composition of Aβ solutions and, their molarities used in in vitro and in vivo conditions, are hard to establish precisely. This is due to the fact that Aβ can easily change conformation after preparation, not to mention that it sticks to the tubes, altering the final concentration of the solution. Finally, it should be stressed that the results found in experimental models using a single Aβ species are not easily transposable in vivo, mainly because the brain normally produces a variety of Aβ peptides and we do not clearly know how and why.
THE STRANGE CASE OF Aβ PEPTIDE: DR. JEKYLL OR MR. HYDE?
As reported in numerous manuscripts, high Aβ levels are involved in AD synaptic dysfunction and memory loss. However, a number of issues remain to be solved before clinical trials aiming to decrease the Aβ load can be undertaken, especially if they are directed at disease “prevention” in healthy subjects. Low Aβ levels are found in the brain throughout life, and the possibility of a physiological role for it is increasingly being investigated by the neuroscience community.
Whereas 20 years ago studies of the physiological function of Aβ peptides were quite limited, the interest has progressively increased in the following two decades. In 1990, Yankner and co-workers emphasized the dual role of Aβ, demonstrating that it could exert a neurotrophic action in differentiating neurons, whereas high concentrations caused neuronal degeneration in mature neurons [38]. Other studies highlighted its neuroprotective role, suggesting that Aβ promotes neuronal growth and survival [39, 40], also protecting against excitotoxic death by activating the phosphatidylinositol-3-kinase pathway [41, 42]. Aβ was shown to serve a double prooxidant/antioxidant role [43–46] and to bind and remove harmful substances by blocking them in plaques [47, 48]. Aβ has also been implicated in neurogenesis and has been suggested to increase the total number of neurons in vitro in a dose-dependent manner [49]. Finally, interesting findings suggest that Aβ is a molecule of innate immunity system because of its antimicrobial activity against common microorganisms [50] and the vulnerability to infections founded in mice lacking β-secretases and in AD patients treated with Aβ-lowering drugs [51, 36]. Aβ is normally found in the brain and in blood. In rodents, normal brain concentrations have been estimated to be in the picomolar range [52, 53]. In humans, the concentrations of Aβ40 and Aβ42 in cerebrospinal fluid (CSF) are around 1,500 pM and 200 pM, respectively; in plasma they are 60 pM and 20 pM, respectively [54]. CSF and plasma concentrations have been used as markers to determine AD prognosis and treatment. However, research outcomes are contradictory, especially when human and animal findings are compared. Notably, Aβ concentrations are higher in the young and decline with age [55]. Moreover, increased CSF levels have been seen in patients with mild cognitive impairment who progressed to AD [56], whereas low levels have been found in AD patients [57, 54]. Aβ concentrations in brain interstitial fluid (ISF) thus seem to correlate with neurological status, and it has been demonstrated that concentrations increase when the neurological status improves, and that they decrease when the cognitive status declines [58]. Aβ levels in brain ISF have been seen to be dynamically influenced by synaptic activity [59], and synaptic transmission has been found to induce more AβPP endocytosis and a consequent increase in Aβ release [60]. In a paper using Sindbis virus to overexpress AβPP, it was shown that neuronal activity stimulates Aβ secretion in hippocampal slice neurons and, in turn, Aβ depresses excitatory synaptic transmission in the same neurons [61]. The endogenously released Aβ seems also to exert a fundamental role in the regulation of neurotransmitter release by modulating vesicle cycling. Indeed, the acute endogenously-released Aβ induced an increase in the number of synapses and in neurotransmitter release whereas a chronic persistence of Aβ, due to a inhibition of its clearance, induced the opposite effect [62]. Other studies suggest that Aβ may stimulate or inhibit the pre-synaptic release of excitatory neurotransmitters such as aspartate and glutamate depending upon the dose [63]. Taken together, these studies suggest that Aβ and neuronal function are closely related. Dependent upon the concentration of Aβ, the peptide might have a positive regulation upon excitatory synaptic transmission from low physiologic concentrations, or a negative regulation from high pathologic concentrations.
Some researchers have explored the possible physiological effect of Aβ by blocking its production via inhibition of secretases or AβPP. Inhibition of β-or γ-secretase activity induced neuronal death that was rescued by preincubation with picomolar concentrations of Aβ [64]. Loss of presenilin function determined LTP and memory deficits [65] and, interestingly, changes in hippocampal synaptic plasticity and cognition in β-secretase-null mice [66] have been prevented by co-expression of AβPP and PS1 transgenes [67]. Interestingly, both the overexpression and the deletion of the β-AβPP cleavage enzyme 1 (BACE1) determined behavioral changes [68]. Even AβPP-deficient mice present impaired LTP and hippocampal memory and marked cortical and hippocampal gliosis [69–74]. However, the complex phenotype of AβPP knock-out (KO) mice (characterized by low body weight, agenesis of the corpus callosum, hypersensitivity to seizures, defects in copper and lipid homeostasis, and impaired grip strength, locomotor and exploratory activity, and cognition) makes them difficult to study, especially where behavioral aspects are concerned. Moreover, use of AβPP-KO animals does not exclude the possibility that other AβPP fragments or AβPP itself other than Aβ might be biologically important. For instance, sAβPP fragments have neurotrophic properties and are required for synaptic plasticity and memory [75–83], and intracellular CTF may regulate gene transcription, calcium signaling, synaptic plasticity, and memory [84–88].
