Abstract
Dipyrone is a common nonopioid analgesic and antipyretic, which, in many countries, is available over the counter and is more widely used than paracetamol or aspirin. However, the exact mechanisms by which dipyrone acts remain inconclusive. Two novel arachidonoyl-conjugated metabolites are formed in mice following the administration of dipyrone that are dependent on the activity of fatty acid amide hydrolase (FAAH), which also represents the major catabolic enzyme of the endogenous cannabinoid ligand anandamide. These arachidonoyl metabolites not only inhibit cyclooxygenase (COX-1/COX-2) but also bind to cannabinoid receptors at low micromolar concentrations. The relative contributions of cannabinoid receptors and FAAH in the overall behavioral response to dipyrone remain untested. Accordingly, the two primary objectives of the present study were to determine whether the behavioral effects of dipyrone would (a) be blocked by cannabinoid receptor antagonists and (b) occur in FAAH−/− mice. Here, we report that thermal antinociceptive, hypothermic, and locomotor suppressive actions of dipyrone are mediated by a noncannabinoid receptor mechanism of action and occurred after acute or repeated administration irrespective of FAAH. These findings indicate that FAAH-dependent arachidonoyl metabolites and cannabinoid receptors are not requisites by which dipyrone exerts these pharmacological effects under noninflammatory conditions.
Keywords: analgesia, antinociception, CB1 and CB2 cannabinoid receptors, dipyrone, fatty acid amide hydrolase, mouse, thermal regulation
Introduction
Dipyrone, a common antipyretic, analgesic, and antispasmodic agent used worldwide, is prohibited in the USA and other countries because of the purported risks of agranulocytosis. However, numerous factors contribute to this condition, which also occurs at relatively comparable frequencies with similar compounds, such as paracetamol and aspirin (Maj and Centkowski, 2004; Ibanez et al., 2005). Although used quite commonly worldwide as an over-the-counter analgesic and antifebrile for almost a century, its mechanism of action remains unknown. Dipyrone is typically not detectable after oral administration, and has a relatively short half-life (Vlahov et al., 1990; Levy et al., 1995). The parent compound and its commonly detected metabolites are relatively low-potency inhibitors of cyclooxygenase (COX-1 and COX-2) isozymes (Campos et al., 1999; Hinz et al., 2007), a common mechanism for most nonsteroidal anti-inflammatory drug. Further evidence shows that dipyrone can produce analgesia through a direct action on the central nervous system (Carlsson et al., 1986; Vanegas et al., 1997).
Interest in the endocannabinoid system as a novel mechanism for mild analgesics began with the discovery of the apparent necessity of cannabinoid receptors in producing paracetamol analgesia (Ottani et al., 2006; Dani et al., 2007). Subsequent investigations showed that an arachidonic acid-conjugated metabolite of paracetamol was formed in the spinal cord and brain of mice through fatty acid amide hydrolase (FAAH), the primary catabolic enzyme regulating the endocannabinoid anandamide. This conjugate, N-(4-hydroxyphenyl)arachidonoylethanol-amide, commonly known as AM404, is an established agonist at vanilloid receptors (TRPV1), enhances the extracellular levels of anandamide, and is an inhibitor of both COX-1 and COX-2 (Hogestatt et al., 2005; Mallet et al., 2008). Although AM404 and cannabinoid receptors appear to be integral to the analgesic effects of paracetamol in preclinical models, the mechanism that produces antipyretic activity remains undetermined (Ayoub et al., 2011).
Similarly, a pair of FAAH-dependent arachidonic acid conjugates are formed in the brain and spinal cord following the administration of dipyrone (Rogosch et al., 2012). These metabolites were modest inhibitors of COX-1 and COX-2, and bound weakly to cannabinoid receptors (CB1/CB2). The first objective of the present study was to determine whether cannabinoid receptors mediate the antinociceptive effects of dipyrone, using the selective CB1 receptor antagonist rimonabant and the CB2 receptor antagonist SR144528. The second goal of this study was to determine whether the analgesic effects of dipyrone were FAAH-dependent, by examining its pharmacological effects after acute or repeated administration in FAAH−/− mice.
Methods
Subjects
Female FAAH+/+ and FAAH−/− littermates of a C57BL6/J background, from the Virginia Commonwealth University (VCU) Center Transgenic Colony, served as subjects. Mice (20–22 g) were housed four mice per cage in a temperature-controlled (20–22°C) and humidity-controlled vivarium, and had free access to food and water. All animal protocols were approved by the VCU Institutional Animal Care and Use Committee.
