Immune thrombocytopenia (ITP) is an acquired immune-mediated disorder characterized by thrombocytopenia in the absence of an underlying cause.1 The patho-physiology of ITP is multifactorial and includes the development of autoantibodies that trigger abnormal thrombopoiesis, enhanced platelet destruction, complement activation and T-cell mediated effects.1–3 Platelet autoantibodies are detected in approximately 50% of patients4 and generally target the fibrinogen receptor αIIbβ3 or the receptor for von Willebrand factor (VWF), the glycoprotein (GP) Ib-V-IX complex. Anti-αIIbβ3 antibodies (70–80% of cases) are thought to induce thrombocytopenia through increased platelet clearance by Fcγ receptor-bearing macrophages. Autoantibodies against GPIb-V-IX (20–40% of cases) often induce a more severe fall in platelet count5 that is less responsive to standard therapies, such as intravenous immunoglobulin G (IVIG).6 Thrombocytopenia induced by GPIb-V-IX autoantibodies has not been characterized in great detail. Some monoclonal antibodies against GPIbα are known to induce platelet activation7 that may lead to accelerated platelet destruction in ITP patients8 with autoantibodies against this receptor. Here we report how an autoantibody against GPIbα, obtained from a patient with ITP, induces recognition signals for macrophages through interplay between glycoprotein Ibα and the low affinity IgG receptor FcγRIIa in lipid rafts.
The patient is a 70-year old Caucasian woman. She had a history of nephrectomy and parathyroidectomy; both surgeries were without bleeding complications. In 2007, routine laboratory investigations revealed thrombocytopenia (20 × 109/L; normal 150–450×109/L). She had recently suffered from increased spontaneous skin hematomas and melena. Her platelet count was low (20×109/L) and disturbed by aggregates. Hemoglobin level, leukocytes and differential were normal. There was no detectable monoclonal protein and immunoglobulin levels were normal. IVIG (30 g per day for 5 days) and platelet transfusions failed to increase platelet count to normal levels. Gastroand colonoscopy did not reveal a clear bleeding focus (Online Supplementary Table S1). A detailed description of the materials and methods used is found in the Online Supplementary Appendix.
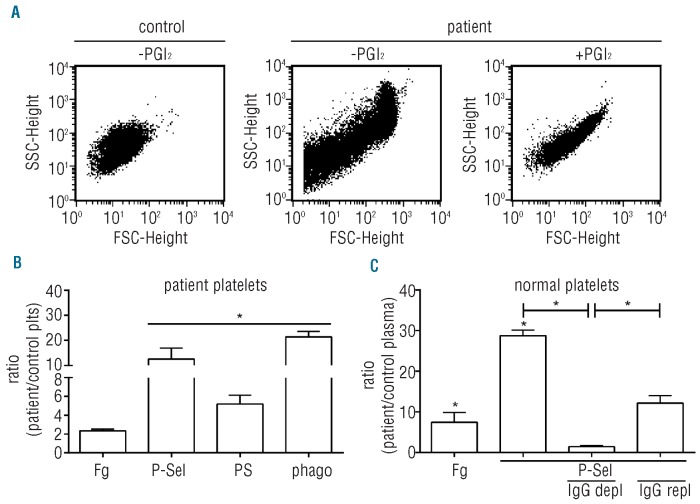
Citrated blood from the ITP patient showed a mixture of single platelets and small aggregates. Inhibition of platelet activation with prostacyclin (PGI2) during blood collection reduced the number of aggregates, but the platelet count remained low (Figure 1A). FACS analysis with gating for single platelets showed increased levels of surface-expressed fibrinogen, P-selectin and PS compared with controls (Figure 1B). Surface-exposed P-selectin and PS are clearance signals, triggering platelet binding to macrophages, followed by their destruction.9 As expected, matured monocytic THP-1 cells phagocytosed 21-fold more patient platelets than control platelets. The activation observed in blood was caused by a plasma constituent, since normal platelets incubated in patient plasma were also activated and showed a strong increase in surface-bound fibrinogen and P-selectin. IgG depletion prevented the increase in P-selectin expression which was restored upon repletion of IgG (Figure 1C).