PHYSIOLOGICAL ROLE OF Aβ IN SYNAPTIC PLASTICITY AND MEMORY: OUR FINDINGS
Because of the problems linked to the complexity of the AβPP processing, we decided to use a different approach starting from the use of exogenous application of different concentrations of Aβ42 preparations containing both monomers and oligomers [53]. In particular, we found that picomolar concentrations of Aβ42 enhanced LTP and hippocampal-dependent memory as tested by the Morris water maze and by fear conditioning. A dose/response curve for the effect of Aβ on LTP showed that perfusion with 200 nM Aβ42 for 20 min impaired LTP at the synapses between Schaeffer collateral fibers and CA1 neurons, whereas lower concentrations enhanced it, with a maximal effect around 200 pM. This effect was not found with scrambled Aβ42, or when the peptide was administered after tetanization. We next investigated the effect of low doses of Aβ42 on memory by injecting 200 pM Aβ42, 200 pM scrambled Aβ42, or vehicle into the hippocampus, and found that low Aβ concentrations improved both reference and contextual memory. Interestingly, a dose-response curve for memory showed a similar biphasic effect of Aβ42, with low doses stimulating and high doses inhibiting reference memory [29]. Our next goal was to inquire into the mechanism by which Aβ improved LTP and memory. We first studied the possible role of NMDA and AMPA receptors, given their involvement in LTP [89]. However, low doses of Aβ did not change current-voltage (I/V) relationships for NMDA and AMPA receptor currents, nor did they alter the amplitude of AMPA receptor-mediated excitatory postsynaptic potentials (EPSCs) or their amplitude distribution. In our experimental conditions, NMDA and AMPA receptors were therefore not involved in Aβ-induced improvement of synaptic function. We also assessed whether Aβ might affect spontaneous neurotransmitter release, but average miniature EPSC frequency and amplitude were not affected by treatment with 200 pM Aβ. Given that mechanisms regulating basal neurotransmission were not affected, we turned our attention toward mechanisms that are involved in synaptic plasticity. In particular, we investigated a presynaptic phenomenon, the transmitter release occurring during the tetanus. We recorded post-tetanic potentiation (PTP), a form of short-term plasticity that reflects the increase in glutamate release from presynaptic terminals due to brief periods of high-frequency stimulation [90]. PTP was increased by perfusion with low Aβ concentrations, suggesting that its favorable effect on synaptic plasticity is exerted through enhancement of transmitter release during the tetanus.
Our next question was: how do picomolar levels of Aβ42 enhance PTP? Aβ might have several targets. We chose to focus onto acetylcholine receptors (AChRs). Indeed, both nicotinic (nAChRs) and muscarinic receptors (mAChRs) play a fundamental role in learning and memory in physiological and pathological conditions such as AD [91–93]. Because of their impaired cholinergic activity pharmacological strategies to improve cholinergic transmission (i.e., cholinesterase inhibitors) have been used in AD patients [94, 95]. Moreover, nAChRs are involved in multiple brain functions including learning and memory. In particular, we concentrated on the central α7-nAChRs, which boost synaptic plasticity and memory [96–98] and enhance transmitter release in several brain structures including hippocampus [99, 100], spinal cord dorsal horn [101], and amygdala [102]. We reasoned that targeting the α7-nAChR subtype might reduce AD symptoms [for a review, see 103], and an association between a genetic variant of the α7-nAChR subunit and AD had recently been documented [104]. Moreover, Aβ has a picomolar affinity for α7-nAChRs [105], may regulate nAChR function by binding with membrane lipids [106] such as lipid rafts [107], or may activate α7-nAchRs at presynaptic nerve endings of synaptosomes [108]. Intriguingly, Aβ might act either as an α7-nAChR agonist [109] or an α7-nAChRs inhibitor [110], with low concentrations activating and high concentrations inhibiting α7-nAChRs [111]. We tried to establish whether α7-nAchRs were involved in Aβ-induced improvement of synaptic plasticity. To do so, we studied the effect of Aβ after pharmacological or genetic blockage of nAChRs. First, blocking them with mecamylamine (MCL) or with the selective α7-nAchR blocker α-bungarotoxin resulted in inhibition of the Aβ-induced increase of PTP. Importantly, MCL or α-bungarotoxin alone did not affect PTP. Finally, perfusion of hippocampal slices with picomolar concentrations of Aβ did not enhance LTP or memory in α7-nAchR-KO mice, providing genetic evidence for the involvement of α7-nAchRs in the enhancing effect of Aβ. Taken all together, these results support the hypothesis that the enhancement of synaptic plasticity and memory by picomolar concentrations of Aβ42 involves neurotransmitter release and α7-nAChRs.
