Accelerated phase chronic myeloid leukemia (AP-CML) is characterized by tyrosine kinase inhibitor (TKI) resistance, additional cytogenetic abnormalities, and tyrosine kinase mutations.1,2 Although the recently approved TKI ponatinib may be effective in some patients with TKI-resistant AP-CML, patients with resistance or intolerance to multiple TKIs may benefit from a non-TKI approach.
Omacetaxine mepesuccinate (“omacetaxine”) provides a unique mechanistic approach to the treatment of relapsed/refractory CML that, unlike TKIs, does not require binding to BCR-ABL and is not affected by resistance-conferring mutations in BCR-ABL.3–6 Functioning as a protein synthesis inhibitor;7,8 omacetaxine reduces levels of multiple oncoproteins, including BCR-ABL, and induces apoptosis in leukemic stem cells.3–6 Omacetaxine has demonstrated clinical activity in chronic phase (CP)-CML patients previously treated with TKIs.9 Here we report the efficacy and safety of omacetaxine in patients with AP-CML who have demonstrated intolerance or resistance to two or more approved TKIs.
Patients with AP-CML enrolled in two international open-label phase II studies of omacetaxine (CML-202 and CML-203) were included in this pooled analysis if they had previously received imatinib and had documented resistance or intolerance to dasatinib and/or nilotinib. AP-CML was defined as: 15–30% blasts, 30% or over blasts and promyelocytes, or 20% or over basophils in peripheral blood or bone marrow; platelet count less than 100 × 109/L unrelated to therapy; or clonal evolution. Primary end points were rates of major hematologic response (MaHR) and major cytogenetic response (MCyR). Treatment and assessments were identical in the two studies. Patients received induction therapy with omacetaxine 1.25 mg/m2 administered subcutaneously twice daily (BID) Days 1 to 14 every 28 days for up to six cycles or until hematologic or cytogenetic response. Patients who achieved hematologic or cytogenetic response were switched to maintenance omacetaxine therapy 1.25 mg/m2 BID for 7 consecutive days every 28 days. In patients who developed grade 4 neutropenia or grade 3 or over thrombocytopenia, treatment was delayed until recovery to values of grade 2 or under, and the number of consecutive days of treatment was reduced by two days in subsequent treatment cycles. Similar adjustments were made for treatment-related non-hematologic toxicities that did not respond to supportive care.
