Abstract
The primary aim of this study was to determine whether different durations of contingency management (CM) in conjunction with psychosocial treatment produced different rates of abstinence among methamphetamine dependent individuals. Participants were randomized to one of four 16-week treatment conditions: standard psychosocial treatment or psychosocial treatment plus one of three durations of CM (one-month, two-month, or four-month). A total of 118 participants were randomized to the four treatment conditions. There were significant differences across treatment conditions for number of consecutive days of methamphetamine abstinence (p < 0.05). These differences were in the hypothesized direction, as participants were more likely to remain abstinent through the 16-week trial as CM duration increased. A significant effect of treatment condition (p < 0.05) and time (p < 0.05) on abstinence over time was also found. Longer durations of CM were more effective for maintaining methamphetamine abstinence.
Methamphetamine use is a public health and criminal justice problem in much of the United States (Rawson, Gonzales, & Brethen, 2002). In 2001, an estimated 9.6 million people in the United States had tried methamphetamine at least once (Substance Abuse and Mental Health Services Administration, 2002). Between 1994 and 1999, the number of individuals admitted into publicly funded treatment programs for primary methamphetamine abuse increased by approximately 58% (Substance Abuse and Mental Health Services Administration, 2001). The continued production, procurement, and consumption of methamphetamine represent a serious public health problem that remains to be satisfactorily addressed.
CM has been used effectively to promote abstinence from many drugs of abuse, including abuse of benzodiazepines (e.g., Stitzer, Bigelow, & Liebson, 1979), cocaine (e.g., Higgins, Budney, et al., 1994), nicotine (e.g., Stitzer, Rand, Bigelow, & Mead, 1986), opioids (e.g., Higgins, Stitzer, Bigelow, & Liebson, 1986; Robles, Stitzer, Strain, Bigelow, & Silverman, 2002), marijuana (e.g., Budney, Higgins, Radonovich, & Novy, 2000; Sigmon, Steingard, Badger, Anthony, & Higgins, 2000), and methamphetamine (e.g., Rawson et al., 2002; Roll et al., 2006). Several variables thought to impact CM efficacy, have been studied: type of substance use disorder (e.g., Childress, McLellan, & O’Brien, 1985; Stitzer & Higgins, 1995), type of reinforcer (e.g., Higgins, Budney, et al., 1994; Iguchi, Stitzer, Bigelow, & Liebson, 1988; Petry & Martin, 2002; Schmitz et al., 1998), reinforcement schedule (e.g., Kirby, Marlowe, Festinger, Lamb, & Platt, 1998; Roll & Higgins, 2000; Roll, Higgins, & Badger, 1996), method of distributing reinforcers (e.g., Petry, 2002), delay (e.g., Reilly, Roll, & Downey, 2000; Schwartz, Lauderdale, Montgomery, Burch, & Gallant, 1987), reinforcer magnitude (e.g., Dallery, Silverman, Chutuape, Bigelow, & Stitzer, 2001; Lussier, Heil, Mongeon, Badger, & Higgins, 2006; Roll, Reilly, & Johanson, 2000; Silverman, Chutuape, Bigelow, & Stitzer, 1999; Stitzer & Bigelow, 1985) and population (e.g., Corby, Roll, Ledgerwood, & Schuster, 2000; McNamara, Schumacher, Milby, Wallace, & Usdan, 2001; Roll, Higgins, Steingard, & McGinley, 1998; Shaner et al., 1997). Despite calls for research into the effects of duration of CM procedures (Bigelow, Stitzer, Griffiths, & Liebson, 1981; Crowley, 1999) the topic has not been well studied (Higgins, 1997; Leal & Galanter, 1995).
Review of the CM literature indicates that duration of interventions range from approximately five days for some feasibility trials (e.g., Corby et al., 2000) to 2-3 years (Glosser, 1983; Silverman et al., 2002). Typically, CM interventions are in effect for a fixed period of time (e.g., 8-24 weeks, see Higgins & Silverman, 1999 for a representative sample of trial lengths); however, they can be open ended (e.g., Crowley, 1999). Those CM procedures that use contrived or secondary reinforcers like vouchers, prizes, or money are more likely to be in effect for shorter periods of time than are the ones that utilize more naturalistic reinforcers like methadone dose changes, some types of contingency contracting, access to housing, and employment opportunities. Often the schedule of reinforcement or the magnitude of the reinforcer changes over the course of a given CM protocol (e.g., Higgins, Bickel, & Hughes, 1994); however, even then each phase is generally in effect for a relatively short fixed period of time (e.g., Preston, Umbricht, Wong, & Epstein, 2001).
However, there is a need for studies that systematically vary the duration of CM interventions. There are reports suggesting that short-term CM interventions delivered at crucial times during a treatment episode such as during a transition from an inpatient to an outpatient setting may produce clinically relevant results (e.g., Jones, Haug, Silverman, Stitzer, & Svikis, 2001), although others have suggested that long-term CM may be the most beneficial procedure (e.g., Crowley, 1999). In a meta-analysis of CM in outpatient methadone treatment settings which reviewed 30 studies it was found that 8 studies had CM procedures in effect for 12 weeks or less, 13 studies had the procedures in effect for 12-18 weeks, and 7 studies had the procedures in effect for more than 18 weeks (Griffith, Rowan-Szal, Roark, & Simpson, 2000). The results from the meta-analysis revealed that there were no differential effects of the duration of CM on drug use during treatment, with all durations promoting abstinence while they were in effect. This is not a surprising outcome given the efficacy of CM. Unfortunately, the Griffith et al. (2000) analysis did not report on drug use following the termination of the CM intervention. Drug use following termination of a CM intervention is an important dependent measure to examine because the duration of abstinence engendered with CM may be a moderate subsequent response to other, concurrently available, treatment regimens (e.g., methadone maintenance, psychosocial treatment). This in turn, may influence subsequent drug use behavior.
The current study examined the effects of CM duration when delivered in combination with a psychosocial treatment program in an outpatient setting for the treatment of methamphetamine dependence. The primary aim of this study was to determine whether different durations of an established CM intervention, when delivered in combination with a psychosocial substance abuse treatment program produced different rates of abstinence among methamphetamine dependent individuals. Additionally, we examined effects on abstinence as confirmed by urinalysis (UA) and patterns of relapse in the year following treatment initiation among methamphetamine dependent individuals.
Methods
Study Procedures
Participants were seeking treatment for methamphetamine dependence. Participants randomized into the study must have met all of the following inclusion criteria: (1) 18-65 years of age, (2) met DSM-IV criteria for methamphetamine dependence, (3) were willing and able to comply with study procedures, and (4) were willing and able to provide written informed consent. Additional exclusion criteria included: (1) a medical condition that, in the study PI’s judgment, may have interfered with safe study participation, (2) a recent (past 30 days) history of suicide attempts and/or current serious suicidal intention or plan, and (3) a history of violent criminal behavior or current parole status, and (4) any other circumstances that, in the opinion of the PI, would interfere with study participation. See Figure 1 for flow diagram through the phases of the randomized controlled trial (i.e., enrollment, intervention allocation, follow-up, and data analysis).
