Abstract
Reports are emerging of hematologic responses associated with iron chelation therapy; however, studies are limited in aplastic anemia patients. Deferasirox reduced iron overload in aplastic anemia patients enrolled in the EPIC (Evaluation of Patients’ Iron Chelation with Exjade®) study (n=116). A post hoc analysis of hematologic responses was conducted on 72 patients with evaluable hematologic parameters (according to UK guideline criteria), 24 of whom received deferasirox without concomitant immunosuppressive treatment. Partial hematologic responses were observed in 11 of 24 (45.8%) patients; all became transfusion-independent. One patient had an additional platelet response and one patient had an additional platelet and hemoglobin response. Mean serum ferritin levels at end of study were significantly reduced in partial hematologic responders (n=11; −3948±4998 ng/mL; baseline 6693±7014 ng/mL; percentage change from baseline −45.7%; P=0.0029). In non-responders, the reduction in serum ferritin was less pronounced (n=13; −2021±3242 ng/mL; baseline 4365±3063 ng/mL; % change from baseline −27.6%; P=0.0171). Alongside reduction in iron overload, deferasirox may, therefore, improve hematologic parameters in a subset of aplastic anemia patients. Further investigation is required to elucidate the mechanisms involved. (Clinicaltrials.gov identifier: NCT00171821)
Introduction
Aplastic anemia (AA) is a rare, heterogeneous bone marrow failure disorder, whereby patients most commonly present with symptoms of anemia.1 Immunosuppressive therapy, including cyclosporin and antithymocyte globulin, is used in patients for whom bone marrow transplantation is not an option, with response rates of 60–80%.1 However, supportive care with red blood cell transfusions is also essential in many patients to maintain adequate hemoglobin levels (70–80 g/L in younger patients or >80 g/L in older patients or those with a history of coronary artery disease),2 particularly since the response to immunosuppressive therapy may take several months. Iron overload can, therefore, become a significant problem in regularly transfused patients, leading to organ damage, particularly in the liver and heart.3 Studies of iron chelation therapy in patients with AA have mainly focused on efficacy in reducing tissue iron and safety.3–6
However, reports are now emerging of hematologic improvements associated with iron chelation therapy in patients with AA.7–10 As iron overload also has a suppressive effect on erythroid progenitors11 and may increase transfusion requirements,12 it is of interest to evaluate hematologic responses in patients with AA receiving iron chelation therapy. The 1-year, prospective EPIC (Evaluation of Patients’ Iron Chelation with Exjade®) study enrolled 116 iron-overloaded AA patients.4,13 Overall, the EPIC study demonstrated a significant and clinically relevant reduction in iron overload as assessed by serum ferritin levels. A post hoc analysis was performed based on hematologic response criteria reported by Camitta14 to evaluate the association between deferasirox treatment and changes in hematologic parameters in iron-overloaded patients with AA.
Design and Methods
EPIC was a prospective, multicenter, open-label, 1-year study conducted by 136 investigators across 23 countries. The study design has been reported previously.4,13 Male or female patients were enrolled with transfusional iron overload as shown by a serum ferritin level of 1000 ng/mL or more, or less than 1000 ng/mL but with a history of multiple transfusions (>20 transfusions or 100 mL/kg of red blood cells) and R2 magnetic resonance imaging-confirmed liver iron concentration of or exceeding 2 mg Fe/g dry weight. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki, and was approved by an Institutional Review Board/Independent Ethics Committee. Written, informed consent was obtained from all patients prior to study participation.
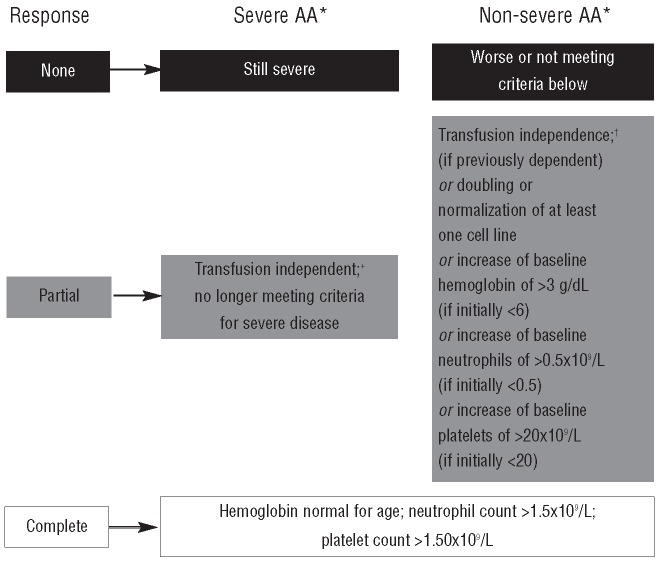
The definition and severity of AA were assessed based on the UK treatment guidelines as summarized here.1 For a definition of AA, patients must exhibit two of the following parameters: i) hemoglobin levels <100 g/L; ii) platelet count <50 x 109/L; and iii) neutrophil count <1.5 x 109/L. Patients were classified as having severe AA if bone marrow cellularity was less than 25%, or 25–50% with less than 30% residual hematopoietic cells (determined by comparison with normal controls), and two of the following were recorded: neutrophil count less than 0.5 x 109/L, platelet count less than 20 x 109/L or reticulocyte count less than 20 x 109/L. Patients were assigned as having non-severe AA if they did not meet the criteria for severe AA. As no bone marrow data were recorded in the EPIC study, AA severity was assessed based on hematologic parameters only. Reticulocyte counts were also not available and, therefore, severe AA was considered when both platelet and neutrophil criteria were fulfilled. Hemoglobin, platelet and neutrophil responses were assessed for patients with severe and non-severe AA according to criteria reported by Camitta14 (Figure 1).
