Abstract
The first total synthesis of stereochemically pure resolvin D3 and aspirin-triggered resolvin D3 is reported. These enzymatic metabolites of docosahexaenoic acid (DHA) have potent anti-inflammatory and pro-resolving actions. The convergent synthetic strategy is based on enantiomerically pure starting materials and it is highly stereocontrolled.
Recent investigations on the resolution of inflammation have revealed the key regulatory roles of some new types of lipid metabolites produced locally via enzymatic pathways from polyunsaturated fatty acids.1 These studies identified a number of anti-inflammatory and pro-resolving lipid mediators,2 including the lipoxins3 formed by arachidonic acid, and the E-series resolvins obtained from eicosapentaenoic acid (EPA).1,4 Several related molecules derived from docosahexaenoic acid (DHA) have also been identified, including the D-series resolvins,1,5 the protectins,1,6 and the maresins.7 Notably, the discovery and study of these potent metabolites of EPA and DHA, which are important omega-3 fatty acids, provided key molecular and biological insights for their well-known benefits against inflammatory diseases.8
An important aspect of these lipid mediators is that they are formed biosynthetically via enzymatic processes, and are often extremely potent (nM to pM range), acting via specific receptors and signaling pathways. Despite some common features, each type of these molecules has a unique structure and distinct biological role. They usually contain multiple C=C bonds with defined Z/E geometry, as well as several hydroxy substituents with specific R/S configurations. Since they are produced in very small quantities in vitro or in vivo, their complete stereochemical characterization requires direct matching with stereochemically pure materials produced via total synthesis. These synthetic efforts are also essential for elucidating the biological functions of these molecules.