Figure 1.
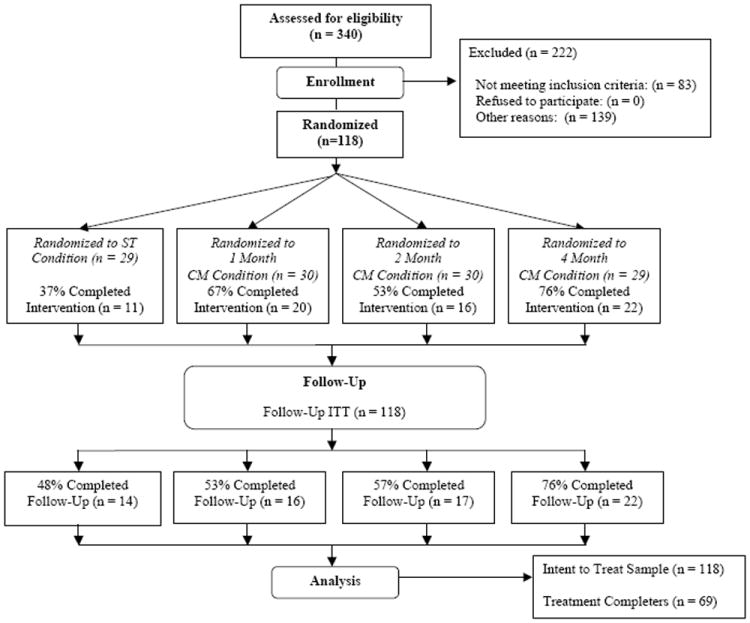
Flow Diagram through the Phases of the Randomized Controlled Trial
Note. ST = Standard Treatment. CM = Contingency Management.
Of the 340 participants assessed for eligibility, a total of 118 participants were eligible for inclusion in the study. Informed consent was obtained and a baseline interview was conducted in which urine samples were collected and participants completed a battery of questionnaires and interviews. The full assessment included measures of drug and alcohol use, physical status, psychological status, and engagement in and compliance with treatment. Participants were randomized to one of the four treatment conditions: Standard psychosocial treatment only, or psychosocial treatment plus one of three durations of CM which was delivered for 1 month, 2 months, or 4 months. Each treatment condition lasted 16 weeks and participants were expected to provide urine samples on a Monday, Wednesday, Friday schedule throughout the course of treatment.
Standard psychosocial treatment consisted of a manualized protocol based on the Matrix Model for methamphetamine abuse (Rawson et al., 1995). The basic elements of the psychosocial treatment approach consisted of a collection of group sessions delivered over a 16-week intensive treatment period. Patients were scheduled three times per week to attend treatment sessions.
The CM intervention used the variable magnitude of reinforcement procedure, frequently referred to as the “fishbowl” technique, which is common in CM research (e.g., Roll et al., 1996). This procedure involved making “draws” from a bowl of chips representing different prize magnitudes. Fifty percent of the chips said “good job” and did not result in the delivery of any tangible reinforcement. 41.8% of the chips resulted in a small reinforcer (worth about $1.00), 8% resulted in a large reinforcer (worth about $20.00) and 0.2% resulted in a jumbo reinforcer (worth about $80.00). The maximum any one person could receive was approximately $500 and the actual maximum payout averaged $250 or less per participant, depending on the percentage of methamphetamine-free urines submitted. Participants in the CM conditions earned at least one draw for each urine sample submitted that was negative for methamphetamine. The number of draws awarded at each urine collection escalated by one chip with consecutive weeks of methamphetamine-negative urine tests (e.g., one draw in week one, two draws in week two). Missing or methamphetamine positive urine samples resulted in a reset to one draw available at the next negative sample submitted. UAs were analyzed for cocaine, opioids, benzodiazepines, and marijuana once per week in addition to methamphetamine; however, the only CM consequence was on the presence or absence of a positive test for methamphetamine. The escalating schedule with a reset contingency has been demonstrated to increase duration of abstinence, but not necessarily decrease probability of relapse (e.g., Roll & Higgins, 2000; Roll et al., 1996).
Participants were contacted to participate in four follow up interviews conducted at 6 months, 8 months, 10 months, and 12 months post randomization. Urine samples were collected and participants completed previously described questionnaires at each follow-up session.
Outcome Measures
Drug use was measured in three ways: 1) longest duration of continuous abstinence during the treatment phase, 2) total percent of UAs indicating abstinence during treatment phase (e.g., 100% and 80%), and 3) proportion of negative methamphetamine UAs submitted across the treatment period (i.e., 3 samples per week for 16 weeks). Given that participants were asked to submit 3 urine specimens per week, each UA represents approximately 2.33 days of abstinence (if negative) or use (if positive). Follow up data was measured by UA result and by whether the participant completed treatment phase of the study or not. Covariates of interest included: age, gender, and ASI composite scores (medical, legal, alcohol, drug, employment, family and psychiatric).
Statistical Analysis
For all baseline comparisons of continuous variables across conditions, ANOVAs were performed. For all baseline comparisons of categorical variables across conditions, chi-square tests were performed. When analyzing continuous outcomes (i.e., longest continuous period of abstinence) across treatment conditions, ANOVAs were performed and Bonferroni correction procedures were implemented for the post hoc tests. The primary analysis involved repeated methamphetamine urine analyses and generalized estimation equations (GEE) (Twisk, 2003). GEE analyses were used for analyzing UA results at follow-up over time as well.
Because substitution methods (i.e., mean substitution) can distort research findings, missing data was handled by imputing a positive methamphetamine UA for those who did not complete the 16-week treatment phase. Patients were allowed to miss one session of treatment per week for approved reasons (e.g., health or child care issues), and thus these missed visits were not considered missing and were not imputed. Given the high percentage of missing data, and the potential confounding of methamphetamine UA by attendance, we also employed multiple imputation procedures to help clarify the analyses. Multiple imputation has several advantages over single imputation or listwise deletion (Schafer & Graham, 2002) in conjunction with GEE analyses and has frequently been used in psychiatric studies with similar levels of missing data (Lapham, Stout, Laxton, & Skipper, 2011; Stein et al., 2004).
Multiple imputation requires the assumption of ‘missing at random.’ This method of dealing with missing data is a more conservative approach compared to the default listwise deletion used in GEE analyses, which assumes ‘missing completely at random’. Preliminary analyses were performed to identify a large set of variables that predicted missing values. We used these variables during the imputation phase of the multiple imputation procedure in order to help ensure that our ‘missing at random’ assumption was tenable. While there is no test for whether missing data are truly ‘missing at random’ versus ‘missing not at random’, our inclusive strategy for auxiliary variables (i.e., variables used during the imputation, but not the analysis phase of multiple imputation) during the imputation phase made for a thorough and consistent treatment of the missing values. Multiple imputation procedures use a regression-based approach to fill in the missing values to produce multiple datasets. In order to maximize the efficiency of our standard errors, 50 datasets were analyzed for each GEE analysis, and we made liberal use of auxiliary variables during the imputation process. Parameters and standard errors were combined using Rubin’s rules (Schafer & Graham, 2002). Lastly, in an additional attempt to help clarify the true relationship between treatment condition and methamphetamine abstinence and treatment attendance, we performed GEE analyses on the treatment completers only. We also conducted post hoc pairwise tests to compare the treatment conditions’ odds ratios to one another in order to assess whether there were significant differences between CM conditions on our primary outcome. Analyses were performed using Stata 11.2 (StataCorp, College Station, TX) and SPSS for Windows 17.0.
