Abstract
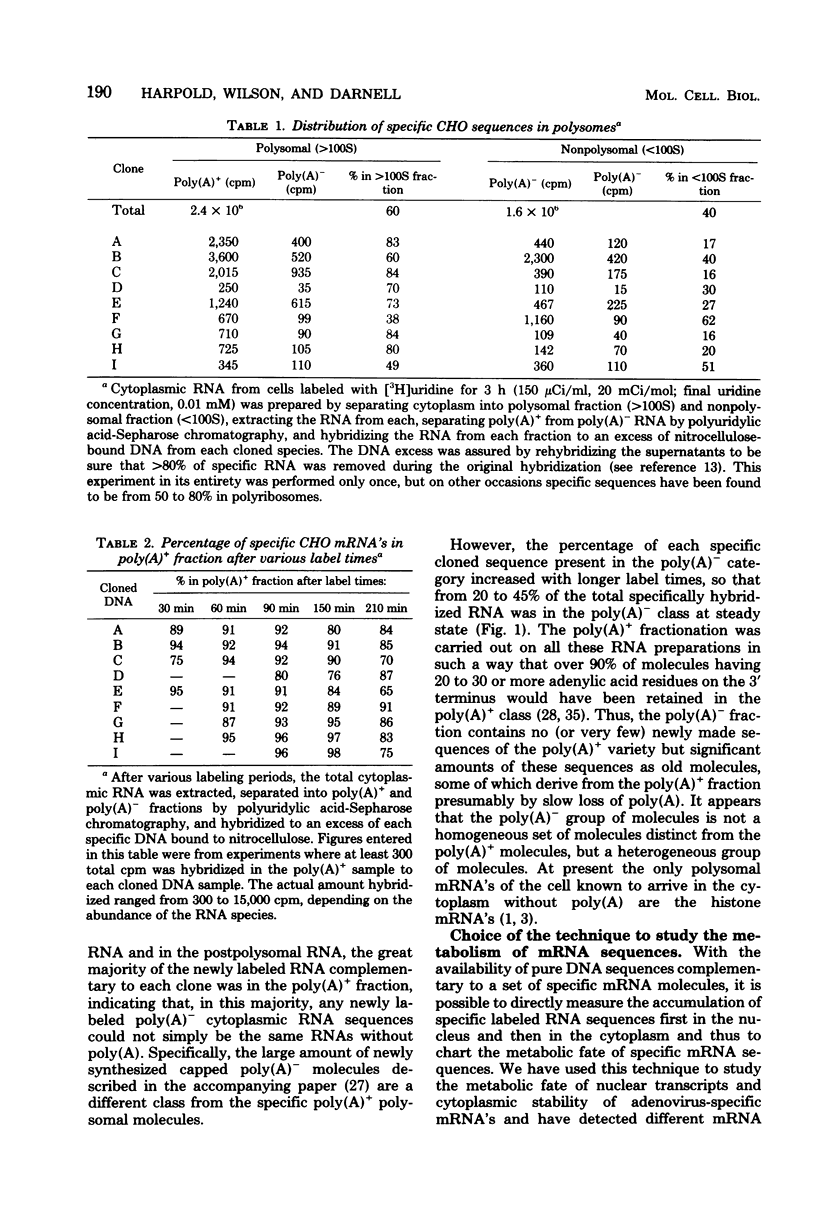
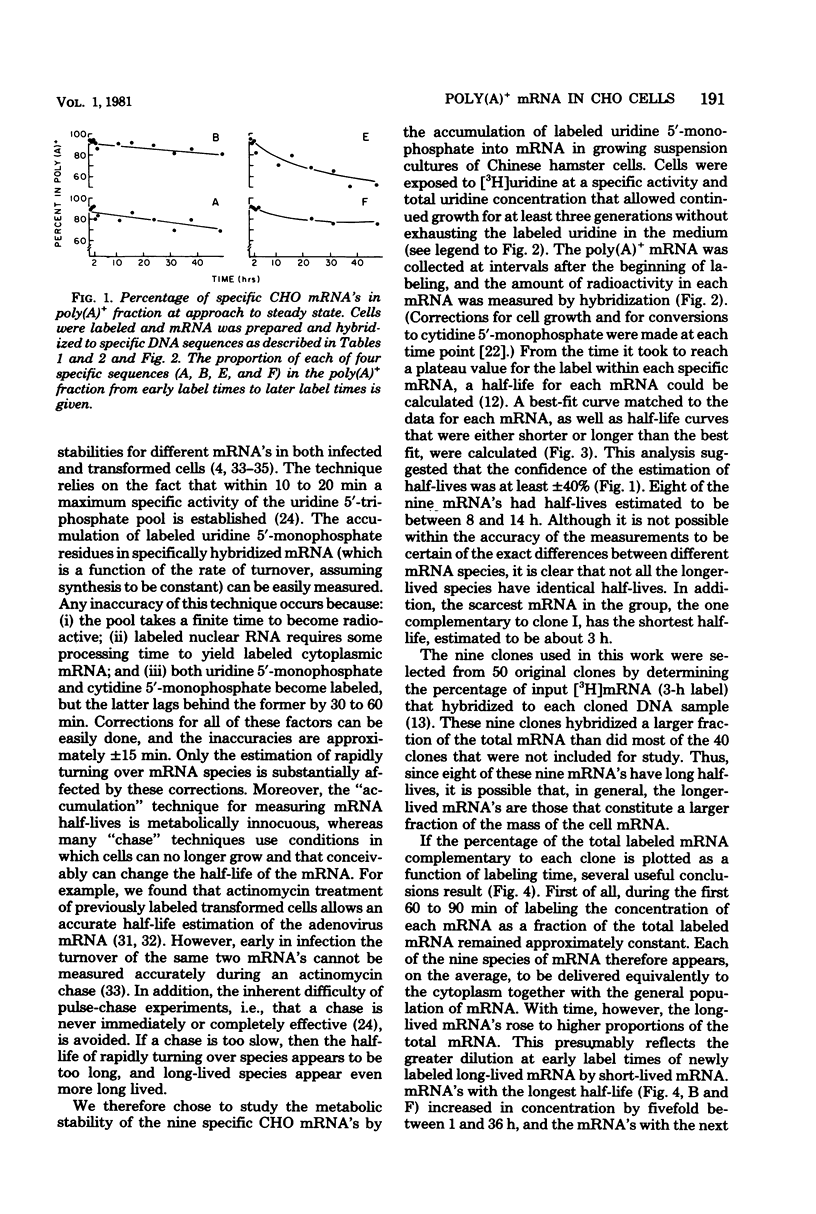
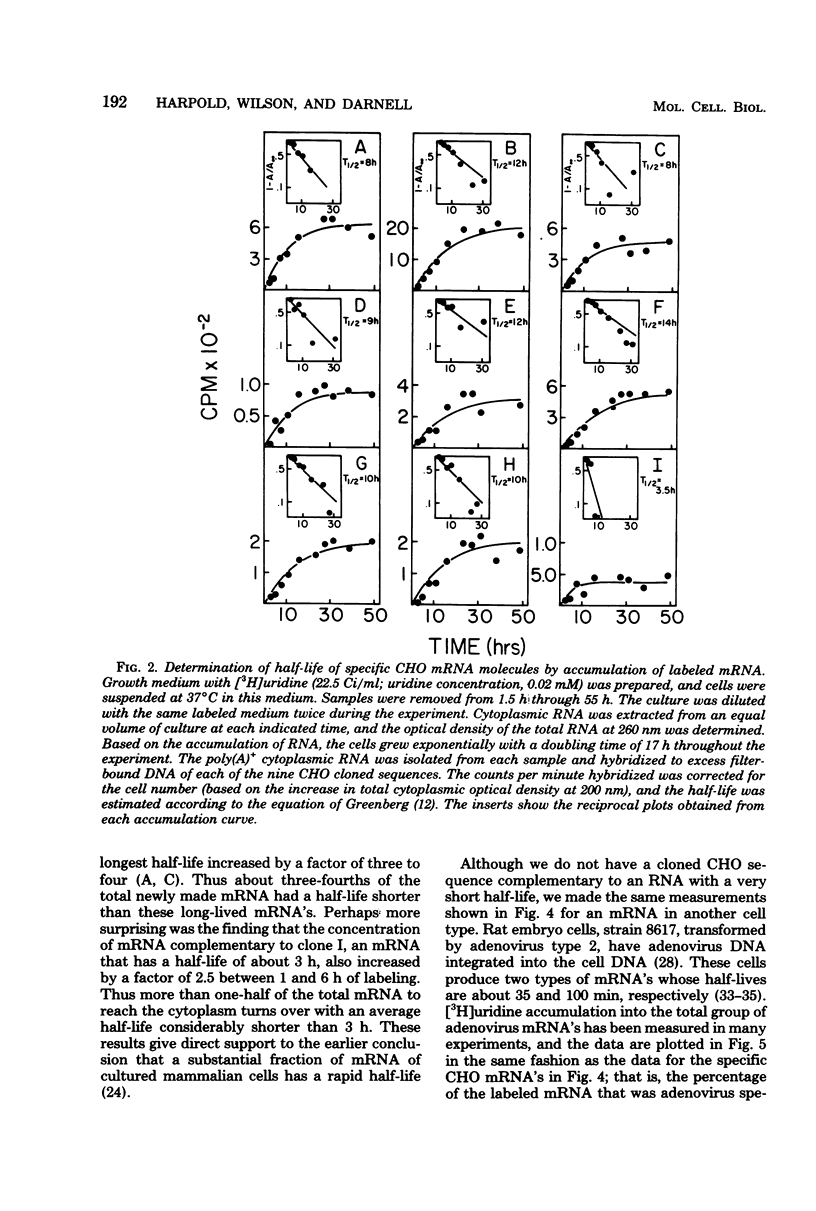
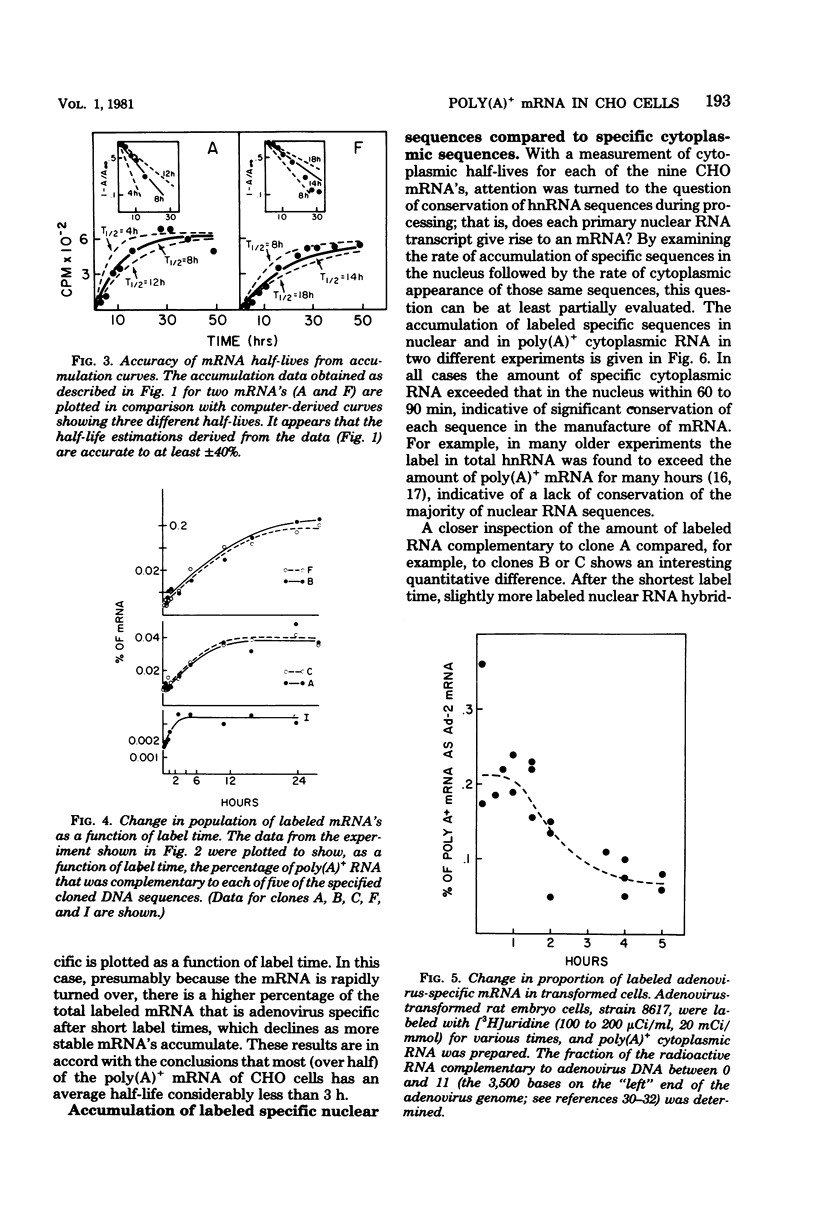
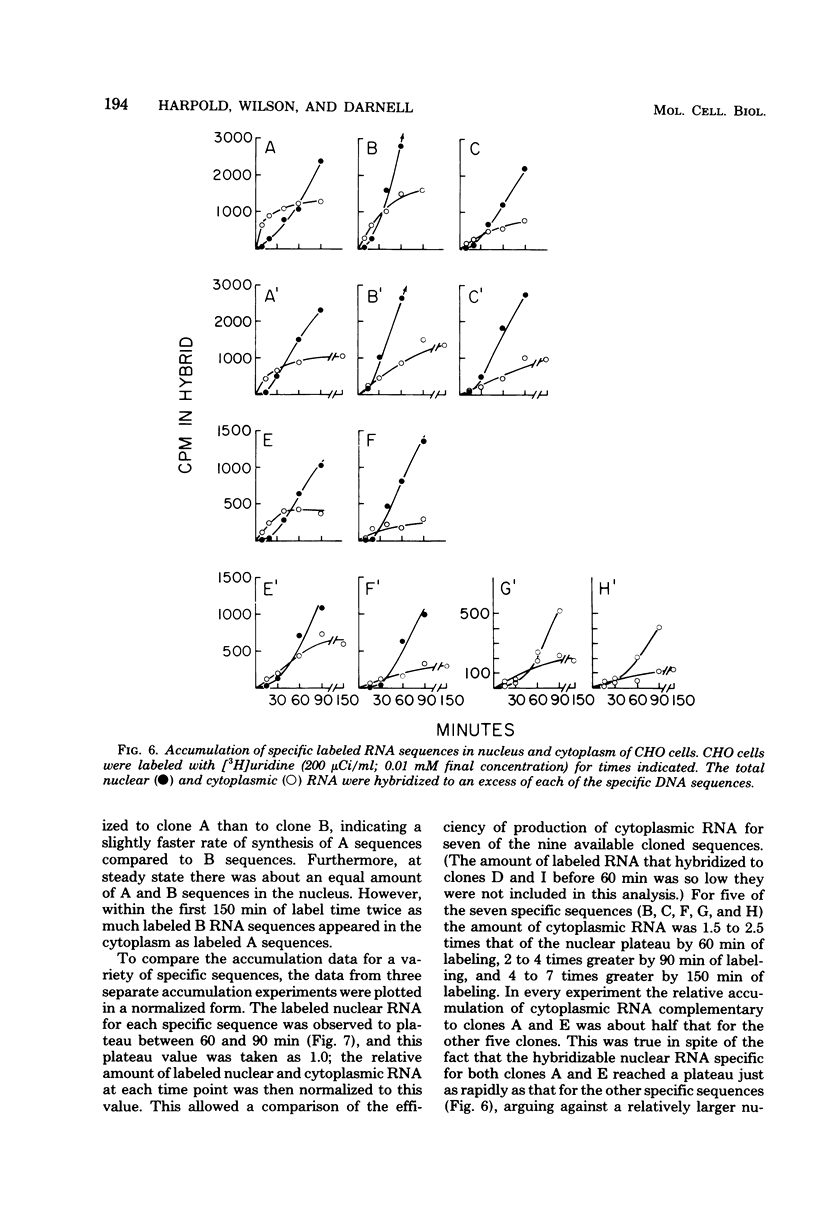
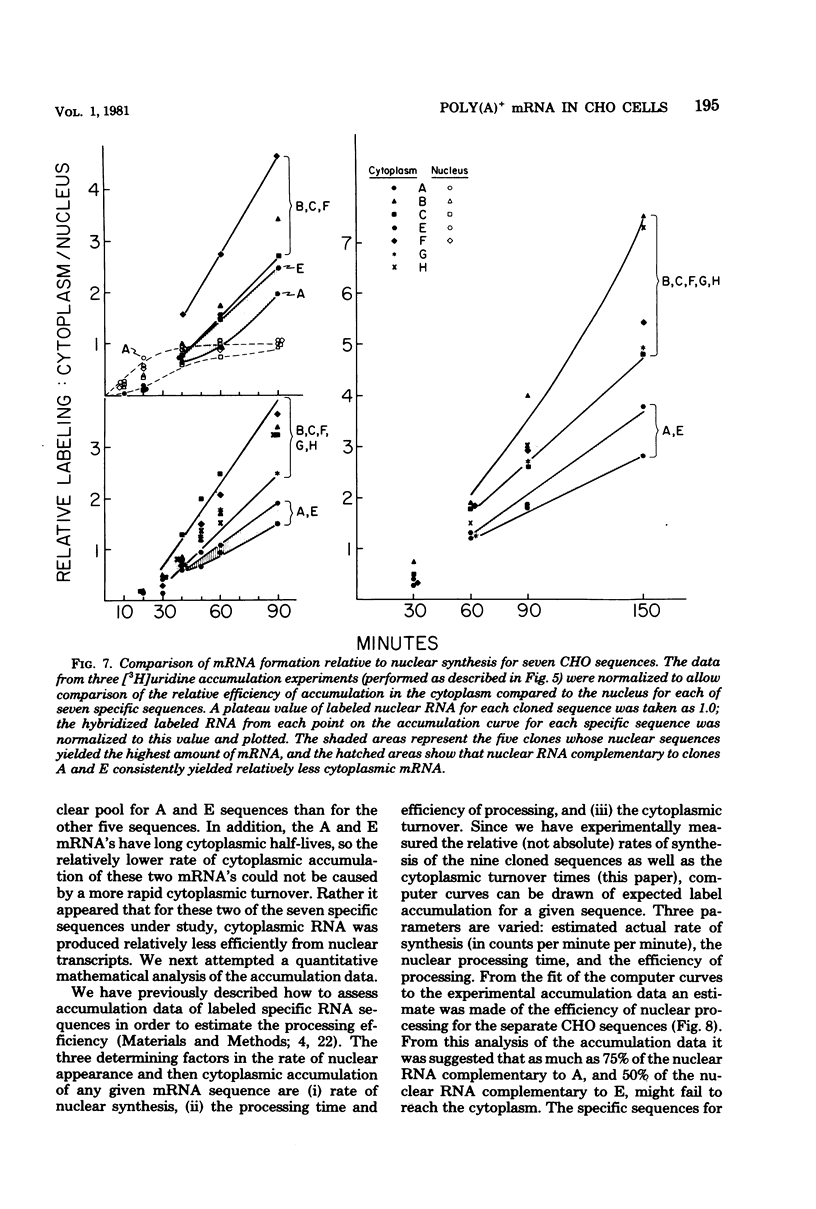
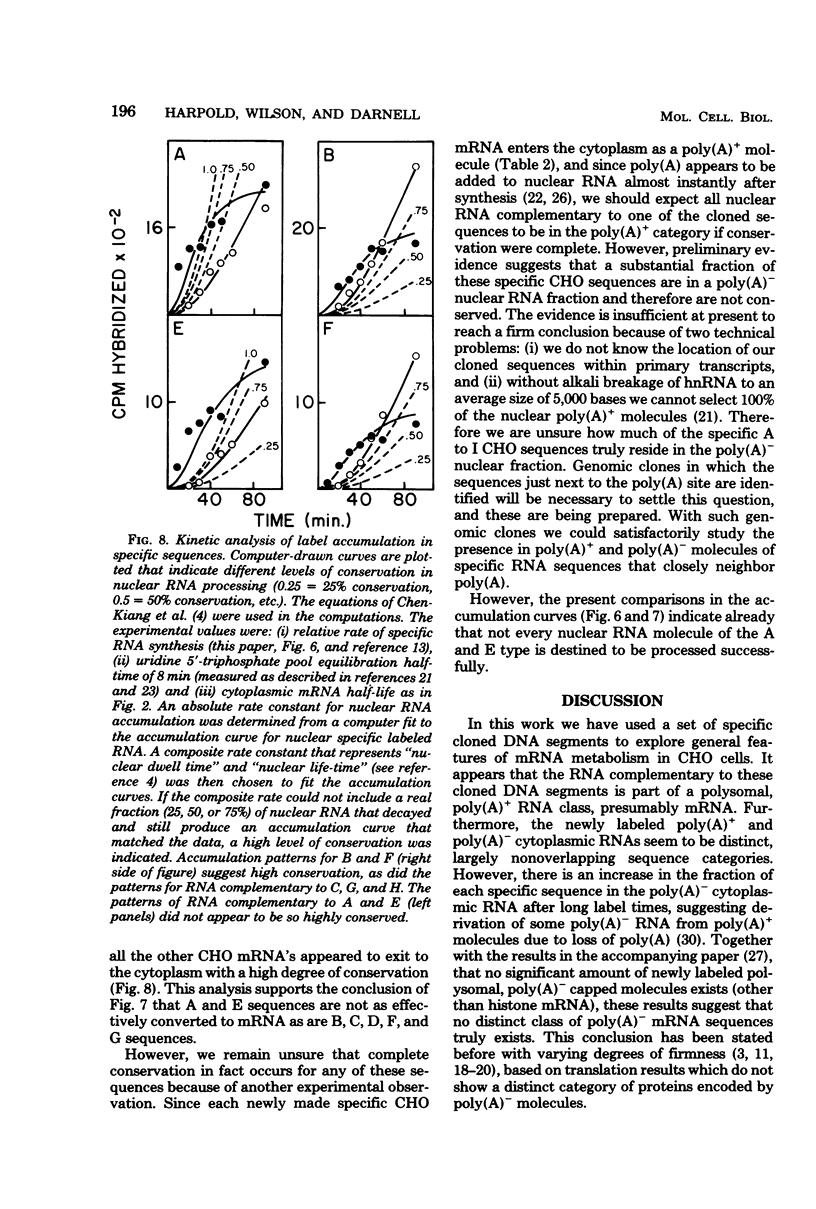
We have further analyzed the metabolism of specific messenger ribonucleic acid (mRNA) sequences within the cytoplasmic and nuclear RNA of Chinese hamster ovary (CHO) cells by using a set of previously constructed complementary deoxyribonucleic acid (DNA) clones (Harpold et al., Cell 17:1025-1035, 1979) as specific molecular probes in a variety of RNA:DNA hybridization experiments. The majority of the labeled mRNA complementary to each of the nine clones was found in the polyribosomes, with some variation between individual sequences. The great majority of each specific mRNA labeled for 3 h or less was in the polyadenylated [poly(A)+] fraction. However, the amount of each sequence increased in the non-poly(A)+ [poly(A)-] fraction after very long label times, suggesting the derivation of the poly(A)- RNA from the poly(A)+ RNA. Eight of the