Another major question that was tackled in a following work [112] was: does endogenous Aβ have a function throughout life in normal healthy individuals? To address this question we blocked endogenously produced Aβ with a monoclonal antibody, JRF/rAb2, which recognizes a rodent-specific epitope within the first 15 AA of rodent Aβ40 and Aβ42. Depletion of endogenously produced Aβ caused a reduction of synaptic plasticity (LTP and PTP) and both reference and contextual memory. Interestingly, application of the antibody immediately after the tetanus or training had no effect, suggesting that Aβ is involved in the induction phase of synaptic plasticity and memory, but not in maintenance or consolidation processes. Because the antibody might act on a target other than Aβ (e.g., on other AβPP fragments, or AβPP itself, or other unknown proteins), we next performed rescue experiments with human Aβ42, which is not recognized by the antibody. Animals treated with JRF/rAb2 and low doses of Aβ exhibited normal synaptic plasticity and memory, confirming that the antibody acted through Aβ, and that Aβ is required for synaptic plasticity and memory. Moreover, a higher Aβ concentration (300 pM) induced a further increase in synaptic plasticity and memory that resembled the enhancement obtained by 200 pM Aβ alone. These findings were confirmed by an independent approach in which we blocked endogenous Aβ by knocking down AβPP expression in mice using an siRNA specific for murine AβPP (AβPP-siRNA). The LTP and memory impairment by intrahippocampal injections of AβPP-siRNA was rescued by picomolar doses of Aβ42. Moreover, neither the antibody nor AβPP-siRNA affected LTP in AβPP-null mice. Consistent with these studies, it was found that low picomolar doses of Aβ42 enhanced memory consolidation in tests of inhibitory avoidance in rats [113]. Interestingly, in the same work pre-incubation of human Aβ42 with an antibody that recognizes the AA sequence 17–24 of human and rodent Aβ which is also present in AβPP as well as in other fragments of its processing, blocked the impairment of memory by the antibody alone [113]. Moreover, in another study, low doses of Aβ enhanced LTP and memory retention, and acetylecholine production in the hippocampus in vivo, and vice versa blocking Aβ with an antibody or DFFVG (which blocks Aβ binding) or decreasing Aβ expression with antisense directed at AβPP reduced LTP and memory [114]. Taken all together these data suggest that Aβ is required for hippocampal synaptic plasticity and memory. What is the minimum dose that is necessary for LTP and PTP induction? To address this question, we injected AβPP-siRNA into mouse hippocampus and after 24 h performed electrophysiological recordings by treating slices with different concentrations of vehicle or synthetic human Aβ42. Given that complete rescue of potentiation was observed with 300 pM Aβ, and that the levels of endogenous Aβ after siRNA treatment were about 80 pM, we estimated that the Aβ42 threshold needed for normal synaptic plasticity is likely to be around 380 pM.
Our finding that Aβ is required for memory induction led us to explore release of Aβ during memory formation. We measured hippocampal Aβ42 in mice trained for contextual fear learning and then sacrificed at different intervals after the electric shock. We found that mice sacrificed at 1 min showed a significant increase in hippocampal Aβ42, lending support to the hypothesis that hippocampal Aβ42 production is enhanced during memory induction.
Another important finding of our work was that a monomer-enriched preparation was unable to rescue LTP in slices that were concomitantly treated with the JRF/rAb2 antibody, suggesting that the “positive” effect of the mixed preparation containing both monomers and oligomers is exerted by oligomeric forms of Aβ.
Next, we studied the involvement of α7-nAChRs in the effect of endogenous Aβ. JRF/rAb2 did not affect PTP or LTP in α7-nAChR-KO mice compared to wild type littermates, confirming that the effect of endogenous Aβ is mediated by α7-nAChRs.
To conclude, our research work demonstrates that picomolar concentrations of Aβ enhance synaptic plasticity and memory, that endogenous Aβ has a critical role in physiological regulation of synaptic plasticity and memory, and that this role is exerted via α7-nAChRs (Fig. 1). Aβ can thus be considered as a Dr. Jekyll/Mr. Hyde molecule exhibiting opposite effects at high or low concentrations. These intricate aspects should be taken into consideration when designing therapeutic strategies for AD, especially where Aβ-lowering therapies are concerned. These findings have a broad scope of application, since they span across different fields, including neurodegenerative disorders, synaptic plasticity, memory, and regulation of neurotransmission by nicotinic receptors. Our future work and that of other scientists will hopefully elucidate the questions that are still unanswered.
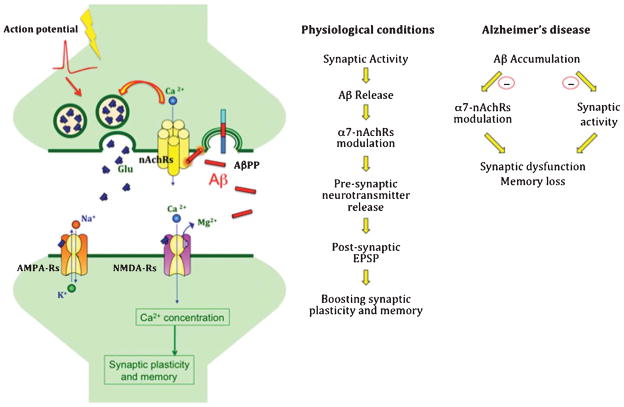
Fig. 1.
Amyloid-β (Aβ) in physiology and pathology. A) Schematic representation of a theoretical model indicating that during neuronal activity the release of Aβ acts on pre-synaptic α7-nAchRs, boosting synaptic plasticity and memory. B) Schematic representation of the role of Aβ in physiology and pathology. In physiologic conditions, synaptic activity triggers Aβ release which, in turn, positively modulates pre-synaptic α7-nAchRs leading to Ca2+ entrance into the presynaptic terminal and enhances releases of neurotransmitter boosting synaptic plasticity and memory. In pathologic conditions, Aβ accumulation has a negative feedback onto synaptic activity and reduces α7-nAchR function, leading to synaptic dysfunction and memory loss. (AMPA-Rs, AMPA receptors; NMDA-Rs, NMDA receptors; Glu, glutamate; α7-nAchRs, alpha-7 nicotinic acetylcholine receptors).
