Abstract
γ-Tocotrienol has attracted great attention due to its multiple health benefits. This study developed and validated a simple, specific, sensitive and reliable LC/MS/MS method to analyze γ-tocotrienol in rat plasma. Plasma samples (50 µL) were extracted with internal standard solution (25 ng/mL of itraconazole) in acetonitrile (200 µL) with an average recovery of 44.7% and an average matrix effect of −2.9%. The separation of γ-tocotrienol and internal standard from the plasma components was achieved with a Waters XTerra® MS C18 column with acetonitrile/water as mobile phases. Analysis was performed under positive ionization electrospray mass spectrometer via the multiple reaction monitoring. The standard curve was linear over a concentration range of 10 – 1000 ng/mL with correlation coefficient values > 0.997. The method was validated with intra- and inter-day accuracy (relative error) ranged from 1.79 to 9.17% and 2.16 to 9.66%, respectively, and precision (coefficient of variation) ranged from 1.94 to 9.25% and 2.37 to 10.08%, respectively. The short-term disability, freeze-thaw stability and the processed sample stability tests were performed. This method was further applied to analyze γ-tocotrienol plasma concentrations in rats at various time points after administration of a 2 mg/kg single intravenous dose, and a pharmacokinetic profile was successfully obtained.
Keywords: γ-tocotrienol, vitamin E, LC/MS/MS, pharmacokinetics, rat plasma
1. INTRODUCTION
Vitamin E refers to a group of naturally occurring lipophilic antioxidants that include 4 tocopherols (α-, β-, γ- and δ-tocopherol) and 4 tocotrienols (α-, β-, γ- and δ-tocotrienol). Tocotrienols differ from tocopherols by having three double bonds in the isoprenoid side chain. In the past few years, γ-tocotrienol has been studied intensively by scientific community because its significant anticancer activities (Ayoub et al., 2011; Kunnumakkara et al., 2010) as well as other health benefits, such as immunomodulatory (Wilankar et al., 2011), radioprotective (Berbee et al., 2012), anti-inflammatory (Nakagawa et al., 2010), antihypercholesterolemic (Das et al., 2012), cardioprotective (Das et al., 2008) and antioxidative (Nowak et al., 2012) effects. Currently, γ-tocotrienol and other vitamin E products are sold as dietary supplements, which are not strictly reviewed by FDA. As a result, limited analytical methods and pharmacokinetic information of γ-tocotrienol are available in the literature. Therefore, a well-designed pharmacokinetic study using a simple yet sensitive and reproducible analytical method is needed.
In previous studies, several chromatographic methods have been reported for γ-tocotrienol analysis in biological fluids; the detection methods have utilized different techniques including fluorescence detection (Huang and Ng, 2011; Panfili et al., 2003; Yap et al., 1999), ultraviolet absorbance (UV) detection (Abuasal et al., 2011), and mass spectrometry (Nagy et al., 2007). The lower limit of quantification of above techniques were 1 µg/mL (Huang and Ng, 2011), 0.45 µg/mL (Panfili et al., 2003), 34 ng/mL (Yap et al., 1999), 100 ng/mL (Abuasal et al., 2011) and 130 nmol/L (Nagy et al., 2007), respectively. However, most of the methods were not specifically designed for γ-tocotrienol analysis, but for simultaneous analysis of vitamin E constituents. Moreover, they used complicated and time-consuming sample preparation procedures and did not provide significant sensitivity for pharmacokinetic study, especially to detect and estimate the amount of rat endogenous γ-tocotrienol.
Herein, we reported the development and validation of a simple, specific, sensitive and reliable LC/MS/MS method to determine γ-tocotrienol concentration in rat plasma. We also demonstrated the applicability of this method for pharmacokinetic study of γ-tocotrienol in rats. The comparisons with previously reported methods were systematically addressed.
2. MATERIALS AND EXPERIMENTS
2.1. Materials
γ-Tocotrienol in ethanol (purity > 98%, 10 mg/mL) was purchased from Cayman Chemical (Ann Arbor, MI). Itraconazole (purity ≥ 98%), formic acid, HPLC grade acetonitrile and water were purchased from Sigma-Aldrich (St. Louis, MO). Freshly obtained rat plasma (not blank plasma, since it contains endogenous γ-tocotrienol) was collected from male Sprague-Dawley rats (Harlan Laboratories, Houston, TX) and stored at −80 °C prior to use.
2.2. Instruments and Conditions
The separation of γ-tocotrienol from the plasma components was achieved with a Waters XTerra® MS C18 column (3.5 µm, 4.6 × 150 mm, Milford, MA) using an Agilent 1200 series HPLC system (Foster City, CA). Mobile phase solvent A was water and solvent B was 0.2% formic acid in acetonitrile. The mobile phase gradient is summarized in Table 1. The injection volume was 20 µL.
Table 1.
Gradient mobile phase timing, flow rate and composition for the reversed-phase chromatographic separation of γ-tocotrienol.
| Time (min) | Flow Rate (µL/min) | Solvent A* (%) | Solvent B* (%) |
|---|---|---|---|
| 0 | 1000 | 70 | 30 |
| 1 | 1000 | 70 | 30 |
| 2 | 1000 | 0 | 100 |
| 8.5 | 1000 | 0 | 100 |
| 9 | 1000 | 70 | 30 |
| 9.5 | 1000 | 70 | 30 |
Solvent A was water, and solvent B was 0.2% formic acid in acetonitrile.
Chromatographic analysis was performed using a 3200 QTRAP® LC/MS/MS system (AB SCIEX, Foster City, CA), which is a hybrid triple quadrupole linear ion trap equipped with a TurboIonSpray ion source. Pure nitrogen was generated by a Parker Balston Source 5000 Tri Gas Generator. The ion source parameters for mass spectrum were set as follows: ionspray voltage, 5500 V; ion source temperature, 700 °C; nebulizer gas, 50 psi; heater gas, 40 psi; curtain gas, 10 psi; and the collision gas, medium. Itraconazole was used as internal standard (IS) to analysis γ-tocotrienol in rat plasma. The product ion scan spectra of γ-tocotrienol and IS are presented in Figure 1. Multiple reaction monitoring (MRM) method in the positive ion mode was used to detect the transition ions from precursor ion to a specific product ion for γ-tocotrienol (m/z 410→m/z 151) and itraconazole (m/z 705→m/z 392). The collision energy was set at 34 and 49 V for γ-tocotrienol and IS, respectively. The LC/MS/MS system was controlled and data was acquired by Analyst® software version 1.5.