Procedure
Mice were measured for baseline nociceptive latencies in the hot plate and tail immersion assays set at 52°C, with cutoff times of 30 and 10 s, respectively. Locomotor activity was measured for 15 min in an enclosed open field 40 min following an injection of dipyrone, using webcam-based immobility detection (ANY-maze software; Stoelting Co., Wood Dale, Illinois, USA) with a threshold immobile time of 3 s. Rectal temperature was evaluated at 55 min, followed by a second set of hot plate and tail immersion testing at 60 min. Catalepsy was evaluated using the elevated bar immobility test with a 10 s cutoff. For further procedural details and equipment descriptions, see Long et al. (2009).
Drugs
Dipyrone was diluted in 0.9% saline, whereas rimonabant (10 mg/kg) and SR144528 (10 mg/kg) were dissolved in a vehicle consisting of ethanol, emulphor, and saline at a ratio of 1 : 1 : 18. The dose of each receptor antagonist used exceeded the dose sufficient to completely reverse the effects of FAAH (Cravatt et al., 2001; Naidu et al., 2010) or MAGL (Schlosburg et al., 2010) inhibitors in mice. In addition, these doses of rimonabant and SR144528 fully reversed the behavioral effects exerted by agonists acting directly at CB1 (Booker et al., 2009; Schlosburg et al., 2010) and CB2 (Kinsey et al., 2011) receptors, respectively. All drug solutions were prepared ~30 min before injection, and administered intraperitoneally in a volume of 10 ml/kg body weight. The antagonists were administered 15 min before dipyrone.
Statistics
Statistical analyses were carried out using either one-way or two-way analysis of variance, with drug and/or genotype as factors. Dunnett's or Tukey's post-hoc tests were carried out on significant analysis of variance to determine individual group differences.
Results
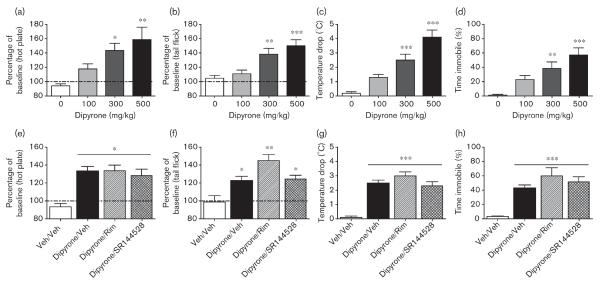
An initial experiment evaluated the acute behavioral effects of dipyrone using a traditional cannabimimetic `tetrad' battery of tests (antinociception, catalepsy, hypothermia, and locomotor suppression), which reflect pharmacological indices highly sensitive to THC and other cannabinoid receptor agonists. Dipyrone (0, 100, 300, and 500 mg/kg) increased both hot plate [F(3,20)=7.1, P<0.001; Fig. 1a] and tail immersion [F(3,20)=10.4, P<0.001; Fig. 1b] latencies, in a dose-responsive manner, by ~50–60% above the baseline. Dipyrone also produced a 3–5°C hypothermic effect [F(3,20)=25.1, P<0.001; Fig. 1c] and decreased open-field locomotor activity ~40–60% [F(3,20)=10.6, P<0.001; Fig. 1d]. The 300 and 500 mg/kg doses differed significantly from the vehicle (Dunnett's post-hoc; P<0.05). However, there were no signs of catalepsy in the bar test (data not shown), and, accordingly, this assay was not used in subsequent experiments. At 6 h following the injection, none of the treatment groups differed significantly from the vehicle (data not shown).
Fig. 1.
Acute behavioral effects of dipyrone are dose dependent, but independent of cannabinoid receptors. Dipyrone showed dose-responsive (0–500 mg/kg) antinociception in hot plate (a) and tail flick (b) latency, while also decreasing body temperature (c) and reducing overall open-field activity (d). Using an active dose of dipyrone, none of these behaviors were altered by the CB1 receptor antagonist, rimonabant (10 mg/kg), or the CB2 receptor antagonist, SR144528 (10 mg/kg). Dipyrone (300 mg/kg) produced significant antinociception, represented by increases from baseline in the (e) hot plate and (f) tail flick assays. The average baseline latencies across all groups in hot plate and tail flick tests were 11.7±0.3 and 2.1±0.1 s, respectively. (g) Dipyrone also led to a substantial decrease in body temperature (baseline: 37.7±0.2°C) and decreased the level of (h) locomotor activity in an open field. However, these differences were still present in both groups concurrently treated with a CB1 or a CB2 antagonist. *P<0.05, **P<0.01, ***P<0.001 versus vehicle (Veh) control on the basis of Dunnett's (dose–response) or Tukey's (antagonist) post-hoc tests. Data are represented as mean±SEM.