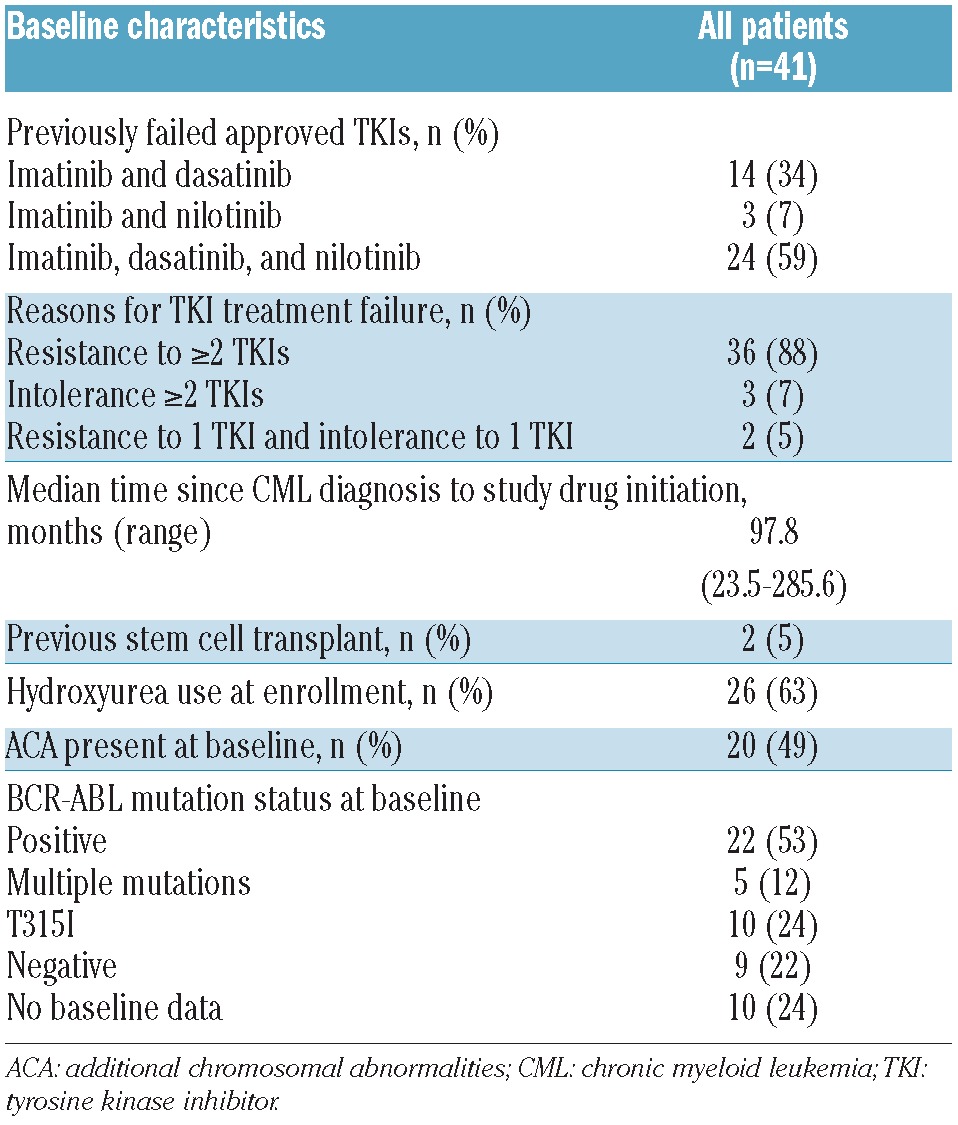
Forty-one patients with AP-CML met inclusion criteria for this analysis; baseline characteristics are shown in Table 1. At the time of data cut off (January 2011), 39 patients (95%) had discontinued the study due to progressive disease (49%), lack of efficacy (17%), death (12%), adverse events (5%), or withdrawal by request (12%). Median duration of follow up was 11.5 months (95%CI: 6.8–16.0 months).
Table 1.
Patients’ baseline characteristics.
Patients received a median of two treatment cycles (range 1–29 cycles); median duration of omacetaxine exposure was 1.9 months (range 0.03–30 months). Two patients remained on study treatment at the time of this analysis, having received 13 and 14 treatment cycles over a period of 31.2 months and 18.1 months, respectively. The median number of treatment days per cycle was 14 (range 1–17 days) in cycles 1 to 3, consistent with induction dosing. The number of patients receiving 14 days of treatment each cycle gradually decreased, from 85% (35 of 41 patients) in cycle 1 to 50% (10 of 20 patients) in cycle 3, with a median of 7 to 8 treatment days in cycles 4 to 6, consistent with a transition to maintenance dosing.
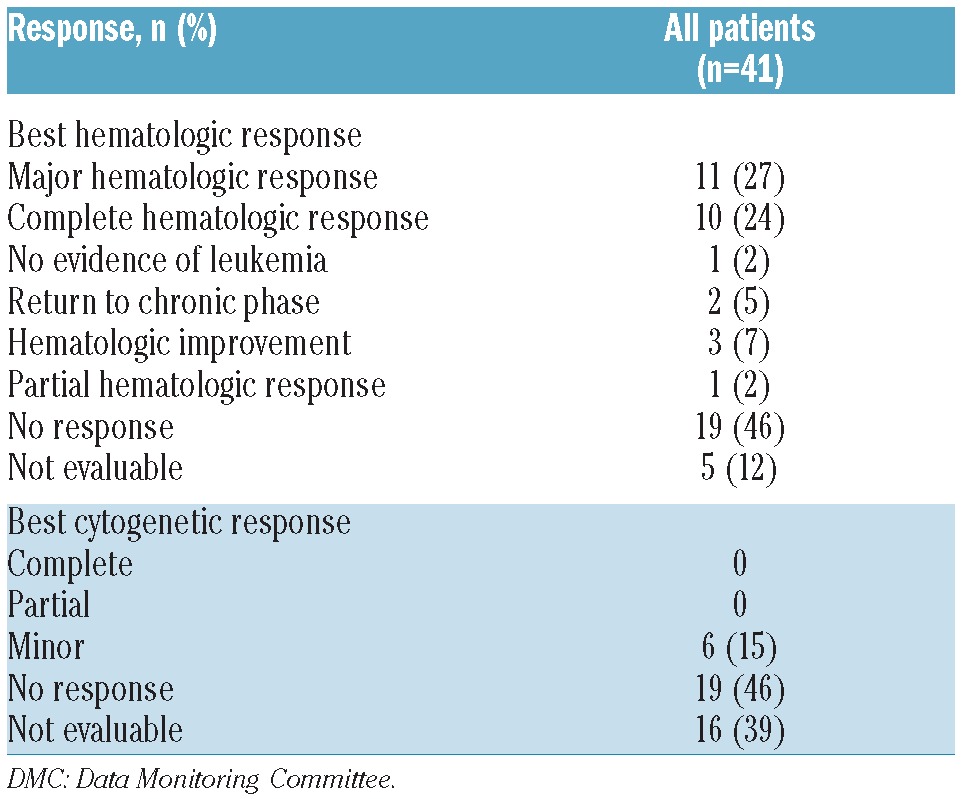
MaHR was achieved or maintained in 11 patients (27%) (Table 2). MaHR was 40% (6 of 15) among patients who were not receiving hydroxyurea at baseline and 19% (5 of 26) in those who were. In an ad hoc efficacy analysis that excluded 6 patients with MaHR at baseline, the overall rate of MaHR was 14% (5 of 35). Among patients with evidence of clonal evolution at baseline, the MaHR rate was 25% (5 of 20); in 2 of these patients, clonal evolution became undetectable with omacetaxine treatment. The rate of MaHR was 32% (7 of 22) in patients with any mutation in BCR-ABL at baseline, 40% (2 of 5) in patients with multiple mutations, and 50% (5 of 10) in patients with T315I. Six patients (15%) achieved minor CyR (Table 2); the median number of cycles necessary to achieve minor CyR in these patients was 1.5 (range 1–3).