Results
Demographics and Baseline Comparisons
No significant (p > .05) differences were found between the treatment conditions on any demographic characteristics. The mean age of participants was 32 (SD = 9.53), and approximately 55% of the sample was male. Table 1 reports the demographics variables for this sample across the different treatment groups. There were also no differences in ASI composite scores across treatment conditions (see Tables 2 and 3). Thus, the randomization of patients across different treatment conditions before the trial began proved effective.
Table 1.
Descriptive statistics of patients with methamphetamine use disorders across treatment conditions with varying CM durations.
| Characteristic | ST (N=29) | 1 Month CM (N=30) | 2 Months CM (N=30) | 4 Months CM (N=29) |
|---|---|---|---|---|
|
| ||||
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Age (years) | 32.8 (10.1) | 31.1 (10.4) | 31.5 (9.3) | 33.5 (8.3) |
| % (N) | % (N) | % (N) | % (N) | |
| Gender | ||||
| Male | 51.7 (15) | 60.0 (18) | 46.7 (14) | 62.1 (18) |
| Female | 48.3 (14) | 40.0 (12) | 53.3 (16) | 37.9 (11) |
| Education | ||||
| Grades 1-8 | 3.5 (1) | 3.3 (1) | 6.7 (2) | 3.5 (1) |
| Grades 9-11 | 20.7 (6) | 13.3 (4) | 23.3 (7) | 17.2 (5) |
| High School Graduate | 34.5 (10) | 43.3 (13) | 26.7 (8) | 34.5 (10) |
| Some College | 34.5 (10) | 40.0 (12) | 40.0 (12) | 44.8 (13) |
| Graduate School | 3.5 (1) | 0.0 (0) | 3.3 (1) | 0.0 (0) |
| Race/Ethnicity | ||||
| Caucasian | 62.0 (18) | 60.0 (18) | 53.3 (16) | 51.7 (15) |
| African American | 3.5 (1) | 0.0 (0) | 6.7 (2) | 0.0 (0) |
| Latino | 34.5 (10) | 33.3 (10) | 36.7 (11) | 41.4 (12) |
| Asian | 0.0 (0) | 6.7 (2) | 3.3 (1) | 0.0 (0) |
| Other | 0.0 (0) | 0.0 (0) | 0.0 (0) | 6.9 (2) |
| Employment | ||||
| Employed for Pay | 24.1 (7) | 26.6 (8) | 53.3 (16) | 34.5 (10) |
| Self-Employed | 13.8 (4) | 3.3 (1) | 0.0 (0) | 6.9 (2) |
| Unemployed > 1 Yr. | 17.2 (5) | 16.7 (5) | 20.0 (6) | 20.7 (6) |
| Unemployed < 1 Yr. | 34.5 (10) | 43.3 (13) | 16.7 (5) | 27.6 (8) |
| Homemaker | 10.3 (3) | 10.0 (3) | 3.3 (1) | 0.0 (0) |
| Unable to Work | 0.0 (0) | 0.0 (30 | 6.7 (2) | 10.3 (3) |
| Income | ||||
| $0 - $9,999 | 41.3 (12) | 36.7 (11) | 40.0 (12) | 48.3 (14) |
| $10,000 - $14,999 | 13.8 (4) | 10.0 (3) | 23.3 (7) | 6.9 (2) |
| $15,000 - $19,999 | 6.9 (2) | 10.0 (3) | 10.0 (3) | 3.5 (1) |
| $20,000 - $24,999 | 17.2 (5) | 6.7 (2) | 10.0 (3) | 13.8 (4) |
| $25,000 - $34,999 | 6.9 (2) | 6.7 (2) | 3.3 (1) | 3.5 (1) |
| $35,000 - $49,999 | 0.0 (0) | 13.3 (4) | 3.3 (1) | 13.8 (4) |
| $50,000 - $74,999 | 10.34 (3) | 0.0 (0) | 6.7 (2) | 6.9 (2) |
| $75,000 + | 3.5 (1) | 16.7 (5) | 3.3 (1) | 3.5 (1) |
| Marital Status | ||||
| Married | 20.7 (6) | 10.0 (3) | 23.3 (7) | 3.5 (1) |
| Divorced | 20.7 (6) | 20.0 (6) | 10.0 (3) | 34.5 (10) |
| Separated | 6.9 (2) | 13.3 (4) | 10.0 (3) | 3.5 (1) |
| Never Married | 34.5 (10) | 46.7 (14) | 56.7 (17) | 51.7 (15) |
| Unmarried Relationship | 17.2 (5) | 10.0 (3) | 0.0 (0) | 6.9 (2) |
Note. ST = Standard Treatment. CM = Contingency Management.
Table 2.
Addiction Severity Index composite scores for patients with methamphetamine use disorders across treatment as usual with varying CM condition at baseline and at the end of treatment.
| Baseline
| |||||
|---|---|---|---|---|---|
| Measures | ST (N=29) | 1 Month CM (N=30) | 2 Months CM (N=30) | 4 Months (N=29) | F value, p value |
| M (SD) | M (SD) | M (SD) | M (SD) | ||
| ASI Medical | 0.057 (0.17) | 0.101 (0.24) | 0.063 (0.18) | 0.062 (0.14) | 0.34, 0.80 |
| ASI Employment | 0.557 (0.30) | 0.552 (0.30) | 0.551 (0.30) | 0.537 (0.28) | 0.14, 0.93 |
| ASI Alcohol | 0.557 (0.30) | 0.080 (0.13) | 0.054 (0.11) | 0.082 (0.22) | 0.23, 0.87 |
| ASI Drug Use | 0.122 (0.10) | 0.112 (0.10) | 0.112 (0.10) | 0.108 (0.28) | 0.11, 0.95 |
| ASI Legal | 0.041 (0.10) | 0.031 (0.10) | 0.087 (0.18) | 0.022 (0.08) | 1.76, 0.16 |
| ASI Family/Social | 0.077 (0.15) | 0.172 (0.26) | 0.087 (0.16) | 0.114 (0.17) | 1.49, 0.22 |
| ASI Psychological | 0.094 (0.16) | 0.103 (0.15) | 0.075 (0.13) | 0.042 (0.09) | 1.19, 0.32 |
|
| |||||
|
End of Treatment
| |||||
| Measures | ST (N=11) | 1 Month CM (N=20) | 2 Months CM (N=16) | 4 Months (N=22) | F value, p value |
| M (SD) | M (SD) | M (SD) | M (SD) | ||
|
| |||||
| ASI Medical | 0.556 (0.18) | 0.108 (0.28) | 0.090 (0.25) | 0.062 (0.14) | 0.21, 0.89 |
| ASI Employment | 0.462 (0.22) | 0.377 (0.29) | 0.538 (0.33) | 0.423 (0.30) | 0.91, 0.44 |
| ASI Alcohol | 0.029 (0.07) | 0.039 (0.11) | 0.031 (0.11) | 0.031 (0.08) | 0.04, 0.94 |
| ASI Drug Use | 0.057 (0.10) | 0.048 (0.08) | 0.053 (0.10) | 0.043 (0.07) | 0.09, 0.97 |
| ASI Legal | 0.041 (0.09) | 0.000 (0.00) | 0.025 (0.07) | 0.024 (0.08) | 0.92, 0.44 |
| ASI Family/Social | 0.165 (0.25) | 0.090 (0.15) | 0.031 (0.07) | 0.057 (0.09) | 2.17, 0.10 |
| ASI Psychological | 0.063 (0.18) | 0.080 (0.13) | 0.037 (0.10) | 0.059 (0.11) | 0.32, 0.81 |
Note. ST = Standard Treatment. CM = Contingency Management. ASI = Addiction Severity Index.