The second experiment examined whether the CB1 receptor antagonist rimonabant (10 mg/kg) or the CB2 receptor antagonist SR144528 (10 mg/kg) would reverse the acute pharmacological effects of dipyrone (300 mg/kg). All dipyrone-treated groups showed significant increases in hot plate latency [F(3,20)=11.9, P<0.001; Fig. 1e], tail withdrawal latency [F(3,20)=10.8, P<0.001; Fig. 1f], hypothermia [F(3,20)=10.1, P<0.001; Fig. 1g], and open-field inactivity [F(3,20)=13.6, P<0.001; Fig. 1h], but neither cannabinoid receptor antagonist significantly attenuated any of these effects.
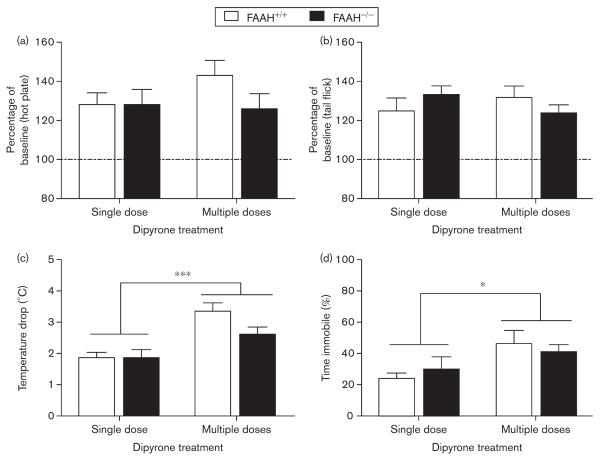
In the final experiment, FAAH+/+ and FAAH−/− mice were administered a daily injection of either saline or dipyrone (300 mg/kg) for 4 days. On day 5, the impact of acute and repeated dipyrone on antinociception, body temperature, and locomotor activity was compared. Five days of dipyrone administration in drinking water led to increased levels of the arachidonoyl-conjugated metabolites in wild-type mice, but they were minimally detectable in FAAH−/− mice (Rogosch et al., 2012), allowing us to test indirectly the relative contributions of these metabolites in the behavioral activity of dipyrone. FAAH+/+ and FAAH−/− mice showed essentially identical dipyrone-induced antinociception in the tail withdrawal (Fig. 2a) and hot plate (Fig. 2b) tests, irrespective of whether dipyrone had been administered acutely or repeatedly. Mice treated repeatedly with dipyrone showed an increased magnitude of hypothermia [Fig. 2c; F(1,28)=22.0, P<0.001] and hypomotility [Fig. 2d; F(1,28)=6.6, P<0.05] compared with the acute treatment group. However, no genotype differences were observed with these measures.
Fig. 2.
The efficacy of dipyrone following acute or repeated administration is mediated through an FAAH-independent mechanism. FAAH+/+ and FAAH−/− mice were evaluated in a variety of cannabimimetic endpoints following either acute (300 mg/kg) or repeated (5 days × 300 mg/kg) administration of dipyrone. (a) Hot plate (baseline: 11.5±0.4 s) and (b) tail flick (baseline: 1.5±0.1 s) assays were unaltered in magnitude because of the length of dipyrone treatment and were unaffected by the FAAH genotype. (c) The magnitude of hypothermia (baseline: 37.7±0.2°C) was greater in mice repeatedly treated with dipyrone, but was not different on the basis of FAAH genotype. Similar results were observed for (d) open-field activity. *P<0.05, ***P<0.001 between acute and multiple dipyrone dosing on the basis of significant one-way analysis of variance. Data are represented as mean±SEM. FAAH, fatty acid amide hydrolase.