Table 2.
Best DMC-adjudicated hematologic and cytogenetic responses to omacetaxine.
The median duration of MaHR was 9.0 months (95%CI: 3.6–14.1 months). Patients who had received two prior TKIs had a longer median duration of response (13.4 months; 95%CI: 5.6–14.1 months) than those who had received three prior TKIs (6.4 months; 95%CI: 3.6 months-NA). The duration of best CyR was 3.0 months (95%CI: 2.3–3.9 months). Median failure-free survival (FFS) was 4.7 months (95%CI: 2.1–7.0 months) and median overall survival (OS) was 16.0 months (95%CI: 8.2–24.6 months). Patients who achieved MaHR had longer median FFS (9.0 vs. 3.5 months) and OS (24.6 vs. 8.9 months) than those without MaHR. Among patients with minor CyR (n=6), median FFS was 7.9 months (95%CI: 1.7-NA) and median OS was 35.8 months (95%CI: 6.8–57.2 months).
The toxicity profile associated with omacetaxine was primarily hematologic. Grade 3/4 hematologic adverse events were reported in 78% of patients (thrombocytopenia 51%; anemia 37%; neutropenia 22%). Febrile neutropenia was reported in 6 patients (15%). Granulocyte-stimulating factors were administered in 5% of patients and erythropoiesis-stimulating agents in 17%. Thirty-one patients (76%) received red blood cells and 24 patients (59%) received platelets. The most common non-hematologic adverse events were infection (all grades, 59%; grade ≥3, 27%), diarrhea (37%), pyrexia (29%), fatigue (24%), asthenia (24%), and nausea (22%). Of the 32 patients receiving at least two cycles of treatment, 20 (63%) had at least one cycle delay during the study. The most common reasons for treatment delays were thrombocytopenia (36% of delays) and neutropenia (20% of delays).
In conclusion, omacetaxine may be a feasible and tolerable treatment option for this patient population. Subcutaneous omacetaxine induced or maintained hematologic response and minor cytogenetic response in a minority of patients with AP-CML who had failed multiple TKIs. Although response duration was limited, the achievement of response may serve as a bridge to allogeneic stem cell transplantation, which remains the best possibility for long-term survival in patients with advanced CML.