Figure 1.
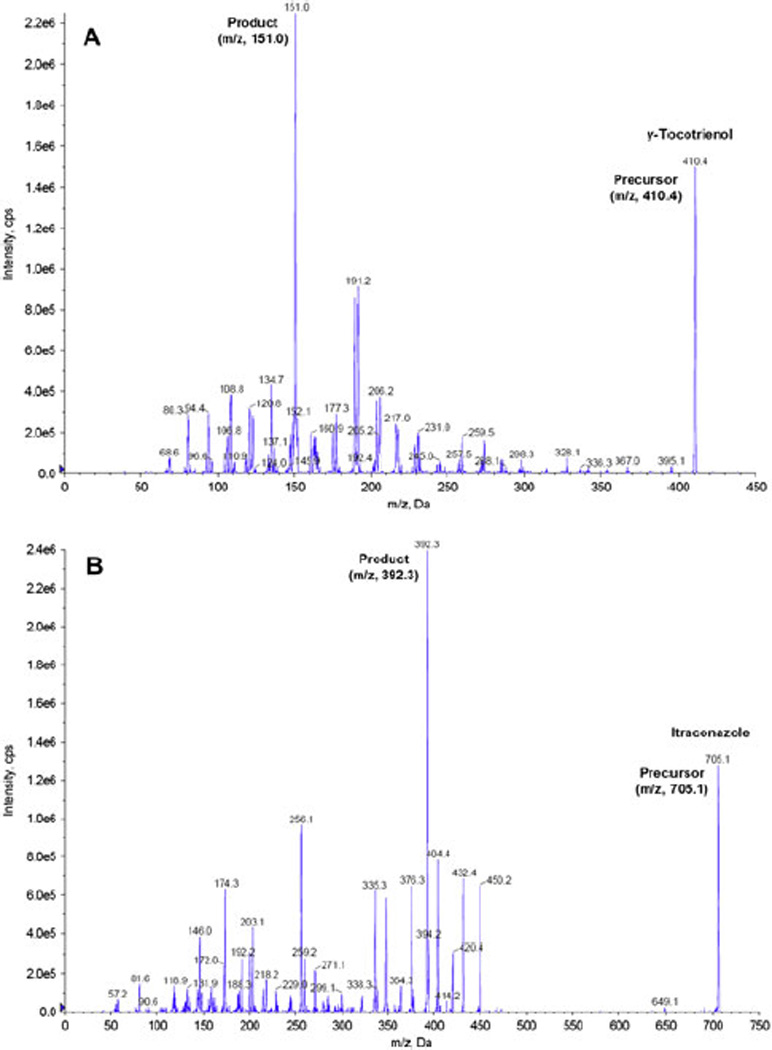
The product ion scan spectra for (A) γ-tocotrienol and (B) itraconazole (IS).
2.3. Standard and Quality Control Samples
Stock solution of γ-tocotrienol was prepared by diluting the original γ-tocotrienol ethanol solution with acetonitrile to the concentration of 1 mg/mL. Stock solution of IS was prepared by dissolving itraconazole in acetonitrile at the concentration of 1 mg/mL. The stock solutions were stored at −80 °C prior to use. Standard samples of γ-tocotrienol were prepared in rat plasma at six different concentrations: 10, 50, 100, 200, 500 and 1000 ng/mL. Quality control (QC) samples of γ-tocotrienol were prepared in rat plasma at low (20 ng/mL), medium (400 ng/mL) and high (800 ng/mL) concentrations. Calibration standard and QC samples were stored at −20 °C until use.
2.4. Plasma Sample Preparation
An aliquot of plasma sample (50 µL) was extracted with 200 µL acetonitrile (containing 25 ng/mL of IS) by vortex for 1 minute. After centrifugation at 13,000 rpm and 4 °C for 10 min, a 20 µL-aliquot of the supernatant was transferred to an autosampler vial, and then injected to the LC/MS/MS for quantitative analysis.
2.5. Method Validation
2.5.1. Linearity and Sensitivity
Linear calibration curves in rat plasma were generated by plotting the peak area ratio of γ-tocotrienol to IS versus six known γ-tocotrienol standard concentrations. Slope, intercept and correlation coefficient of linear regression equation were estimated using least square regression analysis. The lower limit of quantification (LLOQ) was determined close to endogenous γ-tocotrienol level.
2.5.2. Accuracy and Precision
The intra-day accuracy and precision were determined by analyzing six replicates of QC samples with three different concentrations using a calibration curve constructed on the same day. The inter-day accuracy and precision were determined by analyzing six replicates of QC samples with three different concentrations using calibration curves constructed on three different days. The assay accuracy was reflected by relative error from the theoretical drug concentrations and the assay precision was reflected by the coefficient of variation.
The accuracy and precision of diluted rat plasma samples were determined by analyzing six replicates of 10 times dilution of a plasma sample with 5000 ng/mL of γ-tocotrienol and six replicates of 20 times dilution of a plasma sample with 15000 ng/mL of γ-tocotrienol.
2.5.3. Extraction Recovery and Matrix Effect
The extraction recovery and matrix effect was evaluated by analyzing γ-tocotrienol samples with three different concentrations: 20, 400 and 800 ng/mL.
The extraction recovery of γ-tocotrienol was calculated as follows:
where Responseextracted sample is the average area count for γ-tocotrienol, which has been through the extraction process. And Responsepost-extracted spiked sample is the average area count for γ-tocotrienol spiked into extracted matrix after the extraction procedure.