Thus, we have recently developed the first total synthesis that led to the first stereochemical and biological characterization for several of these new lipid mediators.1 Some of our efforts1 with resolvin E1 (RvE1),9 resolvin D1 (RvD1),10 resolvin D2 (RvD2),11 neuroprotectin D1/protectin D1 (NPD1/PD1),12 and maresin 1 (MaR1)13 have been disclosed, while additional studies will be reported in due course.
Herein, we detail our synthetic work towards resolvin D3 (RvD3), an important member of the D-series resolvins1,5 with potent pro-resolving properties, such as reduction of human neutrophil transendothelial migration.5 Notably, the endogenous production of RvD3 was shown to be elevated during ischemic injury of the kidney,14 and in a colitis model with fat-1 transgenic mice15 that produce increased levels of DHA.
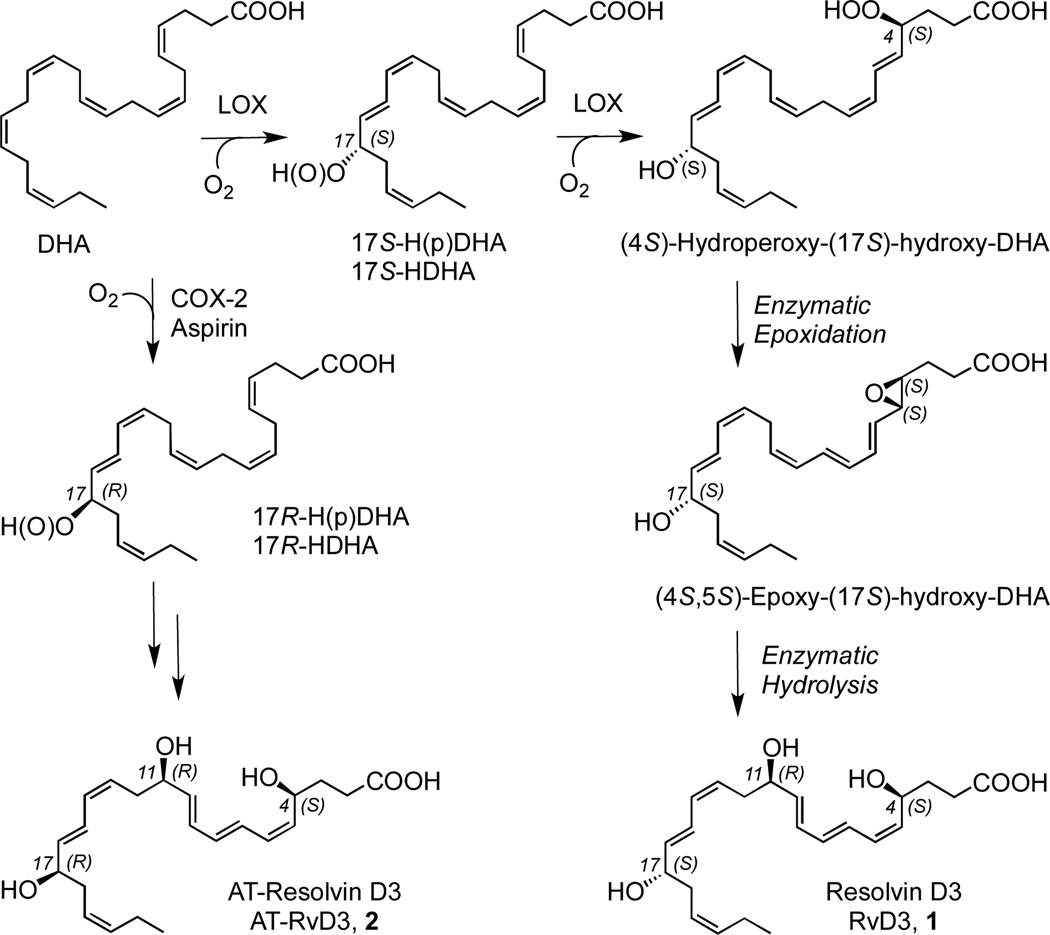
The postulated biosynthesis1,5 of RvD3 from DHA is summarized in Scheme 1. The first step involves the conversion of DHA to (17S)-hydroperoxy DHA (17S-HpDHA) catalyzed by a lipoxygenase enzyme (LOX), such as 15-LOX. A second lipoxygenation of this intermediate or its reduced hydroxy product (17S-HDHA) leads to a new hydroperoxide at the C-4 position, which is converted enzymatically to the 4S,5S epoxide that undergoes enzymatic hydrolysis to form RvD3 1.
Scheme 1.
Biosynthesis of resolvin D3 and AT-resolvin D3
A related biosynthetic pathway1,5 involves the initial oxygenation of DHA with COX-2 in the presence of aspirin to form the (17R)-hydroperoxy DHA (17R-HpDHA) and its reduced hydroxy product 17R-HDHA. Similar lipoxygenation and further transformation of these 17R metabolites leads to the (17R)-epimer of RvD3, named aspirin-triggered RvD3 or AT-RvD3 2.1,5