Table 3.
Addiction Severity Index composite score for patients with methamphetamine use disorders across treatment as usual with varying CM conditions at the first follow-up visit and the last follow-up visit.
| First Follow-Up Visit
| |||||
|---|---|---|---|---|---|
| Measures | ST (N=11) | 1 Month CM (N=19) | 2 Months CM (N=22) | 4 Months (N=23) | F value, p value |
| M (SD) | M (SD) | M (SD) | M (SD) | ||
| ASI Medical | 0.084 (0.25) | 0.118 (0.29) | 0.103 (0.27) | 0.061 (0.14) | 0.22, 0.88 |
| ASI Employment | 0.380 (0.23) | 0.416 (0.26) | 0.422 (0.27) | 0.475 (0.35) | 0.31, 0.82 |
| ASI Alcohol | 0.000 (0.00) | 0.074 (0.18) | 0.040 (0.08) | 0.072 (0.18) | 0.87, 0.46 |
| ASI Drug Use | 0.027 (0.05) | 0.020 (0.05) | 0.050 (0.09) | 0.049 (0.09) | 0.82, 0.49 |
| ASI Legal | 0.036 (0.08) | 0.045 (0.09) | 0.009 (0.04) | 0.035 (0.13) | 0.57, 0.64 |
| ASI Family/Social | 0.055 (0.11) | 0.053 (0.10) | 0.078 (0.14) | 0.085 (0.14) | 0.32, 0.81 |
| ASI Psychological | 0.043 (0.09) | 0.113 (0.16) | 0.095 (0.16) | 0.085 (0.14) | 0.57, 0.64 |
|
| |||||
|
Last Follow-up Visit
| |||||
| Measures | ST (N=14) | 1 Month CM (N=16) | 2 Months CM (N=17) | 4 Months (N=22) | F value, p value |
| M (SD) | M (SD) | M (SD) | M (SD) | ||
|
| |||||
| ASI Medical | 0.032 (0.12) | 0.070 (0.17) | 0.111 (0.21) | 0.079 (0.16) | 0.57, 0.64 |
| ASI Employment | 0.248 (0.27) | 0.411 (0.28) | 0.420 (0.32) | 0.421 (0.32) | 1.17, 0.33 |
| ASI Alcohol | 0.064 (0.12) | 0.007 (0.03) | 0.070 (0.18) | 0.069 (0.18) | 0.71, 0.55 |
| ASI Drug Use | 0.017 (0.06) | 0.013 (0.03) | 0.034 (0.08) | 0.043 (0.09) | 0.76, 0.52 |
| ASI Legal | 0.014 (0.05) | 0.000 (0.00) | 0.006 (0.02) | 0.031 (0.11) | 0.77, 0.51 |
| ASI Family/Social | 0.002 (0.01) | 0.050 (0.09) | 0.017 (0.05) | 0.052 (0.14) | 1.21, 0.31 |
| ASI Psychological | 0.013 (0.03) | 0.055 (0.10) | 0.072 (0.16) | 0.027 (0.08) | 1.03, 0.38 |
Note. ST = Standard Treatment. CM = Contingency Management. ASI = Addiction Severity Index.
Retention and Abstinence from Methamphetamines
As with most substance use disorder clinical trials, retention in this investigation was low for all conditions. The difference in retention rates across treatment conditions was statistically significant (χ2 = 9.807, df = 3, p < 0.05). In addition, follow-up pairwise comparisons (assuming a Bonferonni corrected alpha) revealed that the standard treatment group was significantly different from the 4 month CM condition (χ2 = 8.507, df = 1, p < 0.008), but no other comparisons were statistically significant. In this investigation, the retention rates across treatment condition were as follows: 37% for the standard treatment condition (n=11), 67% for the 1 month CM condition (n=20), 53% for the 2 month CM condition (n=16), and 76% for the 4 month CM condition (n=22). Across all treatment conditions, only 1.6% of the sample attended treatment but did not provide a urine sample during the treatment period.
The overall treatment attendance rate was 64.3%. Of those who attended treatment, 97.4% of the total number of UAs submitted was negative for methamphetamine. An ANOVA revealed significant differences across treatment conditions for the number of consecutive days of abstinence; F(3, 114) = 11.183, p < 0.05. In order to further understand where this significant difference was located (i.e., between which CM conditions) we performed Bonferroni post hoc tests. This post hoc analysis performs all possible pairwise comparisons and conservatively adjusts the alpha level (i.e., α/number of tests performed) in order to account for multiple tests being performed. These post hoc tests revealed that each CM condition was significantly different from every other condition, with the exception of the 1 Month CM condition compared to the 2 Month CM condition (see Figure 2).
Figure 2.
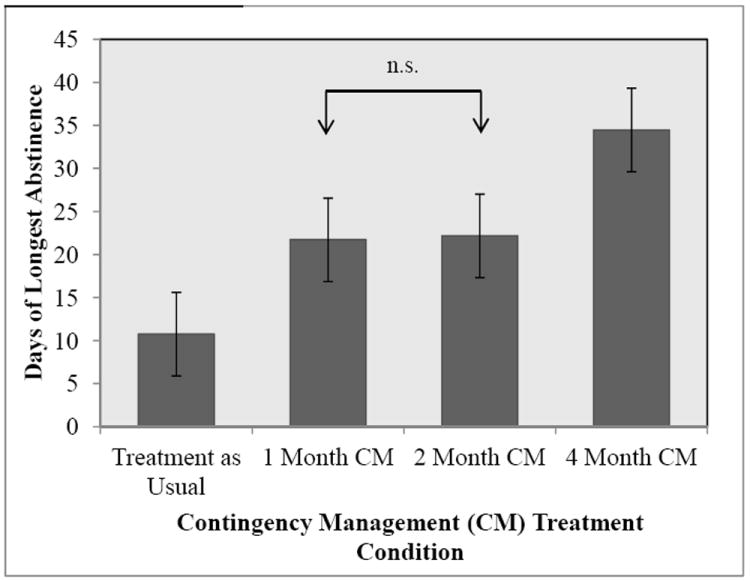
Longest Mean Period of Methamphetamine Abstinence across Initial 16 Weeks of Study (in days).