The matrix effect of γ-tocotrienol was calculated as follows:
where Responsepost-extracted spiked sample is the average area count for the γ-tocotrienol spiked into extracted matrix after the extraction procedure, Responsepost-extracted non-spiked sample is the average area count for the extracted matrix after the extraction procedure without spiking γ-tocotrienol, and Responsenon-extracted neat sample is the average area count for the same concentration of γ-tocotrienol in neat solution (acetonitrile). A positive result of the percentage matrix effect indicates enhancement of the sample signal, and a negative result indicates suppression of the sample signal.
2.5.4. Stability
Short-term stability (bench-top stability) of γ-tocotrienol in rat plasma was evaluated by analyzing rat plasma samples spiked with three different concentrations: 20, 400 and 800 ng/mL. Plasma samples were removed from frozen storage (−20 °C) and remained on the bench-top for three different period of time: 60, 90 and 120 min. And another set of plasma samples were stored at −20 °C for 48 hr. All those samples were compared with freshly prepared samples at the same concentrations.
Freeze–thaw stability of γ-tocotrienol in rat plasma was evaluated by analyzing rat plasma samples spiked with three different concentrations: 20, 400 and 800 ng/mL. Plasma samples that were exposed for two cycles of freeze (at −20 °C) and thaw (at room temperature) were compared with freshly prepared samples at the same concentrations.
Processed sample stability (on-instrument or autosampler stability) of γ-tocotrienol rat plasma extracts was evaluated by analyzing rat plasma samples spiked with three different concentrations: 20, 400 and 800 ng/mL. Two set of plasma samplers were extracted with acetonitrile with and without IS, respectively. The plasma extracts were remained in the autosampler for three different period of time: 60, 90 and 120 min. And all those samples were compared with freshly prepared samples at the same concentrations.
2.6. Pharmacokinetic Study
To further verify the applicability of the developed LC/MS/MS method, a pharmacokinetic study was performed in five male adult Sprague-Dawley rats. The original 10 mg/mL γ-tocotrienol in ethanol solution was diluted to 2 mg/mL by normal saline. The animal experiment and protocol were reviewed and approved by the Institutional Animal Care and Use Committee at Texas Southern University. The jugular veins of five rats weighing from 353 to 373 g were cannulated under anesthesia using a cocktail of ketamine : acetopromazine : xylazine (50 : 3.3 : 3.3 mg/kg) the day before the study. Blood sample collecting tubes were heparinized and dried. Blood samples were collected from each rat right before dosing. Each rat was given a 2 mg/kg dose of γ-tocotrienol intravenously. In order to avoid any possible contamination, after dosing for each rat, two procedures were conducted: (1) cutting of the foremost part of each tubing associated with the needle for dosing; and (2) injection of 0.5 mL normal saline containing 20 U/mL heparin to flush the tubing. Blood samples (250 µL) were collected at 5, 15, 30, 60, 120, 240, 360, 480, 600 and 900 min post injection. The blood samples were centrifuged at 13,000 rpm for 10 min and the supernatants (plasma) were obtained and immediately stored at −80 °C until analysis. Within 48 hr, those plasma samples were analyzed by the LC/MS/MS method developed above to obtain γ-tocotrienol concentrations. Note that the sample preparation time (from removing the sample from the freezer to sample injection) was less than 60 min. After analysis, the plasma drug concentration data were presented as the mean value with standard deviation (SD) and plotted against time. SigmaPlot 10.0 (SYSTAT Software, San Jose, CA) was used for curve fitting and WinNonlin 5.2.1 (Pharsight Corporation, Mountain View, CA) was used to obtain pharmacokinetic parameters.
3. RESULTS AND DISCUSSION
3.1. Method Development
The goal of this work was to develop and validate a simple, specific, sensitive and reliable LC/MS/MS method to determine γ-tocotrienol concentration in rat plasma, suitable for determination of the pharmacokinetics of γ-tocotrienol in rat model. The retention times for γ-tocotrienol and IS were 7.94 min and 3.86 min, respectively. Figure 2 shows typical representative chromatograms from rat plasma without spiking and dosing (A, B and C: A and B showed rat plasma without IS at 410.4/151.1 Da and 705.1/392.4 Da, respectively; C showed rat plasma with IS at both 410.4/151.1 Da and 705.1/392.4 Da; the endogenous γ-tocotrienol in rat was detected and discussed in Section 3.2); from rat plasma spiked with low and high concentrations of γ-tocotrienol (D and E); and from rat plasma obtained 8 hr after a 2 mg/kg intravenous dose of γ-tocotrienol (F). Those chromatograms show very clear peaks for γ-tocotrienol and IS, and very good separation between γ-tocotrienol and IS.
Figure 2.
