Abstract
We have purified to homogeneity and characterized a 55,000-dalton rat cell membrane glycoprotein, gp55. This protein was originally identified in preparations of a defective pseudotype of the Kirsten sarcoma virus and shown to be present in several rodent retrovirus particles. The gp55 was purified from this defective virus by concanavalin A and heparin affinity chromatography, as well as by preparative sodium dodecyl sulfate-gel electrophoresis. Both preparations displayed similar purity and antigenic characteristics. The 125I-labeled gp55 was precipitated by antisera against rodent retroviruses, but not by monospecific antisera against purified type C virus structural proteins, thus indicating that gp55 was retrovirus associated, but unrelated to known retrovirus structural proteins. Competition radioimmunoassay with an anti-rat virus serum which recognized rodent group-specific antigens on gp55 indicated: the presence of gp55 antigens in 15 rodent cell lines, but not 10 nonrodent cell lines; no effect of viral infection or cell transformation on the amount of gp55 expressed; up to 100-fold increases in the concentration of the gp55 antigens in nine rodent retroviruses, but not in five nonrodent viruses, as compared to cells; the presence of gp55 in rodent sera, especially of the NZB mouse, where anti-gp55 antibody was also detected; a lymphoid and epithelial tissue distribution of gp55 in rats and mice. Additional competition radioimmunoassays with a broad-reacting antivirus serum also detected the presence of gp55 in nonrodent, mink, and human cells and thus distinguished rat type, rodent group, and interspecies antigenic determinants on gp55. In conclusion, gp55 is a cell membrane glycoprotein associated in high concentration with retroviruses.
Full text
PDF





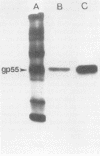
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Boyse E. A., Old L. J., De Harven E., Hämmerling U., Wood H. A. G (Gross) and H-2 cell-surface antigens: location on Gross leukemia cells by electron microscopy with visually labeled antibody. Proc Natl Acad Sci U S A. 1970 Mar;65(3):569–576. doi: 10.1073/pnas.65.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Takahashi T. Viral and cellular surface antigens of murine leukemias and myelomas. Serological analysis by immunoelectron microscopy. J Exp Med. 1972 Mar 1;135(3):443–457. doi: 10.1084/jem.135.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer P., Olshevsky U., Baltimore D., Dolberg D., Fan H. Virus-like 30S RNA in mouse cells. J Virol. 1979 Mar;29(3):1168–1176. doi: 10.1128/jvi.29.3.1168-1176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilello J. A., Strand M., August J. T. Murine sarcoma virus gene expression: transformants which express viral envelope glycoprotein in the absence of the major internal protein and infectious particles. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3234–3238. doi: 10.1073/pnas.71.8.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bubbers J. E., Chen S., Lilly F. Nonrandom inclusion of H-2K and H-2D antigens in Friend virus particles from mice of various strains. J Exp Med. 1978 Feb 1;147(2):340–351. doi: 10.1084/jem.147.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Simmons D. T., Martin M. A., Mora P. T. Identification and partial characterization of new antigens from simian virus 40-transformed mouse cells. J Virol. 1979 Aug;31(2):463–471. doi: 10.1128/jvi.31.2.463-471.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Sawyer R. C., Taylor J. M., Faras A. J., Levinson W. E., Goodman H. M., Bishop J. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. I. Identification of a specific 4S RNA which serves as primer. J Virol. 1974 May;13(5):1126–1133. doi: 10.1128/jvi.13.5.1126-1133.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison B. L., Leighton T., Rabinowitz J. C. Purification of Bacillus subtilis RNA polymerase with heparin-agarose. In vitro transcription of phi 29 DNA. J Biol Chem. 1979 Sep 25;254(18):9220–9226. [PubMed] [Google Scholar]