OUR SCIENTIFIC JOURNEY: OPENING AND ENDING CREDITS
Daniela Puzzo, from the University of Catania, joined Ottavio Arancio at Nathan Kline Institute in Orangeburg and then at Columbia University. At the time, Ottavio’s laboratory was exploring the toxic effect of Aβ on synaptic plasticity and memory; Daniela began her LTP experiments on hippocampal slices using high Aβ concentrations. Surprisingly, slice perfusion with Aβ induced an improvement in LTP, not the expected impairment. An accurate calculation showed that she was actually using 200 pM, not 200 nM Aβ. What could have been an annoying waste of time turned out to be a serendipitous discovery. Our arduous journey into the physiological role of Aβ started there. Since then several colleagues have helped us delve deeper into this fascinating topic and we would like to thank them all. First, Agostino Palmeri, Professor of Physiology and PI at the Department of Bio-medical Sciences, gave us the intellectual and material support to perform part of these studies at the University of Catania. Lucia Privitera helped with the electrophysiological and behavioral experiments and was in charge of colony maintenance and genotyping; Mauro Fa helped with electrophysiology and siRNA preparation but especially contributed with fruitful scientific discussions; Agnieszka Staniszewski performed the behavioral studies; Elena Leznik performed patch clamp studies; Gakuji Hashimoto and Fahad Aziz carried out ELISA assays; Mikako Sakurai studied siRNA in cell cultures; Elena M. Ribe and Carol Troy helped with siRNA-PEN1 conjugation; and Marc Mercken and Sonia Jung provided and studied the anti-Aβ JRF/rAb2 antibody. We are also grateful to Cristina Alberini, Francesca Bartolini, Rusiko Bourtchouladze, Moses V. Chao, Gilbert Di Paolo, Ana Garcia-Osta, Paul M. Mathews, Ipe Ninan, Filippo Palermo, Marina Picciotto, Lorna Role, and David Talmage for helpful comments and discussions; Paul Mathews for suggestions with the use of antirodent antibody; and Julio Pozueta, Luciano Pellizzoni, and Luciano Saieva for suggestions and siRNA preparation.
Acknowledgments
Our work has been supported by the National Institutes of Health (NS049442 and AG034248 to O.A.) and by the Alzheimer’s Association (NIRG-07-59597 and IIRG-09-134220 to D.P.).
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1375).
References
- 1.Glenner GG, Wong CW. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 2.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Müller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1989;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 4.Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St George-Hyslop P, Van Keuren ML, Patterson D, Pagan S, Kurnit DM, Neve RL. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987;235:880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- 5.Olson MI, Shaw CM. Presenile dementia and Alzheimer’s disease in mongolism. Brain. 1969;92:147–156. doi: 10.1093/brain/92.1.147. [DOI] [PubMed] [Google Scholar]
- 6.Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Bots GT, Luyendijk W, Frangione B. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 7.Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, et al. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991;353:844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- 8.Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 9.Levy E, Prelli F, Frangione B. Studies on the first described Alzheimer’s disease amyloid beta mutant, the Dutch variant. J Alzheimers Dis. 2006;9:329–339. doi: 10.3233/jad-2006-9s337. [DOI] [PubMed] [Google Scholar]
- 10.Di Fede G, Catania M, Morbin M, Rossi G, Suardi S, Mazzoleni G, Merlin M, Giovagnoli AR, Prioni S, Erbetta A, Falcone C, Gobbi M, Colombo L, Bastone A, Beeg M, Manzoni C, Francescucci B, Spagnoli A, Cantù L, Del Favero E, Levy E, Salmona M, Tagliavini F. A recessive mutation in the APP gene with dominant-negative effect on amyloidogenesis. Science. 2009;323:1473–1477. doi: 10.1126/science.1168979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spires TL, Hyman BT. Transgenic models of Alzheimer’s disease: Learning from animals. Neuro Rx. 2005;2:423–437. doi: 10.1602/neurorx.2.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 13.Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 14.Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol. 2004;55:801–814. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- 15.Cullen WK, Suh YH, Anwyl R, Rowan MJ. Block of LTP in rat hippocampus in vivo by beta-amyloid precursor protein fragments. Neuroreport. 1997;8:3213–3217. doi: 10.1097/00001756-199710200-00006. [DOI] [PubMed] [Google Scholar]
- 16.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh A, Akaike T, Sokabe M, Nitta A, Iida R, Olariu A, Yamada K, Nabeshima T. Impairments of long-term potentiation in hippocampal slices of beta-amyloid-infused rats. Eur J Pharmacol. 1999;382:167–175. doi: 10.1016/s0014-2999(99)00601-9. [DOI] [PubMed] [Google Scholar]