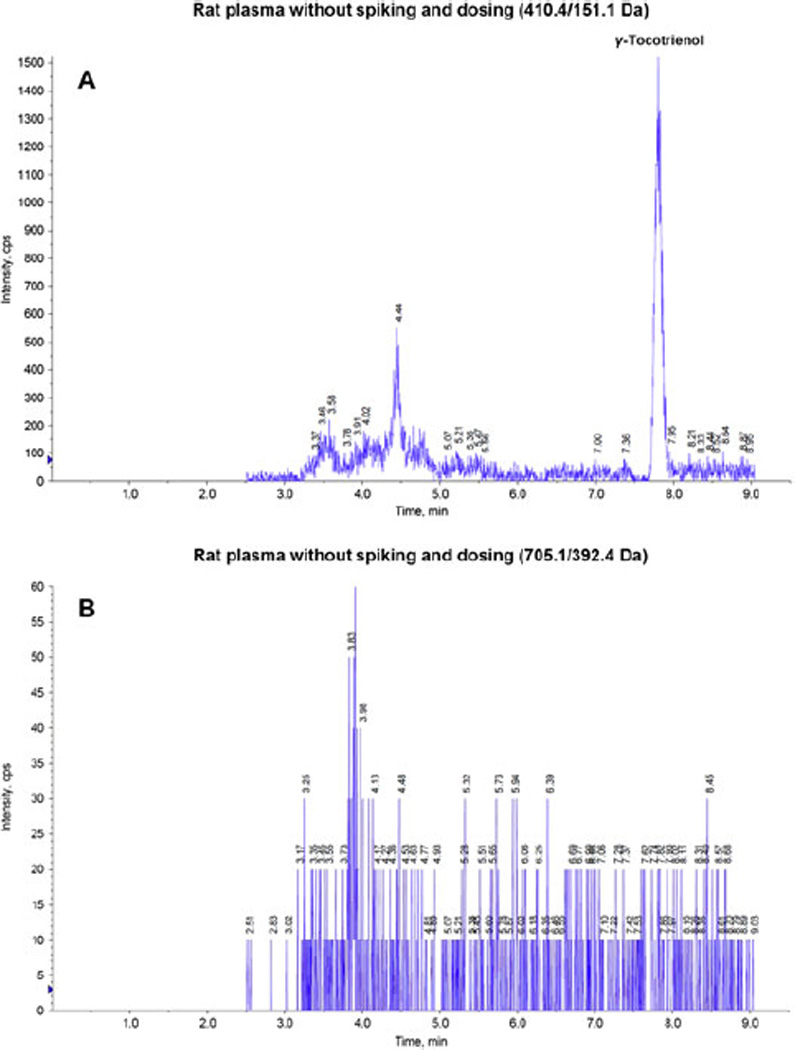
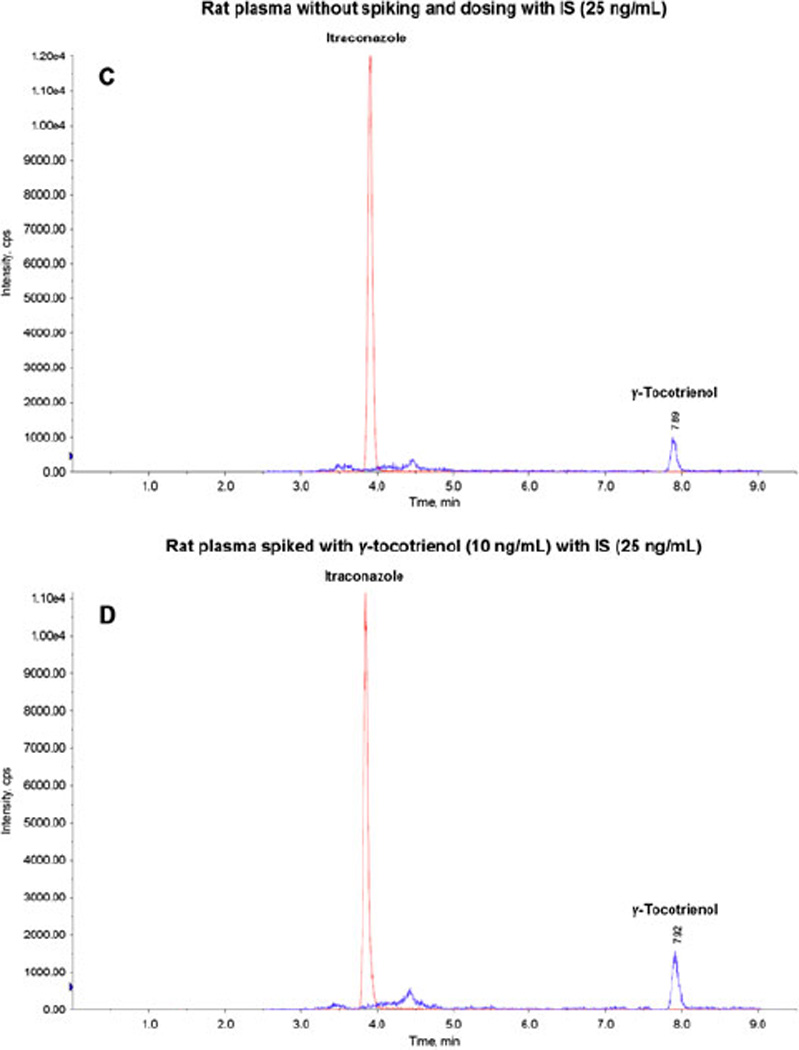
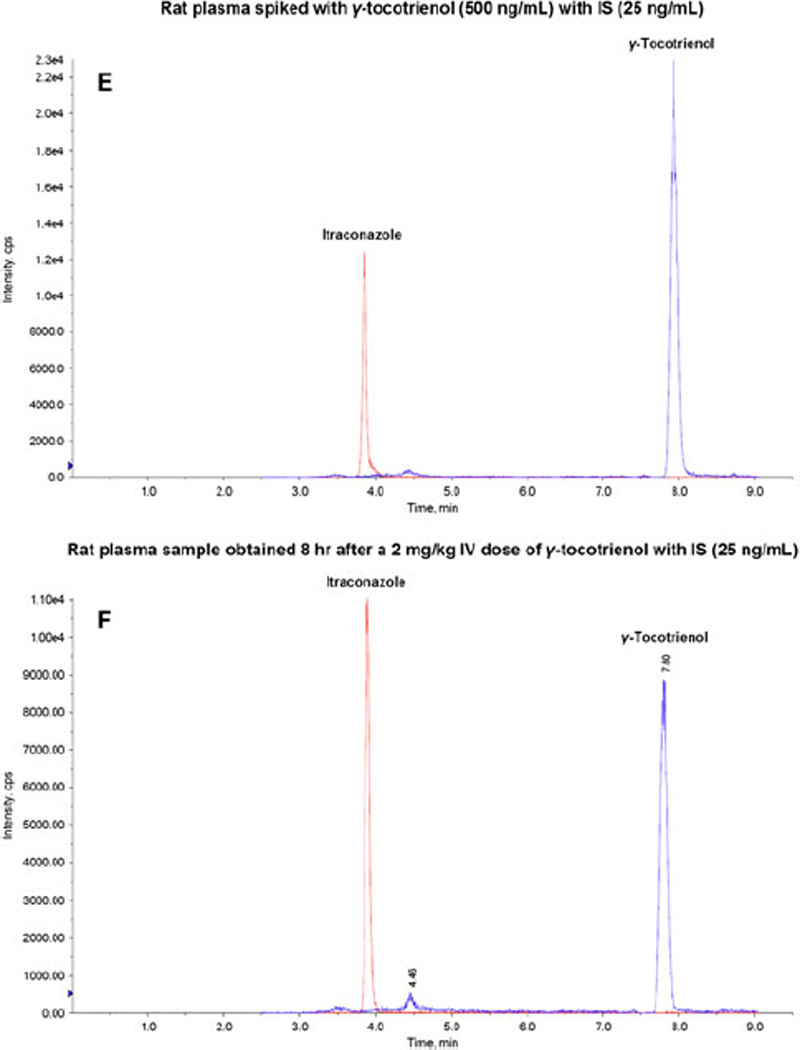
Representative LC/MS/MS chromatograms for (A) rat plasma without spiking and dosing (410.4/151.1 Da); (B) rat plasma without spiking and dosing (705.1/392.4 Da); (C) rat plasma without spiking and dosing with IS (25 ng/mL); (D) rat plasma spiked with γ-tocotrienol (10 ng/mL) with IS (25 ng/mL); (E) rat plasma spiked with γ-tocotrienol (500 ng/mL) with IS (25 ng/mL); and (F) rat plasma sample obtained 8h after a 2 mg/kg intravenous dose of コ-tocotrienol with IS (25 ng/mL).