Note. CM = Contingency Management. Bars represent 95% Confidence Intervals for each of the estimated means.
There was a significant difference across treatment conditions on percent of patients that remained 100% abstinent from methamphetamine throughout the trial; χ2(3) = 10.108, p < 0.05. The trend was in the hypothesized direction, such that 3.4% of the standard treatment condition remained abstinent through the trial, 13.3% of the 1 month CM condition remained abstinent, 20.0% of the 2 month CM condition remained abstinent, and 34.5% of the 4 month CM condition remained abstinent throughout the trial. The only statistically significant result from the follow-up pairwise comparisons (assuming a Bonferonni corrected alpha) revealed that the standard treatment group was significantly different from the 4 month CM condition (χ2 = 9.087, df = 1, p < 0.008).
A similar trend was observed when comparing the percent of subjects in each condition that remained free of methamphetamine for at least 80% of the entire treatment period, χ2(3) = 16.155, p < 0.05. 24.1% of the standard treatment condition, 53.3% of the 1 month CM condition, 43.3% of the 2 month CM condition, and 75.9% of the 4 month CM condition remained abstinent throughout the trial. As demonstrated previous analyses, the only significant result via follow-up pairwise comparisons (assuming a Bonferonni corrected alpha) demonstrated that the standard treatment group was significantly different from the 4 month CM condition (χ2 = 15.517, df = 1, p < 0.008).
Longitudinal Methamphetamine Urine Analysis During Treatment
GEE was used to investigate whether there was a change in methamphetamine abstinence over time (i.e., a total of 48 possible UAs submitted during 16 weeks of treatment trial) and across treatment conditions. The positive UA imputation results revealed that, compared to the standard treatment condition, those in 1 Month CM condition were almost 4 times more likely to submit a negative methamphetamine UA (odds ratio (OR) = 3.58, p < 0.05), the 2 Month CM condition was approximately 2.5 times more likely to submit a negative methamphetamine UA (OR = 2.55, p < 0.05) and the 4 Month CM condition was about 7.25 times more likely to submit a negative methamphetamine UA (OR = 7.25, p < 0.05; see Table 4). Post hoc pairwise tests of the predicted ORs revealed that each CM conditions’ OR was significantly different from one another except for the 1 month CM condition and the 2 month CM condition (p < .05). Figure 3 presents a pictorial description of how individual methamphetamine UAs (averaged across each month of the treatment period for a total of 4 data points per person) changed over time across treatment condition.
Table 4.
Treatment Condition Predicting Negative Methamphetamine Urine Analysis during the Treatment Period and Across Analytic Sample and Missing Data Method.
| Positive UA Imputation | Multiple Imputation | |||
|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| ITT Sample (n = 118) | ||||
| Standard Treatment | 1 | -- | 1 | -- |
| 1 Month CM | 3.58* | 2.41 – 5.32 | 2.64* | 1.56 – 4.47 |
| 2 Month CM | 2.55* | 1.73 – 3.75 | 1.46 | 0.87 – 2.39 |
| 4 Month CM | 7.25* | 4.68 – 11.24 | 2.90* | 1.52 – 5.52 |
|
| ||||
| Completers Only (n = 69) | ||||
| Standard Treatment | 1 | -- | 1 | -- |
| 1 Month CM | 2.10* | 1.40 – 3.16 | 3.08* | 1.24 – 7.63 |
| 2 Month CM | 2.20* | 1.42 – 3.39 | 1.52 | 0.67 – 3.46 |
| 4 Month CM | 9.37* | 2.95 – 6.84 | 5.76* | 1.95 – 17.07 |
Note. CM = Contingency Management.
p < .05.
Figure 3.
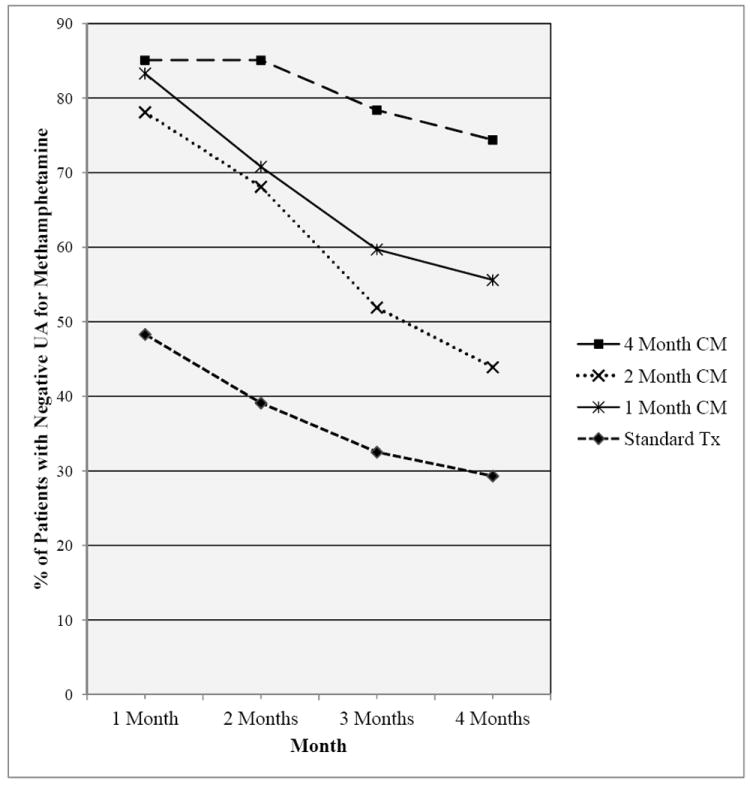
Percent of Negative Methamphetamine UAs across the 4-Month Treatment Period for Patients with Methamphetamine Use Disorders Receiving Standard Treatment or Standard Treatment plus One of Four CM Duration Conditions.
Note. CM = Contingency Management.
When the missing values were handled using the multiple imputation approach, the trend of the ORs followed the same general trend, but were smaller in magnitude. Compared to standard treatment, participants in the 1 month CM condition were approximately 2.5 times more likely to submit a negative methamphetamine UA (OR = 2.64, p < 0.05), those in the 2 month CM condition was not statistically different from standard treatment (OR = 1.46, p > 0.05), and participants in the 4 month CM condition was almost 3 times more likely to submit a negative methamphetamine UA (OR = 2.90, p < 0.05). Post hoc pairwise tests demonstrated that both the 1 month and 4 month CM condition effects were significantly different from the 2 month CM condition (p < 0.05), but they were not significantly different from each other (p > 0.05).