Figure 1.
Platelet activation by patient autoantibodies. (A) Platelet aggregation during blood collection. Blood was collected in citrate supplemented with PGI2 (10 ng/mL) and FSC/SSC-scatter was analyzed by FACS in isolated control platelets (left panel) or patient platelets without and with PGI2 (middle and right panel). (B) Patient platelets are activated during blood collection. FACS analysis of activated αIIbβ3 (bound fibrinogen; Fg), surface P-selectin (P-sel) and PS exposure. CFMDA-labeled platelets were incubated with matured monocytic THP-1 cells and phagocytosis (phago) was determined. (C) Normal platelets were incubated (2 hours, 22°C) with autologous (control plasma) or patient plasma and analyzed for bound fibrinogen and P-selectin expression. Depletion of IgGs from patient plasma (IgG depl) reduced surface P-selectin to control levels, while repletion (IgG repl) increased its expression. Results are expressed as ratio of normal platelets in patient plasma over control plasma. Data are means ± SEM (n=4).
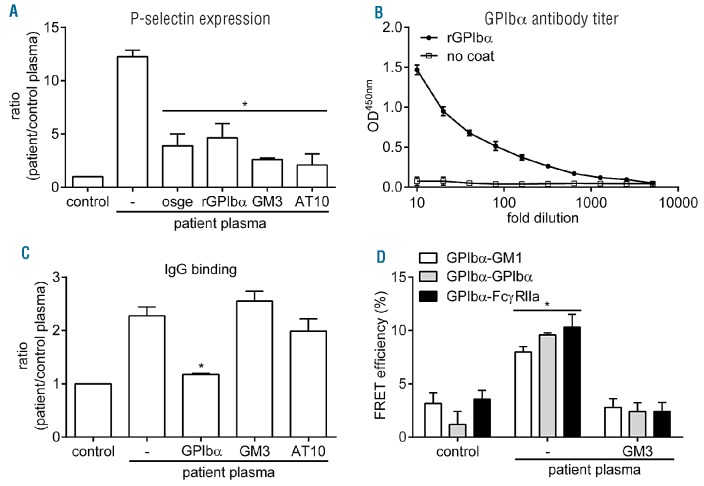
The observed platelet activation suggested the presence of anti-GPIbα autoantibodies, as some monoclonal anti-GPIbα antibodies are known to induce platelet aggregation.7 Indeed, removal of GPIbα ectodomain with o-sialo-glycoprotein endopeptidase (osge) or addition of excess soluble recombinant GPIbα reduced P-selectin expression on normal platelets incubated in patient plasma (Figure 2A). Binding of VWF to GPIbα triggers its translocation to cholesterol-rich domains known as lipid rafts.10 Subsequent platelet activation by GPIbα involves signaling through immunoreceptor tyrosine-based activation motif-containing receptors, such as the Fc receptor γ chain (FcRγ)11 or the low-affinity IgG receptor FcγRIIa.12 To investigate whether a similar mechanism might function in autoantibody-induced platelet activation, suspensions of normal platelets in patient plasma were incubated with GM3 ganglioside, which inhibits GPIbα translocation to lipid rafts,13 or with a neutralizing anti-FcγRIIa antibody (clone AT10). Both treatments strongly suppressed P-selectin expression induced by patient plasma (Figure 2A). Antibody titer determination with immobilized recombinant GPIbα confirmed the presence of GPIbα autoantibodies in patient plasma (Figure 2B). Direct analysis of IgG binding in suspensions of normal platelets in patient plasma confirmed the expected interference by recombinant GPIbα, whereas GM3 and anti-FcγRIIa antibody had no effect (Figure 2C). These data demonstrate that antibody binding is primarily regulated through GPIbα.