- DeLeo A. B., Jay G., Appella E., Dubois G. C., Law L. W., Old L. J. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci U S A. 1979 May;76(5):2420–2424. doi: 10.1073/pnas.76.5.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman N. A., Stepina V. N., Ievleva E. S. H-2 antigens on murine leukemia cells and viruses. Int J Cancer. 1972 May 15;9(3):693–701. doi: 10.1002/ijc.2910090328. [DOI] [PubMed] [Google Scholar]
- Eisenman R. N., Vogt V. M. The biosynthesis of oncovirus proteins. Biochim Biophys Acta. 1978 Apr 6;473(3-4):187–239. doi: 10.1016/0304-419x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Howk R. S., Troxler D. H., Lowy D., Duesberg P. H., Scolnick E. M. Identification of a 30S RNA with properties of a defective type C virus in murine cells. J Virol. 1978 Jan;25(1):115–123. doi: 10.1128/jvi.25.1.115-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashmiri S. V., Rein A., Bassin R. H., Gerwin B. I., Gisselbrecht S. Donation of N- or B-tropic phenotype to NB-tropic murine leukemia virus during mixed infections. J Virol. 1977 Jun;22(3):626–633. doi: 10.1128/jvi.22.3.626-633.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Shaul Y., Kaminchik J., Aviv H. Heterogeneity of "virus-like" genes encoding retrovirus-associated 30S RNA and their organization within the mouse genome. Cell. 1980 Jun;20(2):431–439. doi: 10.1016/0092-8674(80)90629-7. [DOI] [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Huebner R. J. Rescue of the genome of focus forming virus from rat non-productive lines by 5'-bromodeoxyruidine. Nat New Biol. 1971 Nov 3;234(44):12–14. doi: 10.1038/newbio234012a0. [DOI] [PubMed] [Google Scholar]
- Kress M., May E., Cassingena R., May P. Simian virus 40-transformed cells express new species of proteins precipitable by anti-simian virus 40 tumor serum. J Virol. 1979 Aug;31(2):472–483. doi: 10.1128/jvi.31.2.472-483.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lenard J. Virus envelopes and plasma membranes. Annu Rev Biophys Bioeng. 1978;7:139–165. doi: 10.1146/annurev.bb.07.060178.001035. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Wilson C. B., Villano B. C., McConahey P. J., Dixon F. J. Endogenous oncornaviral gene expression in adult and fetal mice: quantitative, histologic, and physiologic studies of the major viral glycorprotein, gp70. J Exp Med. 1976 Jan 1;143(1):151–166. doi: 10.1084/jem.143.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M. M., Benveniste R. E., Livingston D. M., Todaro G. J. Mammalian cells in culture frequently release type C viruses. Science. 1973 Oct 5;182(4107):56–59. doi: 10.1126/science.182.4107.56. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Melero J. A., Stitt D. T., Mangel W. F., Carroll R. B. Identification of new polypeptide species (48-55K) immunoprecipitable by antiserum to purified large T antigen and present in SV40-infected and -transformed cells. Virology. 1979 Mar;93(2):466–480. doi: 10.1016/0042-6822(79)90250-2. [DOI] [PubMed] [Google Scholar]
- Mellors R. C., Shirai T., Aoki T., Huebner R. J., Krawczynski K. Wild-type Gross leukemia virus and the pathogenesis of the glomerulonephritis of New Zealand mice. J Exp Med. 1971 Jan 1;133(1):113–132. doi: 10.1084/jem.133.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson D., Scheinberg D., Klement V., Strand M., August J. T. Characterization of a defective pseudotype particle of Kirsten sarcoma virus. Virology. 1979 Jun;95(2):536–549. doi: 10.1016/0042-6822(79)90507-5. [DOI] [PubMed] [Google Scholar]
- Peeples P. T., Gerwin B. I., Papageorge A. G., Smith S. G. Murine sarcoma virus defectiveness. Viral polymerase expression murine and nonmurine host cells transformed by S+L-type murine sarcoma virus. Virology. 1975 Oct;67(2):344–355. doi: 10.1016/0042-6822(75)90436-5. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Rifkin D. B., Reich E. Lipid studies of Rous sarcoma virus and host cell membranes. Virology. 1972 Nov;50(2):550–557. doi: 10.1016/0042-6822(72)90406-0. [DOI] [PubMed] [Google Scholar]