Similar trends were observed when conducting analyses on only those participants that completed treatment, but the magnitude of the effects were larger when the missing values were treated with positive UA imputation and the multiple imputation procedure (see Table 4). For the completers only analysis, post hoc tests for the positive UA imputation analysis demonstrated that each CM condition’s ORs was significantly different from one another except for the 1 month CM condition and the 2 month CM condition (p < .05). Additionally, post hoc tests for the multiple imputation estimates on the treatment completer sample revealed that the 1 month CM OR is not significantly different from either the 2 month CM OR or the 4 month CM OR. However, the 2 month CM OR is significantly different from the 4 month CM condition.
Longitudinal Methamphetamines Urine Analysis and Urine Collection Rates During Follow-Up
The difference in urine collection (i.e., attendance) rates across treatment conditions was statistically significant (χ2 = 13.715, df = 3, p < 0.05) during follow-up. The overall attendance rates across treatment condition were as follows during the 4 month follow-up period: 42% for the standard treatment condition, 53% for the 1 CM condition, 62% for the 2 CM condition, and 64% for the 4 CM condition. Across all treatment conditions, only 1.9% of the sample attended during follow-up but did not provide a methamphetamine negative urine sample. During follow-up, 95.8% of the urine submitted was negative for methamphetamine, but only 55% of the participants attended their follow-ups visits.
We used GEE to investigate change in methamphetamine abstinence (i.e., provision of a negative methamphetamine UA) during the follow-up period. Using the positive UA imputation approach to missing data for the ITT sample, the only significant difference was between the 4 month CM condition and standard treatment (OR = 2.17, p < .05; see Table 5). No other treatment conditions significantly differed (p > 0.05) from standard treatment or from one another, nor was there an effect of longest consecutive days of abstinence during treatment as a predictor of negative methamphetamine UA submission during follow-up (p > 0.05). As shown in detail in Table 5, no other analysis of methamphetamine UAs (i.e., completers only analyses and multiple imputation analyses) during follow-up evidenced any significant differences between the four treatment conditions. See Figure 4 for a pictorial of these two effects across CM conditions.
Table 5.
Treatment Condition Predicting Negative Methamphetamine Urine Analysis During the Follow-Up Period and Across Analytic Sample and Missing Data Method.
| Positive UA Imputation | Multiple Imputation | |||
|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| ITT Sample (n = 118) | ||||
| Standard Treatment | 1 | -- | 1 | -- |
| 1 Month CM | 1.37 | 0.65 – 2.87 | 0.49 | 0.04 – 5.62 |
| 2 Month CM | 1.71 | 0.80 – 3.60 | 0.35 | 0.03 – 3.78 |
| 4 Month CM | 2.17* | 1.01 – 4.66 | 0.41 | 0.40 – 5.74 |
|
| ||||
| Completers Only (n = 69) | ||||
| Standard Treatment | 1 | -- | 1 | -- |
| 1 Month CM | 1.18 | 0.41 – 3.04 | 0.71 | 0.03 – 16.34 |
| 2 Month CM | 2.21 | 0.75 – 6.51 | 0.38 | 0.03 – 5.33 |
| 4 Month CM | 2.52 | 0.36 – 2.52 | 0.53 | 0.04 – 7.17 |
Note. CM = Contingency Management.
p < .05.
Figure 4.
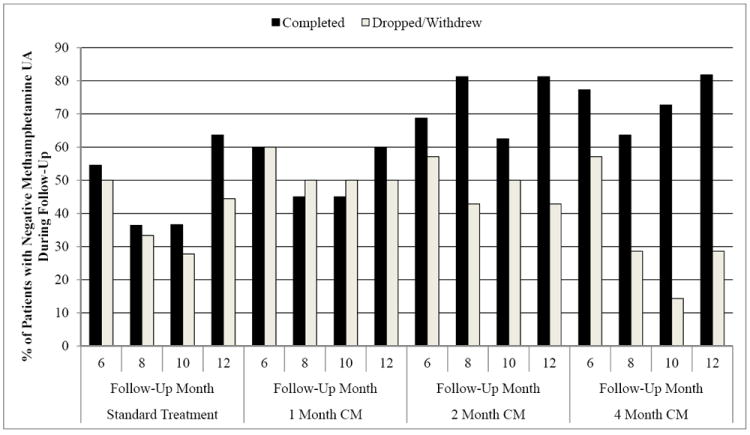
Percent of Patients with Negative Methamphetamine UAs during the Follow-Up Period across Condition and 4-Month Treatment Completion Status Over Time.
Note. CM = Contingency Management.
Discussion
The current study provides information on the effectiveness of varying durations of a CM intervention paired with standard psychosocial treatment, and several findings are of interest. We aimed to determine whether different durations of an established CM intervention (in combination with a psychosocial treatment program) produced different rates of methamphetamine abstinence. Results indicated that attendance, consecutive days of methamphetamine abstinence, and the number of participants who remained 100% abstinent or 80% abstinent throughout the trial increased as CM duration increased. We also observed a change in methamphetamine abstinence over time (i.e., during 16 weeks of treatment trial) and across treatment conditions. Virtually all of the participants who attended treatment also provided a methamphetamine-negative UA. Given the high correlation between attendance and UA-verified abstinence in the current study, it is difficult to completely separate the effect of CM on attendance versus methamphetamine abstinence. While other investigations of a similar type have not always reported the level of overlap between attendance and substance use, this appears to be unusually high relative to previous investigations (Peirce et al., 2006; Petry et al., 2005; Roll et al., 2006).
Additionally, this research aimed to examine the effects on follow-up UA and patterns of relapse in the year following treatment initiation. Rates of attendance were comparable to previous CM studies (Roll et al., 2006) with more participants from the CM conditions attending follow-up sessions overall. Methamphetamine abstinence rates at follow-up were high across conditions. When analyzing the ITT sample, a significant difference was found between the 4-month CM condition and standard treatment indicating that participants in the 4-month CM condition were more likely to attend follow-up sessions and submit a methamphetamine-negative UA than participants in the standard treatment condition. While this finding is promising, additional analyses using the completers only and multiple imputations samples revealed no significant differences between the four treatment conditions. Future research in this area is clearly warranted. Our results provide evidence that the duration of CM intervention has an effect on attendance, which in turn appears to have an effect on abstinence from methamphetamine, and adds to the existing literature on the utility of treating methamphetamine dependence with CM (e.g., Roll et al., 2006). This finding has clear implications for the transfer of CM interventions into community-based treatment settings. All three durations of CM intervention (plus standard psychosocial treatment) implemented in the current study showed significant improvement over treatment as usual, with 4-month CM outperforming the 1-month and 2-month CM groups. Our results add to the body of literature reporting that CM interventions enable participants to initiate and maintain abstinence from methamphetamine (e.g., Prendergast, Podus, Finney, Greenwell, & Roll, 2006), thus enabling them to engage more productively in standard psychosocial treatment services that promote recovery. Treatment providers should view CM as an adjunct to existing empirically-supported psychosocial treatments for substance use disorders that enhances overall effectiveness.