As a potent antioxidant, γ-tocotrienol is unstable in the ambient environment, especially sensitive to temperature and oxygen (Kamal-Eldin and Appelqvist, 1996). So γ-tocotrienol plasma sample preparation was a challenge. Several methods were investigated to extract γ-tocotrienol from rat plasma, including protein precipitation with acetonitrile, liquid–liquid extraction (LLE) and solid–phase extraction (SPE). Usually, LLE and SPE methods can provide better extraction recovery rate than protein precipitation method. However, in previous studies regarding extraction of γ-tocotrienol from rat plasma samples, the maximum recovery was 60% (by LLE method) (Abuasal et al., 2011), which is not a significant improvement over current precipitation method (results in Section 3.4). Most importantly, both LLE and SPE methods showed obvious degradation of γ-tocotrienol during sample preparation, since they involved more complicated procedures, such as drying and reconstitution. On the other hand, the one-step protein precipitation method was simple and quick, and significantly reduced the degradation of γ-tocotrienol during sample preparation. All of our sample preparation was handled in a time and light sensitive fashion. In addition, the high acetonitrile to sample ratio (4:1) offered satisfactory recovery and matrix effect (results in Section 3.4).
Typically, an ideal IS has a structure as close as possible to the analyte, for example, a stable isoform of the analyte. However, all the isoforms of vitamin E are very unstable, and have poor extraction recovery to different extent (Nagy et al., 2007). The assay will be non-reliable and non-repeatable if both the analyte and the IS have poor stability and bad extraction recovery. Therefore in this study, itraconazole was selected as IS mainly because: (1) it is stable (stable in autosampler in 24 h); (2) it has very high extraction recovery (96% recovery by protein precipitation method) (Yao et al., 2001); and (3) it also can be detected by MRM method in the positive ion mode like γ-tocotrienol.
Separation by isocratic elution was demonstrated by Abuasal et al. in their reversed-phase HPLC analysis of γ-tocotrienol (Abuasal et al., 2011). However, this method led to significant matrix effect in LC/MS/MS analysis due to its failure on separation of γ-tocotrienol from the blood matrix components. Therefore, we developed the gradient methods (Section 2.2), which not only completely separated γ-tocotrienol and IS from the blood matrix components but also significantly reduced matrix effect (Section 3.4).
3.2. Linearity and Sensitivity
The calibration curves for γ-tocotrienol in rat plasma was linear in the concentration range of 10 – 1000 ng/mL with correlation coefficient values > 0.997.
The LLOQ of this LC/MS/MS method was 10 ng/mL, which was more than 10 times lower than the HPLC method most recently reported for γ-tocotrienol quantification by Abuasal et al. (Abuasal et al., 2011). And in our study, the amount of rat endogenous γ-tocotrienol was detected and estimated by the standard addition method (Skoog et al., 1995). The amount of rat endogenous γ-tocotrienol was estimated as follows:
The rats used for taking plasma and the rats used in pharmacokinetic study had an average endogenous γ-tocotrienol concentration of 17.4 ± 1.3 ng/mL, which might be contributed by the rats’ diet. Previous study has determined the γ-tocotrienol was found to be present at approximately 30 – 80 ng/mL in human plasma (Yap et al., 1999). To our knowledge, this is the first time that rats’ endogenous γ-tocotrienol concentration was detected and reported, indicating the high sensitivity of this newly developed LC/MS/MS method.
3.3. Accuracy and Precision
Intra- and inter-day accuracy and precision in rat plasma were evaluated by measuring six replicates of QC samples at low, medium and high concentration levels of γ-tocotrienol. The accuracy and precision obtained are summarized in Table 2. These data showed that the accuracy and precision were well within the 15% acceptance range.
Table 2.
Intra- and inter-day accuracy and precision of γ-tocotrienol LC-MS/MS analysis.
| Concentration (ng/mL) |
Intra-day (n=6) | Inter-day (n = 6) | ||
|---|---|---|---|---|
| Accuracy (RE*, %) |
Precision (CV*, %) |
Accuracy (RE, %) |
Precision (CV, %) |
|
| 20 | 9.17 | 9.66 | 9.25 | 10.08 |
| 400 | 2.38 | 2.83 | 2.63 | 3.54 |
| 800 | 1.79 | 2.16 | 1.94 | 2.37 |
RE = relative error; CV = coefficient of variation.