Figure 2.
Autoantibody binding to GPIbα leads to FcγRIIa-mediated platelet activation. (A) Normal platelets were incubated with autologous (control plasma) or patient plasma (2 hours, 22ºC) and surface P-selectin expression was measured following removal of extra-cellular GPIbα (osge; 80 μg/mL), addition of recombinant GPIbα to patient plasma (rGPIbα; 50 μg/mL), GM3 (50 μM) to prevent GPIbα translocation to lipid rafts or a neutralizing anti-FcγRIIa antibody (clone AT10; 50 μg/mL). (B) Determination of GPIbα antibody titer in patient plasma by ELISA. Patient plasma was pre-diluted (1:10) and added to wells coated with or without recombinant GPIbα to determine IgG binding. (C) Determination of IgG binding to platelets by FACS analysis under conditions as described for (A). (D) FRET/FLIM analysis of GPIbα translocation to lipid rafts, clustering and co-localization with FcγRIIa of normal platelets incubated with patient plasma. Platelets were fixed with 2% paraformaldehyde and stained with 6B4-488, 6B4-594 (1 μg/mL), CTB-594 (5 μg/mL) or CD32-488 (1 μg/mL). The fluorescence lifetimes of the donor fluorophore (6B4-488 or AT10-488) were determined in the absence and presence of acceptor fluorophore (6B4-594 or CTB-594) and subsequently used to calculate the FRET efficiency. Normal platelets incubated in autologous plasma have dispersed GPIbα receptors that do not co-localize with GM1, a marker for lipid rafts, or FcγRIIa. Platelet incubation in patient plasma triggers GPIbα translocation to rafts, leading to its clustering and association with FcγRIIa, which is prevented by addition of GM3. Data are means ± SEM (n=3).
Förster resonance energy transfer (FRET) measurement using fluorescence lifetime imaging microscopy (FLIM) is a sensitive technique to analyze protein colocalization on the intact platelet membrane.13 Analysis of normal platelets revealed that little GPIbα was present in lipid rafts (Figure 2D). The receptor was dispersed over the surface and formed few associations with FcγRIIa. Incubation in patient plasma induced GPIbα translocation to rafts, the formation of GPIbα clusters and GPIbα association with FcγRIIa. Addition of GM3 not only induced the expected blockade of GPIbα translocation to rafts but also prevented formation of GPIbα clusters and GPIbα association with FcγRIIa. Collectively, these findings indicate that patient autoantibodies against GPIbα trigger surface expression of GPIbα clusters, P-selectin and PS, which are all ‘eat-me’ signals for macrophages.9,14 The association with lipid rafts is crucial, both for formation of GPIbα clusters and activation of FcγRIIa, whose ligand-binding properties are enhanced by localization to rafts.15 The result is a more severe drop in platelet count compared to patients with anti-αIIbβ3 antibodies.5 The fact that αIIbβ3 does not translocate to rafts upon ligand binding may explain the inability of autoantibodies against this receptor to efficiently activate FcγRIIa to generate additional destruction signals.10 Future studies should reveal whether generation of destruction signals is similar in other ITP patients with autoantibodies against GPIbα. Confirmation of this mechanism in a larger population may open up ways to explore the prevention of autoantibody-induced GPIbα association with FcγRIIa in lipid rafts as a possible therapy in ITP.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding: this study was supported by a grant from the Landsteiner Foundation of Blood transfusion Research (LSBR grant n. 0807). JWNA is supported by The Netherlands Thrombosis Foundation. RTU is a research fellow of the Dutch Heart Foundation (grant n. 2010T068)