Future research that compares CM durations among equivalent groups should examine additional durations within the zero to four month range (e.g., three months) as well as longer durations (e.g., six months, twelve months). A recent meta-analysis on CM for the treatment of substance use disorders reported that shorter treatment duration produced larger effect sizes in a comparison of CM durations ranging from 1-142 weeks (Prendergast et al., 2006). While the results of this meta-analysis seem to indicate that CM is more effective when administered in shorter rather than longer interventions (perhaps due to the duration of sustained abstinence required among a population with a high relapse rate), this finding may also result from the study characteristics of research analyzed or specific methodological differences (Prendergast et al., 2006). Another meta-analysis found no impact of CM duration (Lussier, Heil, Mongeon, Badger, & Higgins, 2006). Perhaps the differences observed in the present study result from the systematic variation of duration within the same protocol. An examination of longer CM durations using a design similar to the present study would help to determine whether the trend in methamphetamine abstinence observed via one-month, two-month, and four-month CM duration conditions remains consistent when new conditions are introduced or whether effectiveness at maintaining methamphetamine abstinence decreases in longer CM durations. In this sample of one-month, two-month, and four-month CM interventions, longer CM duration appears to be most effective for maintaining methamphetamine abstinence, with significant increases from standard treatment to one- and two-month duration to four-month duration. This finding also has implications for the transfer of CM interventions into treatment settings. The results related to the effectiveness of varying CM durations may be useful in determining the optimal length of CM interventions to be implemented by treatment providers aiming to treat clients with methamphetamine dependence. This is important given the concerns commonly expressed by treatment providers that extended durations of CM may be cost prohibitive, particularly in settings with limited funding. Our results indicate that the shortest duration of CM (one-month) in conjunction with standard psychosocial treatment produced significantly higher rates of methamphetamine abstinence than treatment as usual. The four-month CM intervention condition showed significant increases in abstinence over the one and two month conditions, indicating that community-based treatment settings that have the resources to increase the duration of CM interventions to four months may expect even higher rates of methamphetamine abstinence. While we interpret the finding that ITT participants in the 4-month CM condition were more likely to attend follow-up sessions and submit a methamphetamine-negative UA than participants in the standard treatment condition with caution because no significant differences were found for the completers only and multiple imputations samples, this finding may indicate that those treatment settings that can provide 4-month CM interventions could potentially anticipate increases in follow-up abstinence as well. Additional research in this area appears to be warranted, specifically whether the rates of methamphetamine abstinence produced by the CM durations used in the present study extend to stimulant users with more severe ASI composite scores, a larger sample size, populations dependent on other substances of abuse, or to polysubstance dependent populations.
Highlights.
Treatment attendance, consecutive days of methamphetamine abstinence, and the number of participants who remained 100% abstinent or 80% abstinent throughout the trial increased as Contingency Management (CM) duration (i.e., Standard Care with no CM, 1 month CM, 2 month CM, and 4 month CM) increased.
We also observed an increase in methamphetamine abstinence over time (i.e., during 16 weeks of treatment trial) as CM duration increased, with significant differences between the 4 month CM and standard treatment conditions.
These attendance and abstinence trends became even stronger when analyzing only those who completed treatment.
During follow-up, rates of attendance were comparable to previous CM studies with more participants from the CM conditions attending follow-up sessions overall.
Acknowledgments
The authors wish to acknowledge Arturo Garcia, M.P.H and Sarah Wood, M.A. for their assistance with this project.
This work was supported by NIH RO1 DA170884 to Dr. John Roll when he was at FRI., Inc.
Footnotes
Declaration of Interest: The authors report no real or potential conflict(s) of interest, including financial, personal, or other relationships with other organizations or pharmaceutical/biomedical companies that may inappropriately impact or influence the research and interpretation of the findings.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bigelow GE, Stitzer ML, Griffiths RR, Liebson IA. Contingency management approaches to drug self-administration and drug abuse: efficacy and limitations. Addictive behaviors. 1981;6(3):241–252. doi: 10.1016/0306-4603(81)90022-8. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of consulting and clinical psychology. 2000;68(6):1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP. Behavioral therapies for substance abuse. The International journal of the addictions. 1985;20(6-7):947–969. doi: 10.3109/10826088509047760. [DOI] [PubMed] [Google Scholar]
- Corby EA, Roll JM, Ledgerwood DM, Schuster CR. Contingency management interventions for treating the substance abuse of adolescents: a feasibility study. Experimental and Clinical Psychopharmacology. 2000;8(3):371–376. doi: 10.1037//1064-1297.8.3.371. [DOI] [PubMed] [Google Scholar]
- Crowley TJ. Research on contingency management of drug dependence: Clinical implications and future directions. In: S ST, Higgins K, editors. Motivating behavior change among illicit-drug abusers: Research on contingency management interventions. Washington, D.C.: American Psychological Association; 1999. pp. 345–370. [Google Scholar]
- Dallery J, Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of opiate plus cocaine abstinence in treatment-resistant methadone patients: effects of reinforcer magnitude. Experimental and Clinical Psychopharmacology. 2001;9(3):317–325. doi: 10.1037//1064-1297.9.3.317. [DOI] [PubMed] [Google Scholar]
- Glosser DS. The use of token economy to reduce illicit drug use among methadone maintenance clients. Addictive behaviors. 1983;8(2):93–104. doi: 10.1016/0306-4603(83)90001-1. [DOI] [PubMed] [Google Scholar]
- Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: a meta-analysis. Drug and alcohol dependence. 2000;58(1-2):55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- Higgins ST. The influence of alternative reinforcers on cocaine use and abuse: a brief review. Pharmacology, biochemistry, and behavior. 1997;57(3):419–427. doi: 10.1016/s0091-3057(96)00446-7. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Bickel WK, Hughes JR. Influence of an alternative reinforcer on human cocaine self-administration. Life Sciences. 1994;55(3):179–187. doi: 10.1016/0024-3205(94)00878-7. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry. 1994;51(7):568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Silverman K. Motivating behavior change among illicit-drug abusers: Research on contingency management interventions. Washington, DC: American Psychological Association; 1999. [Google Scholar]
- Higgins ST, Stitzer ML, Bigelow GE, Liebson IA. Contingent methadone delivery: effects on illicit-opiate use. Drug and alcohol dependence. 1986;17(4):311–322. doi: 10.1016/0376-8716(86)90080-3. [DOI] [PubMed] [Google Scholar]