This LC/MS/MS method was validated to be accurate and precise in rat plasma over a γ-tocotrienol concentration range of 10 – 1000 ng/mL. In order to analyze sample with a higher concentration, the accuracy and precision of diluted rat plasma samples were also determined by measuring six replicates of 10 and 20 times of dilutions of two high concentration samples, respectively. The accuracy and precision were 4.73% (relative error) and 6.51% (coefficient of variation) respectively for 10 times dilution, and 4.89% (relative error) and 5.95% (coefficient of variation) respectively for 20 times dilution. This result suggests that the concentration estimation from a diluted plasma sample is reliable.
3.4. Recovery and Matrix Effect
The average extraction recovery rate obtained by measuring triplicates of QC samples at low, medium and high concentration levels of γ-tocotrienol in rat plasma were 44.1 ± 2.9%, 44.7 ± 3.2% and 45.2 ± 2.7%, respectively. This recovery rate was less likely due to degradation but more likely due to the high protein binding, because the short-term and processed sample stability tests (see Section 3.5) showed that γ-tocotrienol in rat plasma and plasma extract was stable up to 120 min, and the sample preparation time (from removing the sample from the freezer to sample injection) was less than 60 min. Since this LC/MS/MS assay provided very high sensitivity, the stable recovery rate is satisfactory.
The average percentage matrix effect acquired by measuring triplicates of QC samples at low, medium and high concentration levels of γ-tocotrienol in rat plasma were −4.9 ± 2.2%, 3.5 ± 1.9% and −7.3 ± 1.4%, respectively. These results suggest that there was no measurable matrix effect that interfered with γ-tocotrienol determination in rat plasma via this LC/MS/MS method.
3.5. Stability
Because of the instable nature of γ-tocotrienol, stability issue is very critical for the development of a reliable analytical method. However, very limited rat plasma sample stability information of γ-tocotrienol is available in the literature. We have only found the freeze–thaw stability of γ-tocotrienol in rat plasma was reported previously (Abuasal et al., 2011). In this study, we conducted a more systematical stability study of γ-tocotrienol in rat plasma to insure the reliability of this LC/MS/MS method.
Short-term stability of γ-tocotrienol in rat plasma was evaluated by measuring triplicates of QC samples at low, medium and high concentration levels of γ-tocotrienol after remaining on bench-top for 60, 90 and 120 min. The samples displayed 92 – 95%, 90 – 93% and 86 – 89% recoveries, respectively. These data indicate that the bench-top stability of γ-tocotrienol in rat plasma was acceptable up to 120 min. The other set of plasma samples that were stored at −20 °C for 48 hours and displayed 96 – 99% recoveries, which suggest that the γ-tocotrienol in rat plasma was stable in the freezer for at least 48 hr.
The freeze–thaw stability of γ-tocotrienol in rat plasma during the sample storage was evaluated by measuring triplicates of QC samples at low, medium and high concentration levels of γ-tocotrienol after two freeze-thaw cycles. All the samples displayed 91 – 96% recoveries after the test, which were similar to those reports previously (Abuasal et al., 2011). These data indicate that γ-tocotrienol in rat plasma was stable after two freeze–thaw cycles.
The processed sample stability of γ-tocotrienol rat plasma extracts was evaluated by measuring triplicates of QC samples at low, medium and high concentration levels of γ-tocotrienol after remaining in the autosampler for 60, 90 and 120 min. The samples that were extracted by acetonitrile with IS displayed 93 – 94%, 87 – 92% and 86 – 88% recoveries, respectively. And the samples that were extracted by acetonitrile without IS displayed 91 – 94%, 87 – 91% and 85 – 88% recoveries, respectively. These data indicate that the processed sample stability of γ-tocotrienol rat plasma extracts was up to 120 min, and not affected by the present of the IS.
Due to γ-tocotrienol’s stability issue, all the pharmacokinetic rat plasma samples were stored at −80 °C until analysis and the storage time was less than 48 hr. In addition, the sample preparation time (from removing the sample from the freezer to sample injection) was within 60 min.
3.6. Pharmacokinetic Study: Application of the Assay
Pharmacokinetic study of γ-tocotrienols in rats was performed to evaluate the applicability of the developed LC/MS/MS assay. Yap et al. has done a study to investigate the influence of route of administration on the absorption and disposition of α-, γ- and δ-tocotrienols in rats, and they found oral absorption was incomplete (the oral bioavailability of γ-tocotrienol was 9.1%) and intraperitoneal and intramuscular administration gave negligible absorption (Yap et al., 2003). Therefore intravenous administration was a better option. Akaho et al. reported intravenous injections of 5, 10 and 25 mg/kg of γ-tocotrienols or its prodrug into rats for pharmacokinetics study (Akaho et al., 2007). Qureshi et al. recently reported that they intravenously injected mixture of α-, γ- and δ-tocotrienols (10 mg/kg) to dogs (Qureshi et al., 2011). Based on those previous reports, we chose a 2 mg/kg single intravenous dose for our pharmacokinetic study in rats. Sample preparation (from removing the sample from the freezer to sample injection) was conducted in a dark environment within 60 min to avoid degradation as mentioned above. The Log10 plasma concentration vs. time curve is presented in Figure 3, which shows a similar decay as reported previously (Yap et al., 2003).
Figure 3.
