Since the first report,1 very few monozygotic pairs of twins with concordant leukemia have been described.2 The interval between the onset of overt disease in each of the twins usually varies from some months to some years.2 Genetic changes in childhood acute leukemia are highly variable and patient specific (clone-specific genomic breakpoints and somatic mutations). Therefore, the finding of a shared unique mutation in monozygotic twins would imply the monoclonal origin in one fetus in utero.2–4 In patients with acute myeloblastic leukemia (AML), mutations in CCAAT-enhancer-binding-protein α (C/EBPA) are common, occurring in 5–14% of patients5 and germline mutations in C/EBPA gene have been described.6,7 We present a unique case of monozygotic and monohorionic twins who developed AML with an unusually long difference in disease onset, in whom we investigated the genetic background.
Twin A was diagnosed with M1-AML at the age of 21 months. GTG banding revealed normal karyotype. She was treated according to AML-BFM 83 protocol followed by syngenic bone marrow transplantation (BMT). She is in the first complete remission (CR) 19 years later. Twin B was diagnosed with M1-AML at the age of 15 years. GTG banding revealed normal karyotype. She was treated according to AML-BFM 98 protocol and is in the first CR five years later. Normal karyotype and favorable outcome prompted us to search for C/EBPA gene mutations. DNA was isolated from bone marrow smears of both patients at the time of diagnosis and from buccal swaps and peripheral blood in CR. The C/EBPA gene was polymerase chain reaction (PCR) amplified and direct sequenced with ABI-PRISM 310 sequencer. Molecular studies were negative for FLT3-ITD, NPM1 mutations for both twins.
Both twins carried a germline N-terminal frameshift C/EBPA mutation, a 19-base pair deletion (c.147_165del, p.Glu50fs) (Figure 1A). Interestingly, at the time of diagnosis of AML, both twins carried an additional identical somatic C-terminal mutation: an inframe insertion of aminoacid lysine (c.936_937dupAAG, p.313_314insLys) (Figure 1B). Twin A, also had the third mutation in C-terminal part of C/EBPA gene, somatic inframe deletion of 50 aminoacids (c.911_1060del, p.304-353del) (Figure 1C). At the present time, DNA from buccal swaps and peripheral blood have been examined and while the germline C/EBPA mutation was found in both twins, we did not find either of the somatic mutations.
Figure 1.
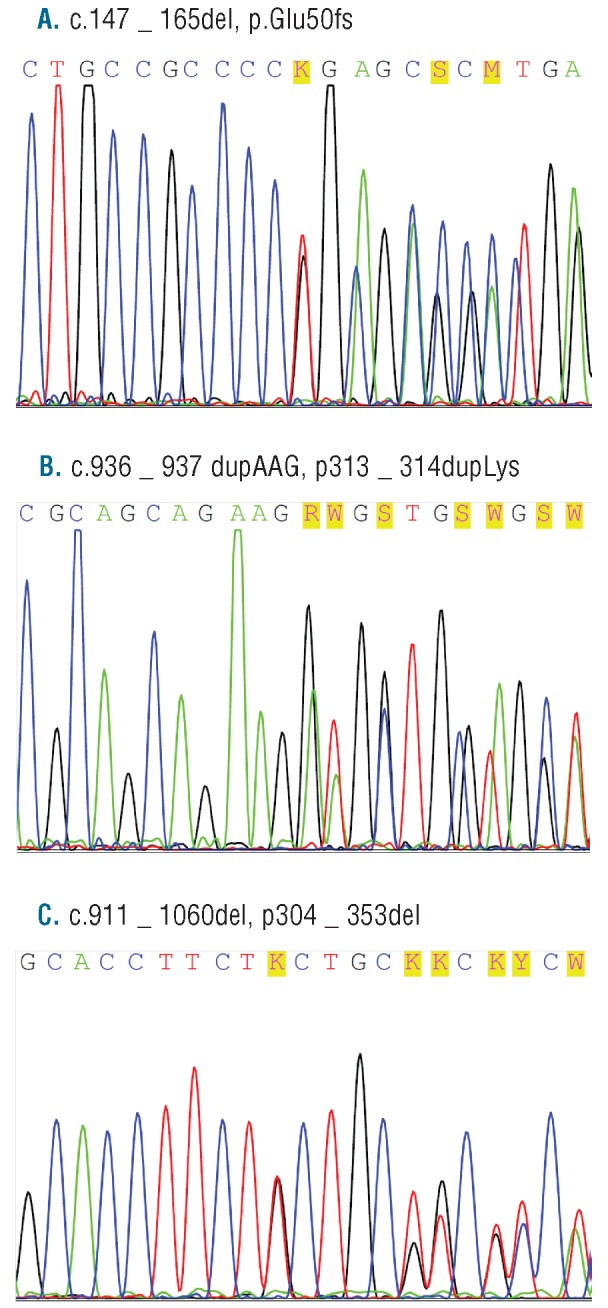
Sequences of CEBPA mutations at diagnosis of AML. (A) Germline N-terminal frameshift CEBPA 19-base pair deletion (c.147_165del, p.Glu50fs). (B) Identical somatic C-terminal an inframe insertion of aminoacid lysine (c.936_937dupAAG, p.313_314insLys). (C) An inframe deletion of 50 aminoacids (c.911_1060del, p.304-353del) present at diagnosis only in twin A.