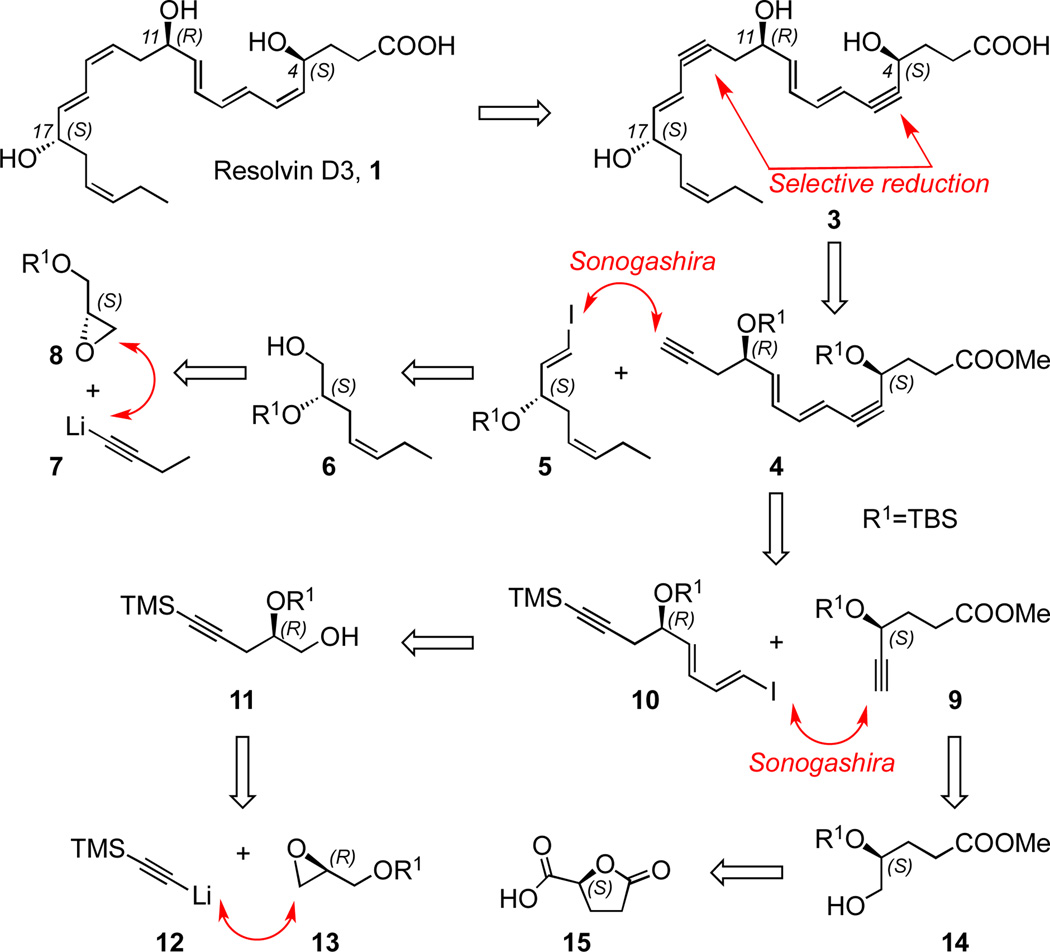
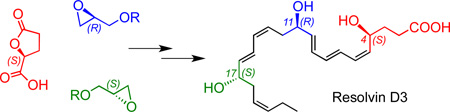
Our convergent and stereocontrolled synthetic strategy for RvD3 1 is outlined in Scheme 2. While the structures of RvD3 and AT-RvD3 were initially deduced by using mass spectrometry,5 their detailed Z/E geometry and R/S configuration remained to be established. Towards this goal, and based on the above biosynthetic hypothesis, we first targeted the total synthesis of the RvD3 isomer shown in Scheme 1, namely (4S,11R,17S)-trihydroxy-5Z,7E,9E,13Z,15E,19Z-docosahexaenoic acid.
Scheme 2.
Retrosynthetic analysis of resolvin D3
In order to prevent Z/E isomerization, our aim was to follow a synthetic route allowing for the sensitive triene and diene moieties to be formed in the final steps of the synthesis. By taking advantage of a mild Zn/Cu/Ag alkyne hydrogenation process16 at the final stage of the synthesis, we envisioned a selective reduction of the bis-acetylenic precursor 3 to create both the 5Z,7E,9E-triene and 13Z,15E-diene units of 1. For the synthesis of 3 we relied on the highly efficient Pd-catalyzed Sonogashira coupling reaction17 of alkyne 4 and vinyl halide 5, which is available from alcohol 6 via oxidation and olefination. Compound 6 can be produced in stereochemically pure form starting with the addition of alkynyl lithium 7 to enantiomerically pure (S)-glycidol derivative 8. The synthesis of the key intermediate 4 involves another Sonogashira reaction17 between alkyne 9 and E,E-dienyl iodide 10, which can be prepared from alcohol 11 that is available via the addition of alkynyl lithium 12 to (R)-glycidol derivative 13. Finally, the synthesis of the alkyne intermediate 9 relies on precursor 14, which is accessible from the commercially available chiral starting material (S)-γ-carboxy butyrolactone 15.