nine mRNA's have cytoplasmic half-lives ranging from 8 to 14 h, whereas one of the mRNA's, the scarcest in the group, has a somewhat shorter half-life of approximately 3 h. The proportion of each of the specific long-lived mRNA's within the total labeled mRNA increased as a function of labeling time, indicating that a large fraction, probably greater than 50%, of the initially labeled poly(A)+ mRNA in CHO cells has a half-life of less than 3 h. A quantitative analysis of the kinetics of labeling of specific nuclear and cytoplasmic sequences indicated that a significant fraction of the mRNA sequences transcribed from genes containing these nine CHO sequences were successfully processed into mRNA. However, two of the CHO mRNA sequences were only partially conserved during nuclear processing to yield mRNA. These studies demonstrated that events at two post-transcriptional levels, differential nuclear processing efficiency of different primary transcripts and cytoplasmic stability of different mRNA's, can be involved in the determination of the cytoplasmic concentrations of different mRNA's.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Darnell J. E. Biogenesis and characterization of histone messenger RNA in HeLa cells. J Mol Biol. 1972 Jun 28;67(3):397–406. doi: 10.1016/0022-2836(72)90458-5. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Bothwell A. L., Knapp M., Siden E., Mather E., Koshland M., Baltimore D. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell. 1980 Jun;20(2):293–301. doi: 10.1016/0092-8674(80)90615-7. [DOI] [PubMed] [Google Scholar]
- Brawerman G. Characteristics and significance of the polyadenylate sequence in mammalian messenger RNA. Prog Nucleic Acid Res Mol Biol. 1976;17:117–148. doi: 10.1016/s0079-6603(08)60068-9. [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S., Nevins J. R., Darnell J. E., Jr N-6-methyl-adenosine in adenovirus type 2 nuclear RNA is conserved in the formation of messenger RNA. J Mol Biol. 1979 Dec 15;135(3):733–752. doi: 10.1016/0022-2836(79)90174-8. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Transcription units for mRNA production in eukaryotic cells and their DNA viruses. Prog Nucleic Acid Res Mol Biol. 1979;22:327–353. doi: 10.1016/s0079-6603(08)60803-x. [DOI] [PubMed] [Google Scholar]