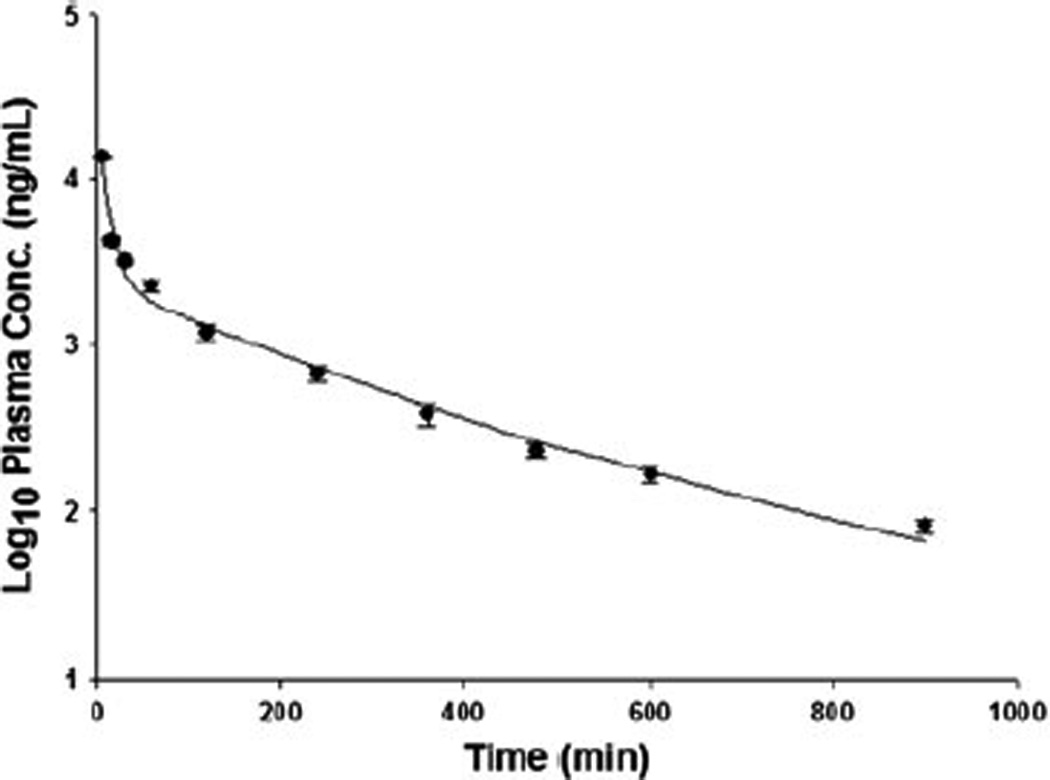
Log10 plasma concentration-time profiles (mean ± SD, n = 5) of γ-tocotrienol after a 2 mg/kg single intravenous dose.
The pharmacokinetic parameters were determined with the WinNonlin 5.2.1 program with a two-compartment model. All previous studies we surveyed used non-compartment model for γ-tocotrienol pharmacokinetic study (Abuasal et al., 2011; Yap et al., 2003; Yap et al., 2001). However, we found that two compartment model had better description of the fit for the intravenous administration, based on the appearance of the observed and predicted concentrations, the reduction in the sums of squares, and the Akaike’s information criterion (Yamaoka et al., 1978). Therefore, we chose the two-compartment model, described by the following equation:
where A and B are the coefficients, α is the distributional rate constant, β is the terminal elimination rate constant, and Ct is the plasma concentration of γ-tocotrienol at time t. The resulting main pharmacokinetic parameters are shown in Table 3.
Table 3.
Pharmacokinetic parameters of γ-tocotrienol in plasma after single intravenous administration at 2 mg/kg to rats (n = 5).
| Cmax* (ng/mL) | 17515 ± 6526 |
| A* (ng/mL) | 15782 ± 6186 |
| T1/2α* (min) | 10.4 ± 9.9 |
| B* (ng/mL) | 1732 ± 350 |
| T1/2β* (min) | 183.6 ± 28.9 |
| AUC0-∞* (min·ng/mL) | 617317 ± 6719 |
| Cl* (mL/kg/min) | 3.24 ± 0.04 |
| V1* (mL/kg) | 138.8 ± 85.2 |
| V2* (mL/kg) | 492.5 ± 29.7 |
| Vss* (mL/kg) | 631.3 ± 59.5 |
Cmax = maximum concentration; A = coefficients of distributional phase; T1/2α = elimination half-life of distributional phase; B = coefficients of terminal phase; T1/2β = elimination half-life of terminal phase; AUC0-∞ = area under curve from time zero to infinity; Cl = total body clearance; V1 = volume of distribution of central compartment; V2 = volume of distribution of peripheral compartment; Vss = volume of distribution at steady state.
After 2 mg/kg intravenous bolus injection, γ-tocotrienol concentration reached a maximum (Cmax) of 17515 ± 6526 ng/mL and then declined rapidly. Although the method was validated over a concentration range of 10 – 1000 ng/mL, we did validate the dilution of rat plasma samples (Section 3.3), so our results are reliable and reproducible. The elimination half-life of distributional phase (T1/2α) was observed to be 10.4 ± 9.9 min and elimination half-life of terminal phase was 183.6 ± 28.9 min (3.1± 0.5 hr), which agreed with the value previously reported by Yap et al. (Yap et al., 2001).
4. CONCLUSION
A simple, specific, sensitive and reproducible LC/MS/MS method for the specific determination of γ-tocotrienol in rat plasma was developed and validated. The method was shown to be accurate and precise over the concentration range of 10 – 1000 ng/mL. This is the first time that the endogenous γ-tocotrienol concentration is detected and estimated. The method was successfully applied to the pharmacokinetic study of intravenously administered γ-tocotrienol using the rat as an animal model. It is ideal for routine analysis in preclinical studies. This method can also be applied to detect plasma concentrations of other vitamin E constituents.
ACKNOLEDGEMENT
This work was supported by Research Starter Grant in Pharmaceutics from the PhRMA Foundation and NIH/RTRN grant (5U54RR022762-05). We also thank Dr. Dong Liang for his technical support on WinNonlin.
Footnotes
We have no conflicts of interest in this work.