Discussion
On the basis of a previous report that dipyrone arachidonoyl-conjugated metabolites are produced through a FAAH-dependent pathway in a manner analogous to those seen following paracetamol (Mallet et al., 2008), we examined the contribution of the endocannabinoid system in dipyrone-induced antinociception. The respective CB1 and CB2 receptor antagonists rimonabant and SR144528 failed to block the antinociceptive, hypothermic, and locomotor inhibitory actions of dipyrone. Also, unlike substances acting at cannabinoid CB1 receptors, dipyrone did not elicit catalepsy. Given that there was no inflammatory insult promoting the tested behaviors, it was unlikely that dipyrone activity was dependent on the activation of CB2 receptors, which are predominantly expressed in immune cells. In contrast to paracetamol (Ottani et al., 2006), CB1 receptors do not play a necessary role in dipyrone-induced antinociception.
Despite the lack of cannabinoid receptor involvement in dipyrone-induced antinociception, and the disparate duration of time required to accumulate sufficient levels of arachidonoyl-conjugated metabolites, it is plausible that these metabolites induce CB1 receptor-mediated antipyretic, antispasmodic, anti-inflammatory, or anti-hyperalgesic actions. In particular, the arachidonoyl conjugate found in paracetamol, AM404, also binds to TRPV1 receptors, which may contribute to its antinociceptive effects (Yue et al., 2004; Mallet et al., 2010). Indeed, as one of these derivatives is also a relatively potent antagonist of TRPV1 channels, which are upregulated during inflammation and participate in pain transmission (White et al., 2011), future studies using inflammatory models of pain could address this possibility. For example, a recent study using the carrageenan model of inflammatory pain in rats reported that microinjection of dipyrone into the periaqueductal grey reduced sensory nerve excitability through a CB1 receptor mechanism of action (Escobar et al., 2011). However, it is unclear whether an intracerebral injection of the parent compound results in the significant formation of any metabolites in the brain that are typical of systemic administration.
In the present study, we used FAAH−/− mice to test indirectly whether dipyrone produces antinociceptive, hypothermic, and locomotor inhibitor effects through its arachidonoyl-conjugated metabolites. The observations that FAAH−/− mice showed no alterations in these pharmacological effects of dipyrone, administered either acutely or following repeated administration, argue against the involvement of these metabolites. Although the results of the current study suggest that these metabolites and cannabinoid receptor activity are not necessary for the thermal antinociceptive effects of dipyrone, it is possible that the FAAH-dependent arachidonoyl-conjugates and cannabinoid receptors contribute toward the other actions of dipyrone that would be found in inflammatory pain models. In conclusion, these data indicate divergent pharmacological mechanisms for dipyrone and paracetamol, and will require further study to understand how one of the world's most commonly used drugs exerts its effects.
Acknowledgements
Funding for the enclosed research was supported by National Institute on Drug Abuse grants P01DA009789, P01DA017259, F31DA026279, and T32DA007027.
Footnotes
Conflicts of interest There are no conflicts of interest.
References
- Ayoub SS, Pryce G, Seed MP, Bolton C, Flower RJ, Baker D. Paracetamol-induced hypothermia is independent of cannabinoids and transient receptor potential vanilloid-1 and is not mediated by AM404. Drug Metab Dispos. 2011;39:1689–1695. doi: 10.1124/dmd.111.038638. [DOI] [PubMed] [Google Scholar]
- Booker L, Naidu PS, Razdan RK, Mahadevan A, Lichtman AH. Evaluation of prevalent phytocannabinoids in the acetic acid model of visceral nociception. Drug Alcohol Depend. 2009;105:42–47. doi: 10.1016/j.drugalcdep.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos C, de Gregorio R, Garcia-Nieto R, Gago F, Ortiz P, Alemany S. Regulation of cyclooxygenase activity by metamizol. Eur J Pharmacol. 1999;378:339–347. doi: 10.1016/s0014-2999(99)00477-x. [DOI] [PubMed] [Google Scholar]