- 18.Chen QS, Kagan BL, Hirakura Y, Xie CW. Impairment of hippocampal long-term potentiation by Alzheimer amyloid beta-peptides. J Neurosci Res. 2000;60:65–72. doi: 10.1002/(SICI)1097-4547(20000401)60:1<65::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 19.Freir DB, Holscher C, Herron CE. Blockade of long-term potentiation by beta-amyloid peptides in the CA1 region of the rat hippocampus in vivo. J Neurophysiol. 2001;85:708–713. doi: 10.1152/jn.2001.85.2.708. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Anwyl R, Suh YH, Djamgoz MB, Rowan MJ. Use-dependent effects of amyloidogenic fragments of (beta)-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. J Neurosci. 2001;21:1327–1333. doi: 10.1523/JNEUROSCI.21-04-01327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malm T, Ort M, Tähtivaara L, Jukarainen N, Goldsteins G, Puoliväli J, Nurmi A, Pussinen R, Ahtoniemi T, Miettinen TK, Kanninen K, Leskinen S, Vartiainen N, Yrjänheikki J, Laatikainen R, Harris-White ME, Koistinaho M, Frautschy SA, Bures J, Koistinaho J. Beta-Amyloid infusion results in delayed and age-dependent learning deficits without role of inflammation or beta-amyloid deposits. Proc Natl Acad Sci U S A. 2006;103:8852–8857. doi: 10.1073/pnas.0602896103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephan A, Laroche S, Davis S. Generation of aggregated beta-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J Neurosci. 2001;21:5703–5714. doi: 10.1523/JNEUROSCI.21-15-05703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid-beta peptide inhibition of the PKA/CREB pathway and long-term potentiation: Reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 25.Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-beta oligomers: Their production, toxicity and therapeutic inhibition. Biochem Soc Trans. 2002;30:552–557. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- 26.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 27.Puzzo D, Vitolo O, Trinchese F, Jacob JP, Palmeri A, Arancio O. Amyloid-beta peptide inhibits activation of the nitric oxide/cGMP/cAMP-responsive element-binding protein pathway during hippocampal synaptic plasticity. J Neurosci. 2005;25:6887–6897. doi: 10.1523/JNEUROSCI.5291-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puzzo D, Privitera L, Palmeri A. Hormetic effect of amyloid-beta peptide in synaptic plasticity and memory. Neurobiol Aging Jan. 2012;25:2012. doi: 10.1016/j.neurobiolaging.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 31.Masliah E. Mechanisms of synaptic dysfunction in Alzheimer’s disease. Histol Histopathol. 1995;10:509–519. [PubMed] [Google Scholar]
- 32.Check E. Nerve inflammation halts trial for Alzheimer’s drug. Nature. 2002;415:462. doi: 10.1038/415462a. [DOI] [PubMed] [Google Scholar]
- 33.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: A case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 34.Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA, Farlow MR, Galvin JE, Peskind ER, Quinn JF, Sherzai A, Sowell BB, Aisen PS, Thal LJ. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch Neurol. 2008;65:1031–1038. doi: 10.1001/archneur.65.8.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 36.Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, Zavitz KH. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: A randomized controlled trial. JAMA. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cummings J. What can be inferred from the interruption of the semagacestat trial for treatment of Alzheimer’s disease? Biol Psych. 2010;68:876–878. doi: 10.1016/j.biopsych.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 38.Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: Reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 39.Bishop GM, Robinson SR. Physiological roles of amyloid-beta and implications for its removal in Alzheimer’s disease. Drugs Aging. 2004;21:621–630. doi: 10.2165/00002512-200421100-00001. [DOI] [PubMed] [Google Scholar]
- 40.Giuffrida ML, Caraci F, De Bona P, Pappalardo G, Nicoletti F, Rizzarelli E, Copani A. The monomer state of beta-amyloid: Where the Alzheimer’s disease protein meets physiology. Rev Neurosci. 2010;21:83–93. doi: 10.1515/revneuro.2010.21.2.83. [DOI] [PubMed] [Google Scholar]
- 41.Luo Y, Sunderland T, Wolozin B. Physiologic levels of beta-amyloid activate phosphatidylinositol 3-kinase with the involvement of tyrosine phosphorylation. J Neurochem. 1996;67:978–987. doi: 10.1046/j.1471-4159.1996.67030978.x. [DOI] [PubMed] [Google Scholar]
- 42.Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A. Beta-amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kontush A, Berndt C, Weber W, Akopyan V, Arlt S, Schippling S, Beisiegel U. Amyloid-β is an antioxidant for lipoproteins in cerebrospinal fluid and plasma. Free Radic Biol Med. 2001;30:119–128. doi: 10.1016/s0891-5849(00)00458-5. [DOI] [PubMed] [Google Scholar]