REFERENCES
- Abuasal B, Thomas S, Sylvester PW, Kaddoumi A. Development and validation of a reversed-phase hplc method for the determination of gamma-tocotrienol in rat and human plasma. Biomedical Chromatography. 2011;25:621–627. doi: 10.1002/bmc.1493. [DOI] [PubMed] [Google Scholar]
- Akaho N, Takata J, Fukushima T, Matsunaga K, Hattori A, Hidaka R, Fukui K, Yoshida M, Fujioka T, Karube Y, Imai K. Preparation and in vivo evaluation of a water-soluble prodrug for 2r-gamma-tocotrienol and as a two-step prodrug for 2,7,8-trimethyl-2s-(beta-carboxyethyl)-6-hydroxychroman (s-gamma-cehc) in rat. Drug Metabolism and Disposition. 2007;35:1502–1510. doi: 10.1124/dmd.106.014365. [DOI] [PubMed] [Google Scholar]
- Ayoub NM, Bachawal SV, Sylvester PW. Gamma-tocotrienol inhibits hgf-dependent mitogenesis and met activation in highly malignant mammary tumour cells. Cell Proliferation. 2011;44:516–526. doi: 10.1111/j.1365-2184.2011.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbee M, Fu Q, Boerma M, Sree Kumar K, Loose DS, Hauer-Jensen M. Mechanisms underlying the radioprotective properties of gamma-tocotrienol: Comparative gene expression profiling in tocol-treated endothelial cells. Genes & Nutrition. 2012;7:75–81. doi: 10.1007/s12263-011-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Lekli I, Das M, Szabo G, Varadi J, Juhasz B, Bak I, Nesaretam K, Tosaki A, Powell SR, Das DK. Cardioprotection with palm oil tocotrienols: Comparision of different isomers. American Journal of Physiology - Heart and Circulatory Physiology. 2008;294:H970–H978. doi: 10.1152/ajpheart.01200.2007. [DOI] [PubMed] [Google Scholar]
- Das S, Mukherjee S, Lekli I, Gurusamy N, Bardhan J, Raychoudhury U, Chakravarty R, Banerji S, Knowlton AA, Das DK. Tocotrienols confer resistance to ischemia in hypercholesterolemic hearts: Insight with genomics. Molecular and Cellular Biochemistry. 2012;360:35–45. doi: 10.1007/s11010-011-1041-9. [DOI] [PubMed] [Google Scholar]
- Huang SH, Ng LT. Quantification of tocopherols, tocotrienols, and gamma-oryzanol contents and their distribution in some commercial rice varieties in taiwan. Journal of Agricultural and Food Chemistry. 2011;59:11150–11159. doi: 10.1021/jf202884p. [DOI] [PubMed] [Google Scholar]
- Kamal-Eldin A, Appelqvist LA. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara AB, Sung B, Ravindran J, Diagaradjane P, Deorukhkar A, Dey S, Koca C, Yadav VR, Tong Z, Gelovani JG, Guha S, Krishnan S, Aggarwal BB. {gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Research. 2010;70:8695–8705. doi: 10.1158/0008-5472.CAN-10-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy K, Courtet-Compondu MC, Holst B, Kussmann M. Comprehensive analysis of vitamin e constituents in human plasma by liquid chromatography-mass spectrometry. Analytical Chemistry. 2007;79:7087–7096. doi: 10.1021/ac0708689. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Shibata A, Maruko T, Sookwong P, Tsuduki T, Kawakami K, Nishida H, Miyazawa T. Gamma-tocotrienol reduces squalene hydroperoxide-induced inflammatory responses in hacat keratinocytes. Lipids. 2010;45:833–841. doi: 10.1007/s11745-010-3458-4. [DOI] [PubMed] [Google Scholar]
- Nowak G, Bakajsova D, Hayes C, Hauer-Jensen M, Compadre CM. Gamma-tocotrienol protects against mitochondrial dysfunction and renal cell death. Journal of Pharmacology and Experimental Therapeutics. 2012;340:330–338. doi: 10.1124/jpet.111.186882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panfili G, Fratianni A, Irano M. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. Journal of Agricultural and Food Chemistry. 2003;51:3940–3944. doi: 10.1021/jf030009v. [DOI] [PubMed] [Google Scholar]
- Qureshi AA, Karpen CW, Qureshi N, Papasian CJ, Morrison DC, Folts JD. Tocotrienols-induced inhibition of platelet thrombus formation and platelet aggregation in stenosed canine coronary arteries. Lipids in Health and Disease. 2011;10:58. doi: 10.1186/1476-511X-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog DA, West DM, Holler FJ. Fundamentals of analytical chemistry. Saunders College Publishing. (7th edition) 1995:557–600. [Google Scholar]
- Wilankar C, Sharma D, Checker R, Khan NM, Patwardhan R, Patil A, Sandur SK, Devasagayam TP. Role of immunoregulatory transcription factors in differential immunomodulatory effects of tocotrienols. Free Radical Biology & Medicine. 2011;51:129–143. doi: 10.1016/j.freeradbiomed.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Nakagawa T, Uno T. Application of akaike's information criterion (aic) in the evaluation of linear pharmacokinetic equations. Journal of Pharmacokinetics and Biopharmaceutics. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]
- Yao M, Chen L, Srinivas NR. Quantitation of itraconazole in rat heparinized plasma by liquid chromatography-mass spectrometry. Journal of Chromatography B: Biomedical Sciences and Applications. 2001;752:9–16. doi: 10.1016/s0378-4347(00)00505-3. [DOI] [PubMed] [Google Scholar]
- Yap SP, Julianto T, Wong JW, Yuen KH. Simple high-performance liquid chromatographic method for the determination of tocotrienols in human plasma. Journal of Chromatography B: Biomedical Sciences and Applications. 1999;735:279–283. doi: 10.1016/s0378-4347(99)00385-0. [DOI] [PubMed] [Google Scholar]
- Yap SP, Yuen KH, Lim AB. Influence of route of administration on the absorption and disposition of alpha-, gamma- and delta-tocotrienols in rats. Journal of Pharmacy and Pharmacology. 2003;55:53–58. doi: 10.1111/j.2042-7158.2003.tb02433.x. [DOI] [PubMed] [Google Scholar]
- Yap SP, Yuen KH, Wong JW. Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. Journal of Pharmacy and Pharmacology. 2001;53:67–71. doi: 10.1211/0022357011775208. [DOI] [PubMed] [Google Scholar]