Acknowledgments
The authors would like to thank the investigators who contributed accelerated-phase patients to this study: Maria Baer, Raghunadharao Digumarti, Laurence Legros, Armin Leitner, Jeffrey Lipton, David Marin, Tamas Masszi, Mauricette Michallet, Candido Rivera, Philippe Rousselot, and Krzysztof Warzocha. We would also like to thank Madeleine Etienne and Elodie Gadolet, CRAs, Hematology department 1G in Pierre Bénite, France, for assistance with study enrollment at this site. The authors would like to thank ChemGenex Pharmaceuticals, now an indirect wholly owned subsidiary of Teva Pharmaceutical Industries Ltd., for study funding and Teva Branded Pharmaceutical Products R&D, Inc. for funding of medical writing assistance. The authors would also like to thank Peter Brown, PhD, of Teva Pharmaceuticals for his critical review of the data and manuscript and Glen Davis of Teva Pharmaceuticals for his dedicated support in the collection and review of additional clinical data.
Footnotes
Funding: FEN reports grants from Novartis and Bristol-Myers Squibb (BMS), and personal fees from Pfizer, Novartis, Teva, Ariad, and BMS, outside the submitted work. HJK has nothing to disclose. LA reports grants and personal fees from Teva/Cephalon during the conduct of the study, and personal fees from BMS, grants and personal fees from Ariad, grants from Merck, grants and personal fees from Novartis, grants from Pfizer, personal fees from Celgene, and grants from Millenium, outside the submitted work. DR reports personal fees from BMS, Novartis, and Teva, outside the submitted work. HK reports grants from ChemGenex during the conduct of the study and grants from Novartis, BMS, Ariad, and Pfizer, outside the submitted work. MB reports other support from Teva, outside the submitted work. AC reports personal fees from ChemGenex, outside the submitted work. A-CB reports other support from ChemGenex during the conduct of the study. JL reports personal fees from Teva Branded Pharmaceutical Products R&D during the conduct of the study. JEC reports grants and non-financial support from ChemGenex during the conduct of the study, and grants and personal fees from Ariad, grants from BMS, grants and personal fees from Pfizer, and grants from Novartis, outside the submitted work.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Branford S, Rudzki Z, Walsh S, Parkinson I, Grigg A, Szer J, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102(1):276–83 [DOI] [PubMed] [Google Scholar]
- 2.Soverini S, Colarossi S, Gnani A, Rosti G, Castagnetti F, Poerio A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006;12(24):7374–9 [DOI] [PubMed] [Google Scholar]
- 3.Allan EK, Holyoake TL, Craig AR, Jorgensen HG. Omacetaxine may have a role in chronic myeloid leukaemia eradication through downregulation of Mcl-1 and induction of apoptosis in stem/progenitor cells. Leukemia. 2011;25(6):985–94 [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Hu Y, Michaels S, Segal D, Brown D, Li S. Inhibitory effects of omacetaxine on leukemic stem cells and BCR-ABL-induced chronic myeloid leukemia and acute lymphoblastic leukemia in mice. Leukemia. 2009;23(8):1446–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuroda J, Kamitsuji Y, Kimura S, Ashihara E, Kawata E, Nakagawa Y, et al. Anti-myeloma effect of homoharringtonine with concomitant targeting of the myeloma-promoting molecules, Mcl-1, XIAP, and beta-catenin. Int J Hematol. 2008;87(5):507–15 [DOI] [PubMed] [Google Scholar]
- 6.Tang R, Faussat AM, Majdak P, Marzac C, Dubrulle S, Marjanovic Z, et al. Semisynthetic homoharringtonine induces apoptosis via inhibition of protein synthesis and triggers rapid myeloid cell leukemia-1 down-regulation in myeloid leukemia cells. Mol Cancer Ther. 2006;5(3):723–31 [DOI] [PubMed] [Google Scholar]
- 7.Chen R, Gandhi V, Plunkett W. A sequential blockade strategy for the design of combination therapies to overcome oncogene addiction in chronic myelogenous leukemia. Cancer Res. 2006;66(22):10959–66 [DOI] [PubMed] [Google Scholar]
- 8.Tujebajeva RM, Graifer DM, Karpova GG, Ajtkhozhina NA. Alkaloid homoharringtonine inhibits polypeptide chain elongation on human ribosomes on the step of peptide bond formation. FEBS Lett. 1989;257(2):254–6 [DOI] [PubMed] [Google Scholar]
- 9.Cortes J, Nicolini FE, Wetzler M, Lipton JH, Akard L, Craig A, et al. Subcutaneous omacetaxine mepesuccinate in patients with chronic-phase chronic myeloid leukemia previously treated with two or more tyrosine kinase inhibitors including imatinib. Clin Lymphoma Myeloma Leuk. 2013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