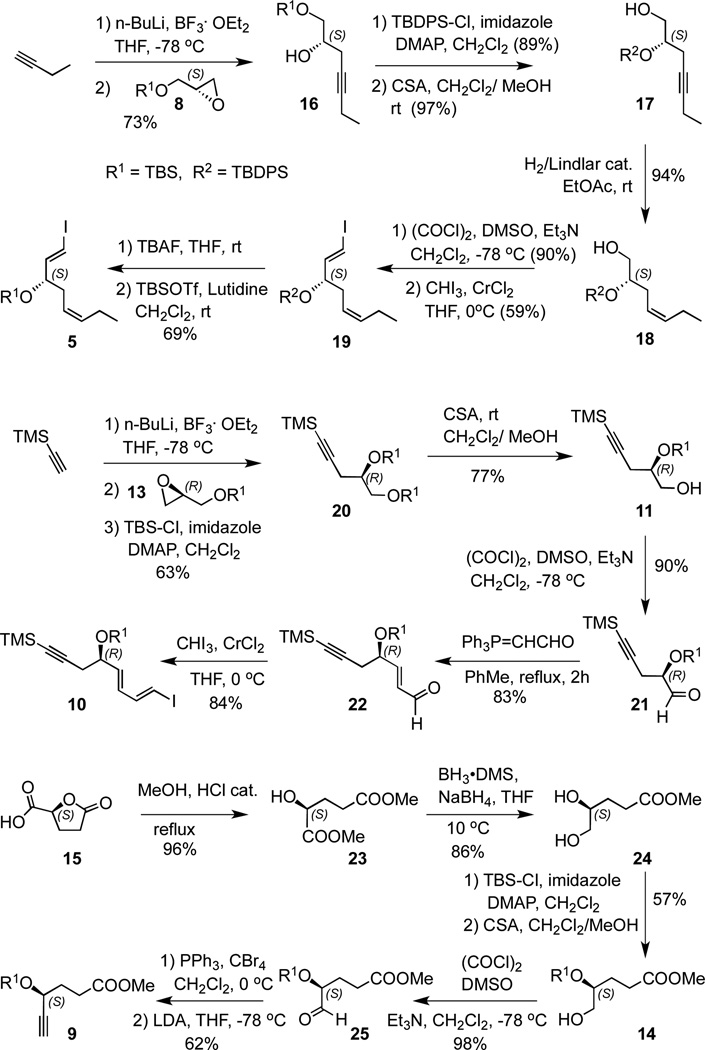
Scheme 3 shows the detailed synthesis of the three key intermediates, alkenyl iodides 5 and 10, and alkyne 9. The synthesis of 5 begun with the addition of lithiated 1-butyne to the (S)-epoxide 8 in the presence of boron trifluoride to afford alcohol 16. Silylation with TBDPS and clean deprotection of the primary alcohol with camphorsulfonic acid (CSA) gave 17, which was selectively hydrogenated under Lindlar conditions to afford intermediate 18. Swern oxidation18 of this alcohol to the corresponding aldehyde, followed by Takai olefination19 led to the vinyl iodide 19. Although this TBDPS-protected compound can be utilized in the final assembly of RvD3, we have found that the final TBDPS removal from the bis-alkynyl product with TBAF leads to product decomposition. Therefore, we converted 19 to the corresponding TBS-protected intermediate 5.20
Scheme 3.
Synthesis of key intermediates 5, 9 and 10
The synthesis of the dienyl iodide 10 relied on the addition of lithiated TMS-acetylene to the (R)-protected glycidol 13, followed by TBS protection to give 20, Selective removal of the primary TBS group converted 20 to alcohol 11. Oxidation under Swern conditions,18 followed by Wittig olefination with triphenylphosphoranylidene acetaldehyde afforded the α,β-unsaturated aldehyde 22. Takai olefination19 of 22 afforded 10 as a labile 9:1 mixture of E/Z alkenyl iodides in 84% yield.
Alkyne 9 was prepared from (S)-γ-carboxy butyrolactone 15,21 starting with ring opening under acid-catalyzed methanol treatment to give the diester 23 in high yield. Selective reduction of the ester adjacent to the hydroxyl group was achieved with borane dimethyl sulfide and a catalytic amount of sodium borohydride to give diol 24.22 TBS di-protection, followed by selective removal of the primary TBS group gave alcohol 14. Swern oxidation,18 afforded aldehyde 25 in very high yield. The direct transformation of 25 to alkyne 9 was achieved via the Corey-Fuchs homologation,23 by using LDA as the base to preserve the integrity of the ester moiety.24 An alternative approach for this transformation employing the Bestmann-Ohira homologation,25 afforded the product in lower yields and with the added disadvantage of multistep preparation of the reagent.