- 44.Butterfield DA. Amyloid β-peptide(1-42)-induced oxidative stress and neurotoxicity: Implications for neurodegeneration in Alzheimer’s disease brain: A review. Free Radic Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- 45.Baruch-Suchodolsky R, Fischer B. Abeta40, either soluble or aggregated, is remarkably potent antioxidant in cell-free oxidative systems. Biochemistry. 2009;48:4354–4370. doi: 10.1021/bi802361k. [DOI] [PubMed] [Google Scholar]
- 46.Nadal RC, Rigby SE, Viles JH. Amyloid beta-Cu2+complexes in both monomeric and fibrillar forms do not generate H2O2 catalytically but quench hydroxyl radicals. Biochemistry. 2008;47:11653–11664. doi: 10.1021/bi8011093. [DOI] [PubMed] [Google Scholar]
- 47.Robinson SR, Bishop GM. Aβ as a bioflocculant: Implications for the amyloid hypothesis of Alzheimer’s disease. Neurobiol Aging. 2002;23:1051–1072. doi: 10.1016/s0197-4580(01)00342-6. [DOI] [PubMed] [Google Scholar]
- 48.Bishop GM, Robinson SR. The amyloid hypothesis: Let sleeping dogmas lie? Neurobiol Aging. 2002;23:1101–1105. doi: 10.1016/s0197-4580(02)00050-7. [DOI] [PubMed] [Google Scholar]
- 49.López-Toledano MA, Shelanski ML. Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J Neurosci. 2004;24:5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010;5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dominguez D, Tournoy J, Hartmann D, Huth T, Cryns K, Deforce S, Serneels L, Camacho IE, Marjaux E, Craessaerts K, Roebroek AJ, Schwake M, D’Hooge R, Bach P, Kalinke U, Moechars D, Alzheimer C, Reiss K, Saftig P, De Strooper B. Phenotypic and biochemical analyses of BACE1-and BACE2-deficient mice. J Biol Chem. 2005;280:30797–30806. doi: 10.1074/jbc.M505249200. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt SD, Nixon RA, Mathews PM. ELISA method for measurement of amyloid-beta levels. Methods Mol Biol. 2005;299:279–297. doi: 10.1385/1-59259-874-9:279. [DOI] [PubMed] [Google Scholar]
- 53.Puzzo D, Privitera L, Leznik E, Fà M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giedraitis V, Sundelöf J, Irizarry MC, Gårevik N, Hyman BT, Wahlund LO, Ingelsson M, Lannfelt L. The normal equilibrium between CSF and plasma amyloid beta levels is disrupted in Alzheimer’s disease. Neurosci Lett. 2007;427:127–131. doi: 10.1016/j.neulet.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 55.Jensen M, Schröder J, Blomberg M, Engvall B, Pantel J, Ida N, Basun H, Wahlund LO, Werle E, Jauss M, Beyreuther K, Lannfelt L, Hartmann T. Cerebrospinal fluid Abeta42 is increased early in sporadic Alzheimer’s disease and declines with disease progression. Ann Neurol. 1999;45:504–511. doi: 10.1002/1531-8249(199904)45:4<504::aid-ana12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 56.Williams JH, Wilcock GK, Seeburger J, Dallob A, Laterza O, Potter W, Smith AD. Non-linear relationships of cerebrospinal fluid biomarker levels with cognitive function: An observational study. Alz Res Ther. 2011;3:5. doi: 10.1186/alzrt64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. Neuro Rx. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, Zipfel GJ, Holtzman DM. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 60.Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 62.Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12:1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- 63.Mura E, Zappettini S, Preda S, Biundo F, Lanni C, Grilli M, Cavallero A, Olivero G, Salamone A, Govoni S, Marchi M. Dual effect of beta-amyloid on α7 and α4β2 nicotinic receptors controlling the release of glutamate, aspartate and GABA in rat hippocampus. PLoS One. 2012;7:e29661. doi: 10.1371/journal.pone.0029661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plant LD, Boyle JP, Smith IF, Peers C, Pearson HA. The production of amyloid-beta peptide is a critical requirement for the viability of central neurons. J Neurosci. 2003;23:5531–5535. doi: 10.1523/JNEUROSCI.23-13-05531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ, 3rd, Kandel ER, Duff K, Kirkwood A, Shen J. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 66.Wang H, Song L, Laird F, Wong PC, Lee HK. BACE1 knock-outs display deficits in activity-dependent potentiation of synaptic transmission at mossy fiber to CA3 synapses in the hippocampus. J Neurosci. 2008;28:8677–8681. doi: 10.1523/JNEUROSCI.2440-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, Borchelt DR, Price DL, Lee HK, Wong PC. BACE1, a major determinant of selective vulnerability of the brain to amyloid-β-amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harrison SM, Harper AJ, Hawkins J, Duddy G, Grau E, Pugh PL, Winter PH, Shilliam CS, Hughes ZA, Dawson LA, Gonzalez MI, Upton N, Pangalos MN, Dingwall C. BACE1 (beta-secretase) transgenic and knockout mice: Identification of neurochemical deficits and behavioral changes. Mol Cell Neurosci. 2003;24:646–655. doi: 10.1016/s1044-7431(03)00227-6. [DOI] [PubMed] [Google Scholar]