- Giorno R., Sauerbier W. A radiological analysis of the transcription units for heterogeneous nuclear RNA in cultured murine cells. Cell. 1976 Dec;9(4 Pt 2):775–783. doi: 10.1016/0092-8674(76)90140-9. [DOI] [PubMed] [Google Scholar]
- Giorno R., Sauerbier W. Units of transcription for cytoplasmic RNA in mouse myeloma cells. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4374–4378. doi: 10.1073/pnas.75.9.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S., Schwartz H., Darnell J. E., Jr Evidence from UV transcription mapping in HeLa cells that heterogeneous nuclear RNA is the messenger RNA precursor. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4520–4523. doi: 10.1073/pnas.74.10.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady L. J., North A. B., Campbell W. P. Complexity of poly(A+) and poly(A-) polysomal RNA in mouse liver and cultured mouse fibroblasts. Nucleic Acids Res. 1978 Mar;5(3):697–712. doi: 10.1093/nar/5.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Paddock G. V., Wall R., Salser W. A general method for cloning eukaryotic structural gene sequences. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3146–3150. doi: 10.1073/pnas.73.9.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Mayrand S., Pederson T. Sequence complexity of nuclear and messenger RNA in HeLa cells. J Mol Biol. 1980 Apr 25;138(4):755–778. doi: 10.1016/0022-2836(80)90064-9. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Adesnik M., Salditt M., Sheiness D., Wall R., Molloy G., Philipson L., Darnell J. E. Further evidence on the nuclear origin and transfer to the cytoplasm of polyadenylic acid sequences in mammalian cell RNA. J Mol Biol. 1973 Apr 15;75(3):515–532. doi: 10.1016/0022-2836(73)90458-0. [DOI] [PubMed] [Google Scholar]
- Johnson L. F., Levis R., Abelson H. T., Green H., Penman S. Changes in RNA in relation to growth of the fibroblast. IV. Alterations in theproduction and processing of mRNA and rRNA in resting and growing cells. J Cell Biol. 1976 Dec;71(3):933–938. doi: 10.1083/jcb.71.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y., Milcarek C., Berissi H., Penman S. HeLa cell poly(A)- mRNA codes for a subset of poly(A)+ mRNA-directed proteins with an actin as a major product. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4801–4805. doi: 10.1073/pnas.74.