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–86 [DOI] [PubMed] [Google Scholar]
- 2.Peerschke EI, Andemariam B, Yin W, Bussel JB. Complement activation on platelets correlates with a decrease in circulating immature platelets in patients with immune thrombocytopenic purpura. Br J Haematol. 2010;148(4):638–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semple JW, Provan D. The immunopathogenesis of immune thrombocytopenia: T cells still take center-stage. Curr Opin Hematol. 2012;19(5):357–62 [DOI] [PubMed] [Google Scholar]
- 4.Stasi R, Evangelista ML, Stipa E, Buccisano F, Venditti A, Amadori S. Idiopathic thrombocytopenic purpura: current concepts in patho-physiology and management. Thromb Haemost. 2008;99(1):4–13 [DOI] [PubMed] [Google Scholar]
- 5.Hou M, Stockelberg D, Kutti J, Wadenvik H. Antibodies against platelet GPIb/IX, GPIIb/IIIa, and other platelet antigens in chronic idiopathic thrombocytopenic purpura. Eur J Haematol. 1995;55 (5):307–14 [DOI] [PubMed] [Google Scholar]
- 6.Go RS, Johnston KL, Bruden KC. The association between platelet autoantibody specificity and response to intravenous immunoglobulin G in the treatment of patients with immune thrombocytopenia. Haematologica. 2007;92(2):283–4 [DOI] [PubMed] [Google Scholar]
- 7.Cauwenberghs N, Schlammadinger A, Vauterin S, Cooper S, Descheemaeker G, Tornai I, et al. Fc-receptor dependent platelet aggregation induced by monoclonal antibodies against platelet glycoprotein Ib or von Willebrand factor. Thromb Haemost. 2001;85(4):679–85 [PubMed] [Google Scholar]
- 8.Yanabu M, Nomura S, Fukuroi T, Soga T, Kondo K, Sone N, et al. Synergistic action in platelet activation induced by an antiplatelet autoantibody in ITP. Br J Haematol. 1991;78(1):87–93 [DOI] [PubMed] [Google Scholar]
- 9.Maugeri N, Rovere-Querini P, Evangelista V, Covino C, Capobianco A, Bertilaccio MT, et al. Neutrophils phagocytose activated platelets in vivo: a phosphatidylserine, P-selectin, and {beta}2 integrin-dependent cell clearance program. Blood. 2009;113(21):5254–65 [DOI] [PubMed] [Google Scholar]
- 10.Shrimpton CN, Borthakur G, Larrucea S, Cruz MA, Dong JF, Lopez JA. Localization of the adhesion receptor glycoprotein Ib-IX-V complex to lipid rafts is required for platelet adhesion and activation. J Exp Med. 2002;196(8):1057–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falati S, Edmead CE, Poole AW. Glycoprotein Ib-V-IX, a receptor for von Willebrand factor, couples physically and functionally to the Fc receptor gamma-chain, Fyn, and Lyn to activate human platelets. Blood. 1999;94(5):1648–56 [PubMed] [Google Scholar]
- 12.Sullam PM, Hyun WC, Szollosi J, Dong J, Foss WM, Lopez JA. Physical proximity and functional interplay of the glycoprotein Ib-IX-V complex and the Fc receptor FcgammaRIIA on the platelet plasma membrane. J Biol Chem. 1998;273(9):5331–6 [DOI] [PubMed] [Google Scholar]
- 13.Gitz E, Koekman CA, van den Heuvel DJ, Deckmyn H, Akkerman JW, Gerritsen HC, et al. Improved platelet survival after cold storage by prevention of glycoprotein Ibalpha clustering in lipid rafts. Haematologica. 2012;97(12):1873–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmeister KM, Felbinger TW, Falet H, Denis CV, Bergmeier W, Mayadas TN, et al. The clearance mechanism of chilled blood platelets. Cell. 2003;112(1):87–97 [DOI] [PubMed] [Google Scholar]
- 15.Bournazos S, Hart SP, Chamberlain LH, Glennie MJ, Dransfield I. Association of FcgammaRIIa (CD32a) with lipid rafts regulates ligand binding activity. J Immunol. 2009;182(12):8026–36 [DOI] [PubMed] [Google Scholar]