- 69.Müller U, Cristina N, Li ZW, Wolfer DP, Lipp HP, Rülicke T, Brandner S, Aguzzi A, Weissmann C. Behavioral and anatomical deficits in mice homozygous for a modified beta-amyloid precursor protein gene. Cell. 1994;79:755–765. doi: 10.1016/0092-8674(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 70.Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJ, Hopkins R, Smith DW, Heavens RP, Dawson GR, Boyce S, Conner MW, Stevens KA, Slunt HH, Sisoda SS, Chen HY, Van der Ploeg LH. Beta-amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 71.Dawson GR, Seabrook GR, Zheng H, Smith DW, Graham S, O’Dowd G, Bowery BJ, Boyce S, Trumbauer ME, Chen HY, Van der Ploeg LH, Sirinathsinghji DJ. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience. 1999;90:1–13. doi: 10.1016/s0306-4522(98)00410-2. [DOI] [PubMed] [Google Scholar]
- 72.Phinney AL, Calhoun ME, Wolfer DP, Lipp HP, Zheng H, Jucker M. No hippocampal neuron or synaptic bouton loss in learning-impaired aged beta-amyloid precursor protein-null mice. Neuroscience. 1999;90:1207–1216. doi: 10.1016/s0306-4522(98)00645-9. [DOI] [PubMed] [Google Scholar]
- 73.Seabrook GR, Smith DW, Bowery BJ, Easter A, Reynolds T, Fitzjohn SM, Morton RA, Zheng H, Dawson GR, Sirinathsinghji DJ, Davies CH, Collingridge GL, Hill RG. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology. 1999;38:349–359. doi: 10.1016/s0028-3908(98)00204-4. [DOI] [PubMed] [Google Scholar]
- 74.Ring S, Weyer SW, Kilian SB, Waldron E, Pietrzik CU, Filippov MA, Herms J, Buchholz C, Eckman CB, Korte M, Wolfer DP, Müller UC. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci. 2007;27:7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Araki W, Kitaguchi N, Tokushima Y, Ishii K, Aratake H, Shimohama S, Nakamura S, Kimura J. Trophic effect of beta-amyloid precursor protein on cerebral cortical neurons in culture. Biochem Biophys Res Commun. 1991;181:265–271. doi: 10.1016/s0006-291x(05)81412-3. [DOI] [PubMed] [Google Scholar]
- 76.Huber G, Martin JR, Löffler J, Moreau JL. Involvement of amyloid precursor protein in memory formation in the rat: An indirect antibody approach. Brain Res. 1993;603:348–352. doi: 10.1016/0006-8993(93)91261-p. [DOI] [PubMed] [Google Scholar]
- 77.Mattson MP. Secreted forms of beta-amyloid precursor protein modulate dendrite outgrowth and calcium responses to glutamate in cultured embryonic hippocampal neurons. J Neurobiol. 1994;25:439–450. doi: 10.1002/neu.480250409. [DOI] [PubMed] [Google Scholar]
- 78.Mucke L, Masliah E, Johnson WB, Ruppe MD, Alford M, Rockenstein EM, Forss-Petter S, Pietropaolo M, Mallory M, Abraham CR. Synaptotrophic effects of human amyloid beta protein precursors in the cortex of transgenic mice. Brain Res. 1994;666:151–167. doi: 10.1016/0006-8993(94)90767-6. [DOI] [PubMed] [Google Scholar]
- 79.Smith-Swintosky VL, Pettigrew LC, Craddock SD, Culwell AR, Rydel RE, Mattson MP. Secreted forms of beta-amyloid precursor protein protect against ischemic brain injury. J Neurochem. 1994;63:781–784. doi: 10.1046/j.1471-4159.1994.63020781.x. [DOI] [PubMed] [Google Scholar]
- 80.Furukawa K, Sopher BL, Rydel RE, Begley JG, Pham DG, Martin GM, Fox M, Mattson MP. Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J Neurochem. 1996;67:1882–1896. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- 81.Ishida A, Furukawa K, Keller JN, Mattson MP. Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport. 1997;8:2133–2137. doi: 10.1097/00001756-199707070-00009. [DOI] [PubMed] [Google Scholar]
- 82.Meziane H, Dodart JC, Mathis C, Little S, Clemens J, Paul SM, Ungerer A. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc Natl Acad Sci U S A. 1998;95:12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mileusnic R, Lancashire CL, Johnston AN, Rose SP. APP is required during an early phase of memory formation. Eur J Neurosci. 2000;12:4487–4495. [PubMed] [Google Scholar]
- 84.Cao X, Sudhof TC. A transcriptionally active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 85.Gao Y, Pimplikar SW. The gamma-secretase-cleaved C-terminal fragment of amyloid precursor protein mediates signaling to the nucleus. Proc Natl Acad Sci U S A. 2001;98:14979–14984. doi: 10.1073/pnas.261463298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kimberly WT, Zheng JB, Guénette SY, Selkoe DJ. The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem. 2001;276:40288–40292. doi: 10.1074/jbc.C100447200. [DOI] [PubMed] [Google Scholar]
- 87.Leissring MA, Murphy MP, Mead TR, Akbari Y, Sugarman MC, Jannatipour M, Anliker B, Müller U, Saftig P, De Strooper B, Wolfe MS, Golde TE, LaFerla FM. A physiologic signaling role for the gamma- secretase-derived intracellular fragment of APP. Proc Natl Acad Sci U S A. 2002;99:4697–4702. doi: 10.1073/pnas.072033799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma H, Lesné S, Kotilinek L, Steidl-Nichols JV, Sherman M, Younkin L, Younkin S, Forster C, Sergeant N, Delacourte A, Vassar R, Citron M, Kofuji P, Boland LM, Ashe KH. Involvement of beta-site APP cleaving enzyme 1 (BACE1) in amyloid precursor protein-mediated enhancement of memory and activity-dependent synaptic plasticity. Proc Natl Acad Sci U S A. 2007;104:8167–8172. doi: 10.1073/pnas.0609521104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lisman J, Raghavachari S. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci STKE. 2006;2006(356):re11. doi: 10.1126/stke.3562006re11. [DOI] [PubMed] [Google Scholar]