- Iguchi MY, Stitzer ML, Bigelow GE, Liebson IA. Contingency management in methadone maintenance: effects of reinforcing and aversive consequences on illicit polydrug use. Drug and alcohol dependence. 1988;22(1-2):1–7. doi: 10.1016/0376-8716(88)90030-0. [DOI] [PubMed] [Google Scholar]
- Jones HE, Haug N, Silverman K, Stitzer M, Svikis D. The effectiveness of incentives in enhancing treatment attendance and drug abstinence in methadone-maintained pregnant women. Drug and alcohol dependence. 2001;61(3):297–306. doi: 10.1016/s0376-8716(00)00152-6. [DOI] [PubMed] [Google Scholar]
- Kirby KC, Marlowe DB, Festinger DS, Lamb RJ, Platt JJ. Schedule of voucher delivery influences initiation of cocaine abstinence. Journal of consulting and clinical psychology. 1998;66(5):761–767. doi: 10.1037//0022-006x.66.5.761. [DOI] [PubMed] [Google Scholar]
- Lapham SC, Stout R, Laxton G, Skipper BJ. Persistence of Addictive Disorders in a First-Offender Driving While Impaired Population. Archives of General Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal J, Galanter M. The use of contingency contracting to improve outcome in methadone maintenance. Substance Abuse. 1995;16:155–167. [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- McNamara C, Schumacher JE, Milby JB, Wallace D, Usdan S. Prevalence of nonpsychotic mental disorders does not affect treatment outcome in a homeless cocaine-dependent sample. The American journal of drug and alcohol abuse. 2001;27(1):91–106. doi: 10.1081/ada-100103120. [DOI] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2006;63(2):201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Petry NM. Discounting of delayed rewards in substance abusers: relationship to antisocial personality disorder. Psychopharmacology. 2002;162(4):425–432. doi: 10.1007/s00213-002-1115-1. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. Journal of consulting and clinical psychology. 2002;70(2):398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Archives of General Psychiatry. 2005;62(10):1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Wong CJ, Epstein DH. Shaping cocaine abstinence by successive approximation. Journal of consulting and clinical psychology. 2001;69(4):643–654. doi: 10.1037//0022-006x.69.4.643. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Gonzales R, Brethen P. Treatment of methamphetamine use disorders: an update. Journal of substance abuse treatment. 2002;23(2):145–150. doi: 10.1016/s0740-5472(02)00256-8. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Shoptaw SJ, Obert JL, McCann MJ, Hasson AL, Marinelli-Casey PJ, et al. An intensive outpatient approach for cocaine abuse treatment. The Matrix model. Journal of substance abuse treatment. 1995;12(2):117–127. doi: 10.1016/0740-5472(94)00080-b. [DOI] [PubMed] [Google Scholar]
- Reilly MP, Roll JM, Downey KK. Impulsivity and voucher versus money preference in polydrug-dependent participants enrolled in a contingency-management-based substance abuse treatment program. Journal of substance abuse treatment. 2000;19(3):253–257. doi: 10.1016/s0740-5472(00)00105-7. [DOI] [PubMed] [Google Scholar]
- Robles E, Stitzer ML, Strain EC, Bigelow GE, Silverman K. Voucher-based reinforcement of opiate abstinence during methadone detoxification. Drug and alcohol dependence. 2002;65(2):179–189. doi: 10.1016/s0376-8716(01)00160-0. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug and Alcohol Dependence. 2000;58(1-2):103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of applied behavior analysis. 1996;29(4):495–504. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Steingard S, McGinley M. Use of monetary reinforcement to reduce the cigarette smoking of persons with schizophrenia: a feasibility study. Experimental and Clinical Psychopharmacology. 1998;6(2):157–161. doi: 10.1037//1064-1297.6.2.157. [DOI] [PubMed] [Google Scholar]
- Roll JM, Petry NM, Stitzer ML, Brecht ML, Peirce JM, McCann MJ, et al. Contingency management for the treatment of methamphetamine use disorders. The American journal of psychiatry. 2006;163(11):1993–1999. doi: 10.1176/ajp.2006.163.11.1993. [DOI] [PubMed] [Google Scholar]
- Roll JM, Reilly MP, Johanson CE. The influence of exchange delays on cigarette versus money choice: a laboratory analog of voucher-based reinforcement therapy. Experimental and Clinical Psychopharmacology. 2000;8(3):366–370. doi: 10.1037//1064-1297.8.3.366. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- Schmitz JM, Rhoades HM, Elk R, Creson D, Hussein I, Grabowski J. Medication take-home doses and contingency management. Experimental and Clinical Psychopharmacology. 1998;6(2):162–168. doi: 10.1037//1064-1297.6.2.162. [DOI] [PubMed] [Google Scholar]
- Schwartz B, Lauderdale RM, Montgomery ML, Burch EA, Gallant DM. Immediate versus delayed feedback on urinalyses reports for methadone maintenance patients. Addictive behaviors. 1987;12(3):293–295. doi: 10.1016/0306-4603(87)90043-8. [DOI] [PubMed] [Google Scholar]
- Shaner A, Roberts LJ, Eckman TA, Tucker DE, Tsuang JW, Wilkins JN, et al. Monetary reinforcement of abstinence from cocaine among mentally ill patients with cocaine dependence. Psychiatric services. 1997;48(6):807–810. doi: 10.1176/ps.48.6.807. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Steingard S, Badger GJ, Anthony SL, Higgins ST. Contingent reinforcement of marijuana abstinence among individuals with serious mental illness: a feasibility study. Experimental and Clinical Psychopharmacology. 2000;8(4):509–517. doi: 10.1037//1064-1297.8.4.509. [DOI] [PubMed] [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: effects of reinforcement magnitude. Psychopharmacology. 1999;146(2):128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- Silverman K, Svikis D, Wong CJ, Hampton J, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: three-year abstinence outcomes. Experimental and Clinical Psychopharmacology. 2002;10(3):228–240. doi: 10.1037//1064-1297.10.3.228. [DOI] [PubMed] [Google Scholar]
- Stein MD, Solomon DA, Herman DS, Anthony JL, Ramsey SE, Anderson BJ, et al. Pharmacotherapy plus psychotherapy for treatment of depression in active injection drug users. Archives of General Psychiatry. 2004;61(2):152–159. doi: 10.1001/archpsyc.61.2.152. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent reinforcement for reduced breath carbon monoxide levels: target-specific effects on cigarette smoking. Addictive behaviors. 1985;10(4):345–349. doi: 10.1016/0306-4603(85)90030-9. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE, Liebson I. Reducing benzodiazepine self-administration with contingent reinforcement. Addictive behaviors. 1979;4(3):245–252. doi: 10.1016/0306-4603(79)90034-0. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Higgins ST. Behavioral treatment of drug and alcohol abuse. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The fourth generation of progress. New York: Raven Press Ltd; 1995. pp. 1807–1819. [Google Scholar]
- Stitzer ML, Rand CS, Bigelow GE, Mead AM. Contingent payment procedures for smoking reduction and cessation. Journal of applied behavior analysis. 1986;19(2):197–202. doi: 10.1901/jaba.1986.19-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Treatment Episode Data Set (TEDS): 1994-1999. National Admissions to Substance Abuse Treatment Services. Rockville, MD: Office of Applied Studies; 2001. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Summary of Findings from the 2001 National Household Survey on Drug Abuse: Technical Appendices and Selected Data Tables. II. Rockville, MD: Office of Applied Studies; 2002. [Google Scholar]
- Twisk JWR. Applied longitudinal data analysis for epidemiology: A practical guide. Cambridge, UK: Cambridge University Press; 2003. [Google Scholar]