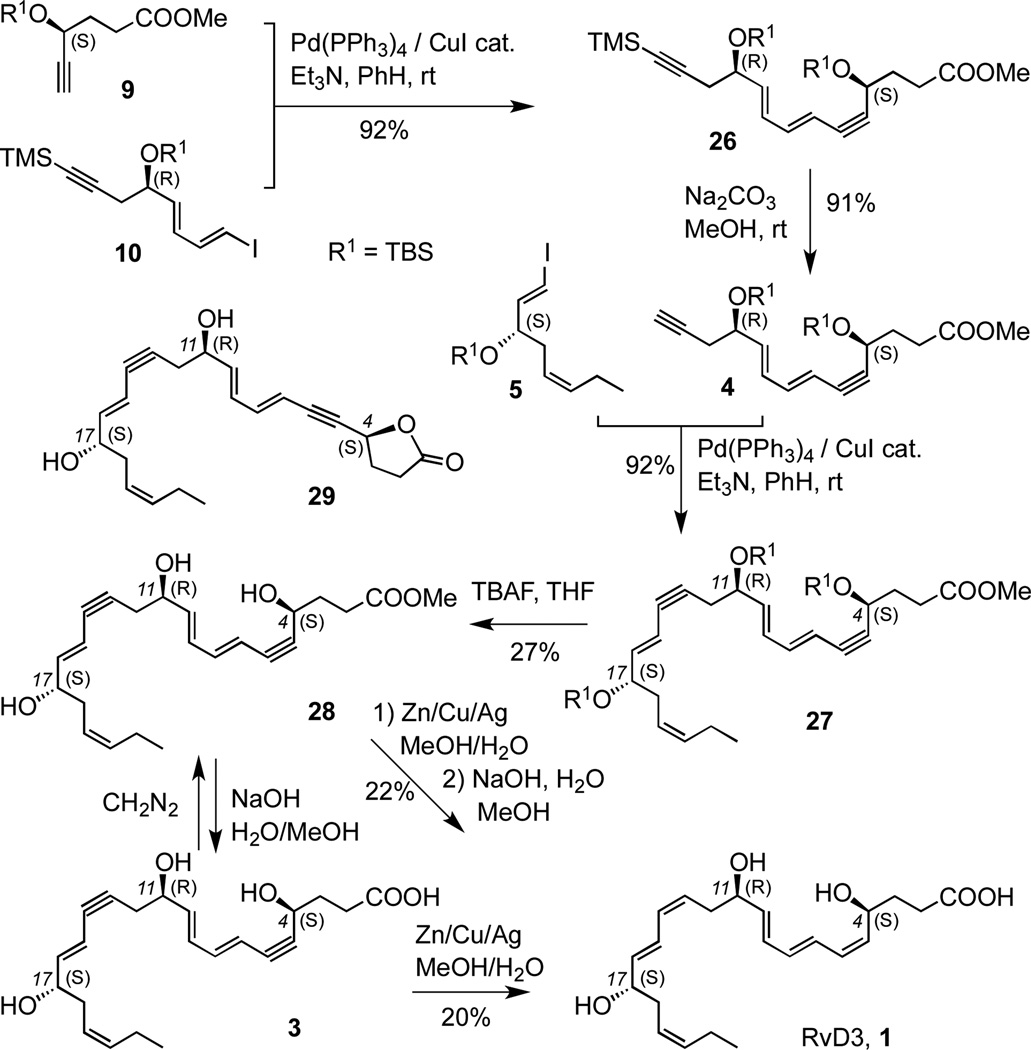
The final stages of the synthesis of RvD3 from intermediates 5, 9 and 10 are shown in Scheme 4. Sonogashira coupling17 of alkyne 9 with dienyl iodide 10 afforded isomerically pure product 26 in excellent yield. Removal of the TMS group gave the terminal alkyne 4. The second Sonogashira reaction17 between 4 and vinyl iodide 5 to form 27 also proceeded in high yield. However, the removal of the silyl protective groups was problematic, forming the expected ester derivative 28 in a 3:1 ratio with lactone 29, as well as the free acid 3 which was esterified with diazomethane back to 28. Although this type of desilylation has been more efficient in other related lipid derivatives,1 presumably in 27 the closer proximity of the C-4 hydroxyl group with the ester moiety favors such side reactions. Treatment of 28 with freshly prepared Zn/Cu/Ag amalgam16 led to the selective hydrogenation of both alkyne groups to the Z-alkenes, but still formed a mixture of ester and lactone products. Basic hydrolysis followed by HPLC purification led to a single stereoisomer of RvD3 1, albeit in low yield. Direct hydrogenation of compound 3 gave similar results.
Scheme 4.