- Rosok M. J., Watson K. F. Fractionation of two protein kinases from avian myeloblastosis virus and characterization of the protein kinase activity preferring basic phosphoacceptor proteins. J Virol. 1979 Mar;29(3):872–880. doi: 10.1128/jvi.29.3.872-880.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Scheinberg D. A., Strand M. A brain membrane protein similar to the rat src gene product. Proc Natl Acad Sci U S A. 1981 Jan;78(1):55–59. doi: 10.1073/pnas.78.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg D. A., Strand M. Transformation-related proteins associated with Kirsten sarcoma virus. Virology. 1980 Oct 30;106(2):335–348. doi: 10.1016/0042-6822(80)90257-3. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Maryak J. M., Parks W. P. Levels of rat cellular RNA homologous to either Kirsten sarcoma virus or rat type-C virus in cell lines derived from Osborne-Mendel rats. J Virol. 1974 Dec;14(6):1435–1444. doi: 10.1128/jvi.14.6.1435-1444.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal S. P., Hartley J. W., Rowe W. P. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3065–3067. doi: 10.1073/pnas.74.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Oncornavirus envelope glycoprotein in serum of mice. Virology. 1976 Nov;75(1):130–144. doi: 10.1016/0042-6822(76)90012-x. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Polypeptide maps of cells infected with murine type C leukemia or sarcoma oncovirus. Cell. 1978 Feb;13(2):399–408. doi: 10.1016/0092-8674(78)90208-8. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Polypeptides of cells transformed by RNA or DNA tumor viruses. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2729–2733. doi: 10.1073/pnas.74.7.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Protein kinase and phosphate acceptor proteins in Rauscher murine leukaemia virus. Nat New Biol. 1971 Sep 29;233(39):137–140. doi: 10.1038/newbio233137a0. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Purification and analysis of a Gross murine oncornavirus protein with a molecular weight of about 12,000 specific for the Gross virus subgroup. Virology. 1977 Jun 1;79(1):129–143. doi: 10.1016/0042-6822(77)90340-3. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of mammalian oncogenic RNA viruses: multiple antigenic determinants of the major internal protein and envelope glycoprotein. J Virol. 1974 Jan;13(1):171–180. doi: 10.1128/jvi.13.1.171-180.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of ribonucleic acid tumor viruses. Purification of envelope, core, and internal components. J Biol Chem. 1976 Jan 25;251(2):559–564. [PubMed] [Google Scholar]
- Strand M. Transformation-related antigens identified by monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3234–3238. doi: 10.1073/pnas.77.6.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., Wilsnack R., August J. T. Structural proteins of mammalian oncogenic RNA viruses: immunological characterization of the p15 polypeptide of Rauscher murine virus. J Virol. 1974 Dec;14(6):1575–1583. doi: 10.1128/jvi.14.6.1575-1583.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEG D. E., BECKER C., BEARD J. W. VIRUS OF AVIAN MYELOBLASTOSIS (BAI STRAIN A). XXV. ULTRACYTOCHEMICAL STUDY OF VIRUS AND MYELOBLAST PHOSPHATASE ACTIVITY. J Natl Cancer Inst. 1964 Jan;32:201–235. [PubMed] [Google Scholar]
- Troxler D. H., Ruscetti S. K., Linemeyer D. L., Scolnick E. M. Helper-independent and replication-defective erythroblastosis-inducing viruses contained within anemia-inducing Friend virus complex (FV-A). Virology. 1980 Apr 15;102(1):28–45. doi: 10.1016/0042-6822(80)90067-7. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L. Polypeptides of Moloney sarcoma-leukemia virions: their resolution and incorporation into extracellular virions. Virology. 1974 Oct;61(2):575–587. doi: 10.1016/0042-6822(74)90291-8. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W., Kimata K., Pratt R. M. Characterization of fibronectin interactions with glycosaminoglycans and identification of active proteolytic fragments. J Biol Chem. 1980 Jul 10;255(13):6055–6063. [PubMed] [Google Scholar]