According to the accepted hypothesis, concordant leukemia arises as a consequence of intraplacental spread of an initiated pre-leukemic clone from one twin to the other. The transcription factor C/EBPα is a regulator of myeloid development, directing granulocyte and monocyte differentiation. Findings suggest that C/EBPA germline mutation predispose to the development of leukemia and that the second ‘hit’ is an acquired mutation in the remaining C/EBPA allele.6 Sporadic mutations in C/EBPA occur in patients with AML8 and can be categorized into 2 types. N-terminal frameshift mutations result in expression of a truncated dominant negative C/EBPαp30 isoform. C-terminal in-frame insertions or deletions result in alteration of the leucine zipper domain preventing dimerization of transcription factor and DNA binding.5,9 Most AML patients with C/EBPA mutations have both mutations simultaneously and display a favorable outcome.9,10 It was shown in families in which several members had the same germline CEBPA mutation and different somatic CEBPA mutations that somatic mutations represented the second event for development of AML.6,7 At the time of diagnosis, the twins shared, in addition to the CEBPA germline mutation, also the same somatic CEBPA mutation, namely inframe insertion of lysine (p.313_314insLys), which has not been described in the literature. It is hard to speculate that there is a common mechanism for development of this somatic mutation. We suspect that this mutation has arisen in one twin and has been intraplacentaly transferred to the other twin in utero. The interval between AML onset in our twins is considerably longer than reported elsewhere. The syngenic bone marrow donor twin developed AML 13 years after the diagnosis of her twin. Both sisters still carry germline CEBPA mutation and are, therefore, at risk for the second hit and development of novel AML.
The implications of this are debatable. Some authors6 propose that BMT should be considered in patients with AML with germline CEBPA mutation to replace the mutated hematopoetic stem cells and eliminate the risk of subsequent C-terminal somatic mutations and secondary AML. However, it seems clear that molecular scrutiny of the healthy monozygotic co-twin is important if the individual is considered as a bone marrow donor for a twin with leukemia. Therefore, in monozygotic twins, we propose searching for germline CEBPA mutations before considering syngenic BMT.
Acknowledgments
We thank both patients for their kind participation in our study. First and second authors contributed equally to the work. The authors stated that they had no interests that might be perceived as posing a conflict or bias.
Footnotes
Funding: The study was supported in part by the Slovenian Research Agency grants J3–4220, and P3–0343.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Senator H. Zur Kenntniss der Leukämie und Pseudoleukämie im Kindesalter. Berliner Klinische Wochenschrift. 1882;35:533–6 [Google Scholar]
- 2.Greaves MF, Maia AT, Wiemels JL, Ford AM. Leukemia in twins: lessons in natural history. Blood. 2003;102(7):2321–33 [DOI] [PubMed] [Google Scholar]
- 3.Greaves MF, Wiemels J. Origins of chromosome translocations in childhood leukaemia. Nat Rev Cancer. 2003;3(9):639–49 [DOI] [PubMed] [Google Scholar]
- 4.Clarkson B, Boyse EA. Possible explanation of the high concordance for acute leukaemia in monozygotic twins. Lancet. 1971;1(7701): 699–701 [DOI] [PubMed] [Google Scholar]
- 5.Paz-Priel I, Friedman A. C/EBPα dysregulation in AML and ALL. Crit Rev Oncog. 2011;16(1–2):93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stelljes M, Corbacioglu A, Schlenk RF, Döhner K, Frühwald MC, Rossig C, et al. Allogeneic stem cell transplant to eliminate germline mutations in the gene for CCAAT-enhancer-binding protein α from hematopoietic cells in a family with AML. Leukemia. 2011;25(7): 1209–10 [DOI] [PubMed] [Google Scholar]
- 7.Sellick GS, Spendlove HE, Catovsky D, Pritchard-Jones K, Houlston RS. Further evidence that germline CEBPA mutations cause dominant inheritance of acute myeloid leukaemia. Leukemia. 2005;19(7):1276–8 [DOI] [PubMed] [Google Scholar]
- 8.Pabst T, Mueller BU, Zhang P, Radomska HS, Narravula S, Schnittger S, et al. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPalpha), in acute myeloid leukemia. Nat Genet. 2001;27(3):263–70 [DOI] [PubMed] [Google Scholar]
- 9.Pabst T, Eyholzer M, Fos J, Mueller BU. Heterogeneity within AML with CEBPA mutations; only CEBPA double mutations, but not single CEBPA mutations are associated with favourable prognosis. Br J Cancer. 2009;100(8):1343–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renneville A, Boissel N, Gachard N, Naguib D, Bastard C, de Botton S, et al. The favorable impact of CEBPA mutations in patients with acute myeloid leukemia is only observed in the absence of associated cytogenetic abnormalities and FLT3 internal duplication. Blood. 2009;113(21):5090–3 [DOI] [PubMed] [Google Scholar]