Synthesis of RvD3 from intermediates 5, 9 and 10
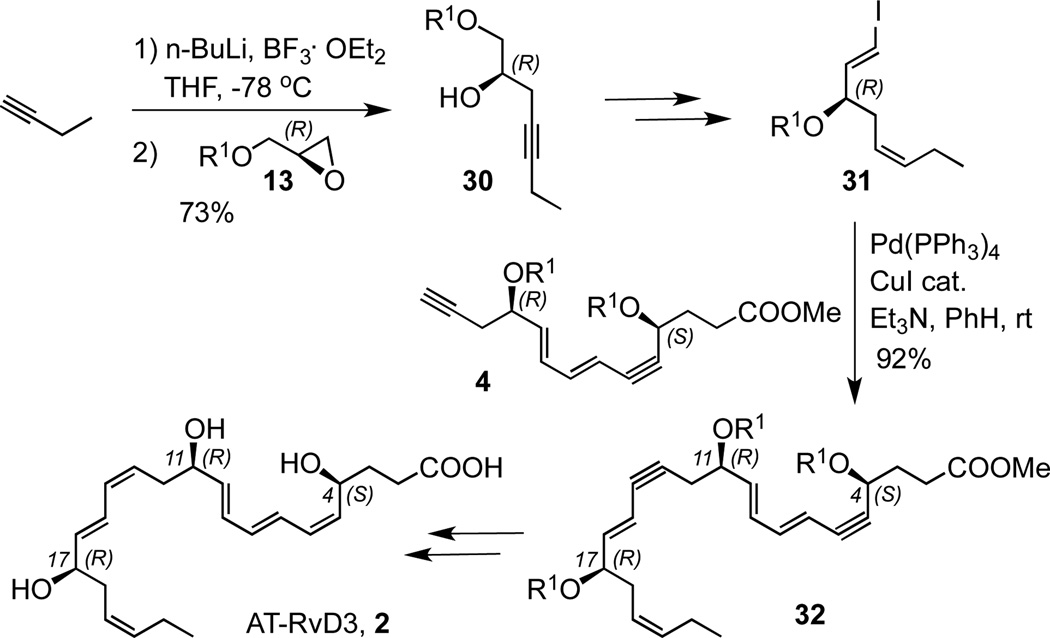
The same type of strategy was employed for the total synthesis of AT-RvD3 2, summarized in Scheme 5. The (R)-glycidol derivative 13 was converted to alcohol 30 which was transformed to the vinyl iodide 31. Sonogashira coupling of 31 with alkyne 4 gave the bis-acetylenic compound 32, which was deprotected and hydrogenated, as above, to afford AT-RvD3 2.
Scheme 5.
Total synthesis of AT-resolvin D3 (2)
The structure and complete stereochemistry of RvD3 1 and AT-RvD3 2 were confirmed by LC/MS and NMR, including 600MHz 2-D COSY. The spectroscopic and biological properties of these synthetic compounds, matched those of biogenically-derived molecules from DHA, and helped to unambiguously establish their Z/E and R/S configurations for the first time.26
Using synthetic materials, the biological profile of this type of lipid mediator has been investigated further. Preliminary data revealed that RvD3 1 and AT-RvD3 2 are potent immunoresolvents, regulating neutrophil actions, and promoting macrophage-mediated activities.26
In summary, the first total synthesis of RvD3 1 and AT-RvD3 2 has been achieved, helping to fully assign their stereochemistry. The reported strategy is convergent and highly stereocontrolled, forming these molecules in stereochemically pure form by using enantiomerically pure starting materials. The synthetic availability of these lipid mediators will help elucidate their role during inflammation and confirm their novel anti-inflammatory and pro-resolving properties. Overall, these data offer new insights for the biological roles of aspirin and DHA.
Supplementary Material
Acknowledgment
We thank NIH for financial support (Grants P50-DE016191 and P01-GM095467 to C.N.S. and N.A.P.).
Footnotes
Supporting Information Available: Experimental procedures and spectroscopic data for new compounds are available free of charge at http://pubs.acs.org.