- 90.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 91.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Levey AI. Muscarinic acetylcholine receptor expression in memory circuits: Implications for treatment of Alzheimer disease. Proc Natl Acad Sci U S A. 1996;93:13541–13546. doi: 10.1073/pnas.93.24.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- 94.Clader JW, Wang Y. Muscarinic receptor agonists and antagonists in the treatment of Alzheimer’s disease. Curr Pharm Des. 2005;11:3353–3361. doi: 10.2174/138161205774370762. [DOI] [PubMed] [Google Scholar]
- 95.Oddo S, LaFerla FM. The role of nicotinic acetylcholine receptors in Alzheimer’s disease. J Physiol Paris. 2006;99:172–179. doi: 10.1016/j.jphysparis.2005.12.080. [DOI] [PubMed] [Google Scholar]
- 96.Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: Correlating physiology with function. Trends Neurosci. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- 97.Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berl) 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- 98.Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 99.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 100.Radcliffe KA, Dani JA. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. J Neurosci. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Genzen JR, McGehee DS. Short- and long-term enhancement of excitatory transmission in the spinal cord dorsal horn by nicotinic acetylcholine receptors. Proc Natl Acad Sci U S A. 2003;100:6807–6812. doi: 10.1073/pnas.1131709100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Girod R, Barazangi N, McGehee D, Role LW. Facilitation of glutamatergic neurotransmission by presynaptic nicotinic acetylcholine receptors. Neuropharmacology. 2000;39:2715–2725. doi: 10.1016/s0028-3908(00)00145-3. [DOI] [PubMed] [Google Scholar]
- 103.Ondrejcak T, Klyubin I, Hu NW, Barry AE, Cullen WK, Rowan MJ. Alzheimer’s disease amyloid beta-protein and synaptic function. Neuromol Med. 2010;12:13–26. doi: 10.1007/s12017-009-8091-0. [DOI] [PubMed] [Google Scholar]
- 104.Fehér A, Juhász A, Rimanóczy A, Csibri E, Kálmán J, Janka Z. Association between a genetic variant of the alpha-7 nicotinic acetylcholine receptor subunit and four types of dementia. Dement Geriatr Cogn Disord. 2009;28:56–62. doi: 10.1159/000230036. [DOI] [PubMed] [Google Scholar]
- 105.Wang HY, Lee DH, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. Beta-Amyloid(1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J Biol Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 106.Small DH, Maksel D, Kerr ML, Ng J, Hou X, Chu C, Mehrani H, Unabia S, Azari MF, Loiacono R, Aguilar MI, Chebib M. The beta-amyloid protein of Alzheimer’s disease binds to membrane lipids but does not bind to the alpha7 nicotinic acetylcholine receptor. J Neurochem. 2007;101:1527–1538. doi: 10.1111/j.1471-4159.2006.04444.x. [DOI] [PubMed] [Google Scholar]
- 107.Khan GM, Tong M, Jhun M, Arora K, Nichols RA. Beta-Amyloid activates presynaptic alpha7 nicotinic acetylcholine receptors reconstituted into a model nerve cell system: Involvement of lipid rafts. Eur J Neurosci. 2010;31:788–796. doi: 10.1111/j.1460-9568.2010.07116.x. [DOI] [PubMed] [Google Scholar]
- 108.Dougherty JJ, Wu J, Nichols RA. Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex. J Neurosci. 2003;23:6740–6747. doi: 10.1523/JNEUROSCI.23-17-06740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fodero LR, Mok SS, Losic D, Martin LL, Aguilar MI, Barrow CJ, Livett BG, Small DH. Alpha7-nicotinic acetylcholine receptors mediate an Abeta(1-42)-induced increase in the level of acetylcholinesterase in primary cortical neurones. J Neurochem. 2004;88:1186–1193. doi: 10.1046/j.1471-4159.2003.02296.x. [DOI] [PubMed] [Google Scholar]
- 110.Grassi F, Palma E, Tonini R, Amici M, Ballivet M, Eusebi F. Amyloid beta(1-42) peptide alters the gating of human and mouse alpha-bungarotoxin-sensitive nicotinic receptors. J Physiol. 2003;547:147–157. doi: 10.1113/jphysiol.2002.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dineley KT, Bell KA, Bui D, Sweatt JD. Beta-amyloid peptide activates alpha 7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Biol Chem. 2002;277:25056–25061. doi: 10.1074/jbc.M200066200. [DOI] [PubMed] [Google Scholar]
- 112.Puzzo D, Privitera L, Fa’ M, Staniszewski A, Hashimoto G, Aziz F, Sakurai M, Ribe EM, Troy CM, Mercken M, Jung SS, Palmeri A, Arancio O. Endogenous amyloid-β is necessary for hippocampal synaptic plasticity and memory. Ann Neurol. 69:819–830. doi: 10.1002/ana.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garcia-Osta A, Alberini CM. Amyloid beta mediates memory formation. Learn Mem. 2009;16:267–272. doi: 10.1101/lm.1310209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morley JE, Farr SA, Banks WA, Johnson SN, Yamada KA, Xu L. A physiological role for amyloid-beta protein: Enhancement of learning and memory. J Alzheimers Dis. 2010;19:441–449. doi: 10.3233/JAD-2009-1230. [DOI] [PubMed] [Google Scholar]