Figure 1.
Overview of hematologic response criteria used in the analysis.14 *Each criterion was confirmed with no measure within 28 days that disproved the response. †Transfusion independence was defined as at least one 8-week period (56 days) without any transfusion. Patients with no transfusions in the year prior to study entry were excluded from transfusion responders.
Serum ferritin changes from baseline to end of study were assessed for hematologic responders and non-responders. Transfusions were recorded during the study and pre-transfusion blood counts were also assessed. Patients who received at least one dose of deferasirox with both baseline and end of study assessments were included in the analysis. Reported P values are based on a Wilcoxon rank test.
Results and discussion
Patients’ characteristics
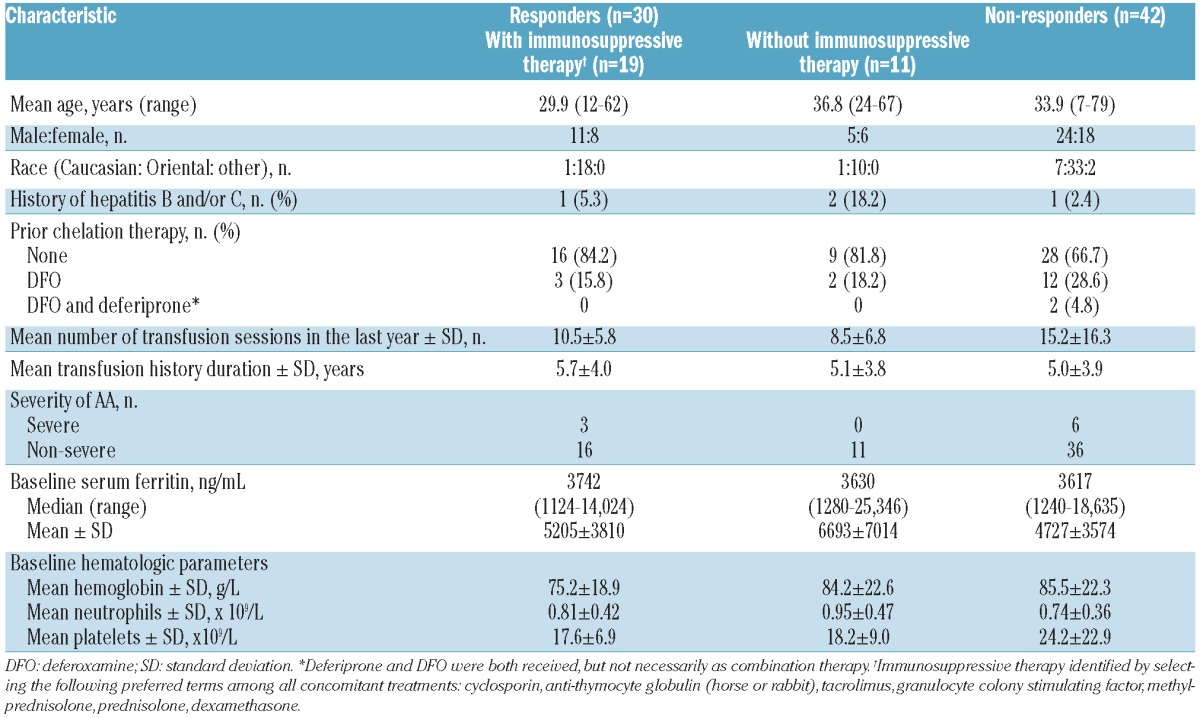
Of the 116 iron-overloaded patients with AA enrolled in the EPIC study, 72 patients were evaluable for hematologic responses according to the UK criteria; 9 (12.5%) and 63 (87.5%) were considered to have severe AA and non-severe AA, respectively (Table 1). Forty-eight (66.6%) patients received at least one concomitant immunosuppressive therapy: 7 patients with severe AA and 41 with non-severe AA.