References
- 1.Serhan CN, Petasis NA. Chem. Rev. 2011;111:5922. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Serhan CN, Savill J. Nature Immunol. 2005;6:1191. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]; (b) Serhan CN, Yacoubian S, Yang R. Annu. Rev. Pathol. Mech. Dis. 2008;3:279. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Serhan CN. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:141. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]; (b) Petasis NA, Akritopoulou-Zanze I, Fokin VV, Bernasconi G, Keledjian R, Yang R, Uddin J, Nagulapalli KC, Serhan CN. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:301. doi: 10.1016/j.plefa.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. J. Exp. Med. 2000;192:1197. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R-L. J. Exp. Med. 2002;196:1025. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Hong S, Gronert K, Devchand PR, Moussignac R-L, Serhan CN. J. Biol. Chem. 2003;278:14677. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]; (b) Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. J. Biol. Chem. 2003;278:43807. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 7.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. J. Exp. Med. 2009;206:15. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simopoulos AP. J. Am. Coll. Nutr. 2002;21:495. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 9.(a) Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. J. Exp. Med. 2005;201:713. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Petasis NA. 6,949,664. US Patent. 2005; (c) Arita M, Oh SF, Chonan T, Hong S, Elangovan S, Sun Y-P, Uddin J, Petasis NA, Serhan CN. J. Biol. Chem. 2006;281:22847. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 10.(a) Sun Y-P, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. J. Biol. Chem. 2007;282:9323. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]; (b) Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. J. Immunol. 2008;181:8677. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee C-H, Yang R, Petasis NA, Serhan CN. Proc. Natl. Acad. Sci. USA. 2010;107:1660. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Nature. 2009;461:1287. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Petasis NA, Winkler JW, Nagengast ES, Uddin J, Serhan CN. 237th ACS National Meeting. Salt Lake City, UT: 2009. Abstract Medi-056. [Google Scholar]
- 12.(a) Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. J. Immunol. 2006;176:1848. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]; (b) Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, Bazan NG, Zhu M, Winkler JW, Petasis NA. Chem. Biol. 2011;18:976. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Petasis NA, Yang R, Winkler JW, Zhu M, Uddin J, Bazan NG, Serhan CN. Tetrahedron Lett. 2012;53:1695. doi: 10.1016/j.tetlet.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Serhan CN, Dalli J, Karamnov S, Choi A, Park C-K, Xu Z-Z, Ji R-R, Zhu M, Petasis NA. FASEB J. 2012;26:1755. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhu M, Serhan CN, Petasis NA. 243rd ACS National Meeting. San Diego, CA: 2012. Abstract Biol-225. [Google Scholar]
- 14.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. J Immunol. 2006;177:5902. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 15.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11276. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Boland W, Schroer N, Sieler C, Feigel M. Helv. Chim. Acta. 1987;70:1025. [Google Scholar]; (b) Chemin D, Linstrumelle G. Tetrahedron. 1992;48:1943. [Google Scholar]
- 17.Chinchilla R, Najera C. Chem. Rev. 2007;107:874. doi: 10.1021/cr050992x. [DOI] [PubMed] [Google Scholar]
- 18.Tidwell TT. Org. React. 1990;39:297. [Google Scholar]
- 19.(a) Takai K, Nitta K, Utimoto K. J. Am. Chem. Soc. 1986;108:7408. doi: 10.1021/ja00279a068. [DOI] [PubMed] [Google Scholar]; (b) Petasis NA. Science of Synthesis. 2009;47:161. [Google Scholar]
- 20.An alternative approach to 5 involving TBS protection of 16 to afford a di-TBS product, followed by selective removal of the primary TBS group was less efficient and gave a mixture of products.
- 21.Purchased from Sigma-Aldrich.
- 22.(a) Saito S, Hasegawa T, Inaba M, Nishida R, Fujii T, Nomizu S, Moriwake T. Chem. Lett. 1984;13:1389. [Google Scholar]; (b) Saito S, Ishikawa T, Kuroda A, Koga K, Moriwake T. Tetrahedron. 1992;48:4067. [Google Scholar]; (c) Fox ME, Jackson M, Lennon IC, McCague R. J. Org. Chem. 2005;70:1227. doi: 10.1021/jo048035v. [DOI] [PubMed] [Google Scholar]
- 23.Corey EJ, Fuchs PL. Tetrahedron Lett. 1972;13:3769. [Google Scholar]
- 24.Lu W, Zheng G, Gao D, Cai J. Tetrahedron. 1999;55:7157. [Google Scholar]
- 25.(a) Ohira S. Synth. Commun. 1989;19:561. [Google Scholar]; (b) Müller S, Liepold B, Roth GJ, Bestmann HJ. Synlett. 1996;1996:521. [Google Scholar]
- 26.Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng C-YC, Chiang N, Petasis NA, Serhan CN. Chemistry & Biology. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.