- Carlsson KH, Helmreich J, Jurna I. Activation of inhibition from the periaqueductal grey matter mediates central analgesic effect of metamizol (dipyrone) Pain. 1986;27:373–390. doi: 10.1016/0304-3959(86)90161-2. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. PNAS. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani M, Guindon J, Lambert C, Beaulieu P. The local antinociceptive effects of paracetamol in neuropathic pain are mediated by cannabinoid receptors. Eur J Pharmacol. 2007;573:214–215. doi: 10.1016/j.ejphar.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Escobar W, Ramirez K, Avila C, Limongi R, Vanegas H, Vazquez E. Metamizol, a non-opioid analgesic, acts via endocannabinoids in the PAGRVM axis during inflammation in rats. Eur J Pain. 2012;16:676–689. doi: 10.1002/j.1532-2149.2011.00057.x. [DOI] [PubMed] [Google Scholar]
- Hinz B, Cheremina O, Bachmakov J, Renner B, Zolk O, Fromm MF, et al. Dipyrone elicits substantial inhibition of peripheral cyclooxygenases in humans: new insights into the pharmacology of an old analgesic. FASEB J. 2007;21:2343–2351. doi: 10.1096/fj.06-8061com. [DOI] [PubMed] [Google Scholar]
- Hogestatt ED, Jonsson BA, Ermund A, Andersson DA, Bjork H, Alexander JP, et al. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005;280:31405–31412. doi: 10.1074/jbc.M501489200. [DOI] [PubMed] [Google Scholar]
- Ibanez L, Vidal X, Ballarin E, Laporte JR. Agranulocytosis associated with dipyrone (metamizol) Eur J Clin Pharmacol. 2005;60:821–829. doi: 10.1007/s00228-004-0836-y. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Mahadevan A, Zhao B, Sun H, Naidu PS, Razdan RK, et al. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology. 2011;60:244–251. doi: 10.1016/j.neuropharm.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Zylber-Katz E, Rosenkranz B. Clinical pharmacokinetics of dipyrone and its metabolites. Clin Pharmacokinet. 1995;28:216–234. doi: 10.2165/00003088-199528030-00004. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj S, Centkowski P. A prospective study of the incidence of agranulocytosis and aplastic anemia associated with the oral use of metamizole sodium in Poland. Med Sci Monit. 2004;10:PI93–PI95. [PubMed] [Google Scholar]
- Mallet C, Daulhac L, Bonnefont J, Ledent C, Etienne M, Chapuy E, et al. Endocannabinoid and serotonergic systems are needed for acetaminophen-induced analgesia. Pain. 2008;139:190–200. doi: 10.1016/j.pain.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Mallet C, Barriere DA, Ermund A, Jonsson BA, Eschalier A, Zygmunt PM, et al. TRPV1 in brain is involved in acetaminophen-induced antinociception. PloS One. 2010;5:9. doi: 10.1371/journal.pone.0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Kinsey SG, Guo TL, Cravatt BF, Lichtman AH. Regulation of inflammatory pain by inhibition of fatty acid amide hydrolase. J Pharmacol Exp Ther. 2010;334:182–190. doi: 10.1124/jpet.109.164806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur J Pharmacol. 2006;531:280–281. doi: 10.1016/j.ejphar.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Rogosch T, Sinning C, Podlewski A, Watzer B, Schlosburg J, Lichtman AH, et al. Novel bioactive metabolites of dipyrone (metamizol) Bioorg Med Chem. 2012;20:101–107. doi: 10.1016/j.bmc.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas H, Tortorici V, Eblen-Zajjur A, Vasquez E. PAG-microinjected dipyrone (metamizol) inhibits responses of spinal dorsal horn neurons to natural noxious stimulation in rats. Brain Res. 1997;759:171–174. doi: 10.1016/s0006-8993(97)00360-0. [DOI] [PubMed] [Google Scholar]
- Vlahov V, Badian M, Verho M, Bacracheva N. Pharmacokinetics of metamizol metabolites in healthy subjects after a single oral dose of metamizol sodium. Eur J of Clin Pharmacol. 1990;38:61–65. doi: 10.1007/BF00314805. [DOI] [PubMed] [Google Scholar]
- White JP, Urban L, Nagy I. TRPV1 function in health and disease. Curr Pharm Biotechnol. 2011;12:130–144. doi: 10.2174/138920111793937844. [DOI] [PubMed] [Google Scholar]
- Yue HY, Fujita T, Kawasaki Y, Kumamoto E. AM404 enhances the spontaneous release of l-glutamate in a manner sensitive to capsazepine in adult rat substantia gelatinosa neurones. Brain Res. 2004;1018:283–287. doi: 10.1016/j.brainres.2004.05.090. [DOI] [PubMed] [Google Scholar]