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Jacobson A., Firtel R., Alton T., Tuchman J. Synthesis of messenger RNA and chromosome structure in the cellular slime mold. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5103–5108. doi: 10.1073/pnas.71.12.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Gros F. Coding potential of non-polyadenylated messenger RNA in mouse Friend cells. J Mol Biol. 1980 May 5;139(1):61–83. doi: 10.1016/0022-2836(80)90116-3. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Puckett L., Darnell J. E. Essential factors in the kinetic analysis of RNA synthesis in HeLa cells. J Cell Physiol. 1977 Mar;90(3):521–534. doi: 10.1002/jcp.1040900315. [DOI] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Harpold M., Sawicki S., Nevins J., Darnell J. E., Jr Addition of poly(A) to nuclear RNA occurs soon after RNA synthesis. J Cell Biol. 1980 Sep;86(3):844–848. doi: 10.1083/jcb.86.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S. G., Jelinek W., Darnell J. E. 3'-Terminal addition to HeLa cell nuclear and cytoplasmic poly (A). J Mol Biol. 1977 Jun 15;113(1):219–235. doi: 10.1016/0022-2836(77)90051-1. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Van Ness J., Maxwell I. H., Hahn W. E. Complex population of nonpolyadenylated messenger RNA in mouse brain. Cell. 1979 Dec;18(4):1341–1349. doi: 10.1016/0092-8674(79)90244-7. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Nevins J. R., Blanchard J. M., Ginsberg H. S., Darnell J. E., Jr Metabolism of mRNA from the transforming region of adenovirus 2. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):447–455. doi: 10.1101/sqb.1980.044.01.048. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Sawicki S. G., Salditt-Georgieff M., Darnell J. E. Adenovirus type 2 mRNA in transformed cells: map positions and difference in transport time. J Virol. 1978 Jan;25(1):97–103. doi: 10.1128/jvi.25.1.97-103.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. C., Sawicki S. G., White P. A., Darnell J. E., Jr A correlation between the rate of poly(A) shortening and half-life of messenger RNA in adenovirus transformed cells. J Mol Biol. 1978 Nov 25;126(1):23–36. doi: 10.1016/0022-2836(78)90277-2. [DOI] [PubMed] [Google Scholar]