Table 1.
Demographics and baseline characteristics of patients evaluable for a hematologic response according to the UK criteria.
Hematologic responses following deferasirox therapy
No patient, with or without concomitant immunosuppressive therapy, achieved a complete response. Overall, a partial hematologic response was observed in 11 of 24 (45.8%) patients not receiving concomitant immunosuppressive therapy and 19 of 48 (39.6%) patients who did receive concomitant immunosuppressive therapy (Table 1, Figure 2). In total, 42 patients (58.3%) did not have a hematologic response (29 and 13 patients with and without immunosuppressive therapy, respectively). The mean number of transfusion sessions in the year prior to study start was considerably higher in the non-responders compared with the hematologic responders and included a larger proportion of patients classified with severe AA (Table 1). Completion rate was 81.8% in responders without concomitant immunosuppressive therapy and 89.5% in responders with concomitant immunosuppressive therapy. In all non-responders, the completion rate was 66.7%. As immunosuppressive therapy can influence hematologic response, analyses were focused on patients receiving deferasirox without concomitant immunosuppressive therapy. Twenty-four patients (2 with severe AA and 22 with non-severe AA) received deferasirox without concomitant immunosuppressive therapy. All subsequent analyses shown are for patients treated with deferasirox without concomitant immunosuppressive therapy.
Figure 2.
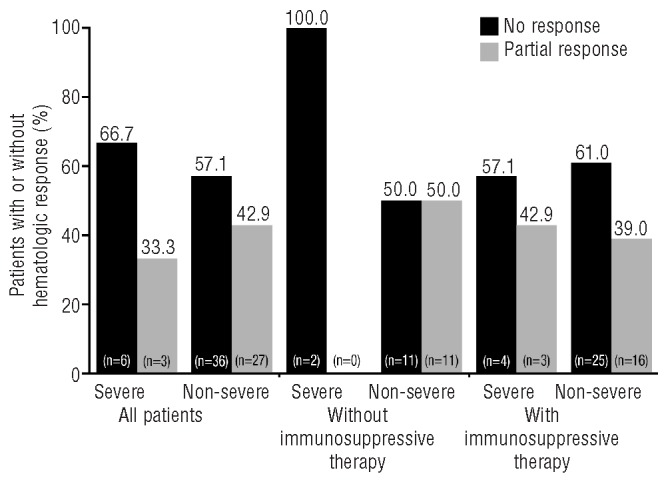
Hematologic responses with or without immunosuppressive treatment in patients with severe AA and non-severe AA.
Partial hematologic response was observed in 11 of 24 (45.8%) patients with non-severe AA, all of whom became transfusion independent during the study, defined as at least one 8-week period without any transfusions. Median time to response onset was 85 days (range 1–277). Nine patients achieved a transfusion response only (median time to response 85 days, range 1–277); 4 of these patients did not receive any transfusions during the study, having received between four and seven transfusions in the year prior to study entry. One patient had a transfusion and an additional platelet response (time to response 111 days) and one patient had a transfusion, platelet and hemoglobin response (time to response 83 days) as defined by the criteria reported by Camitta.14
At the end of study in the responders, mean±standard deviation (SD) platelet count was 28.1±13.9x109/L; mean±SD neutrophil count was 1.5±0.6x109/L, and mean hemoglobin was 93.0±23.9 g/L.
Hematologic responses according to efficacy of iron chelation
Mean serum ferritin levels at end of study were significantly reduced in partial hematologic responders (n=11; − 3948±4998 ng/mL; baseline 6693±7014 ng/mL; percentage change from baseline −45.7%; P=0.0029). In non-responders, the reduction in serum ferritin was less pronounced (n=13; −2021±3242 ng/mL; baseline 4365±3063 ng/mL; percentage change from baseline −27.6%; P=0.0171). The mean serum ferritin level at end of study was 2744±2514 ng/mL in partial responders and 2345±1892 ng/mL in non-responders. Mean deferasirox dose received in the responders was 19.0±3.9 mg/kg/day (median deferasirox exposure 367 days, range 320–394) and in non-responders was 18.4±5.50 mg/kg/day (median deferasirox exposure 365 days, range 77–390).
Conclusions
Although reports are emerging on hematologic improvements associated with iron chelation therapy in patients with myelodysplastic syndromes,8,15–21 there are only a few small studies reporting such improvements in patients with AA.7–10 This post hoc analysis of data from the EPIC trial presented here indicates that in addition to reducing iron burden, treatment with deferasirox for 1 year in patients with AA is associated with improvements in hematologic parameters, whether administered concomitantly with immunosuppressive therapy or not. In patients without concomitant immunosuppressive therapy, hematologic responses were only observed in patients with non-severe AA and in all cases included the development of transfusion independence, with 2 patients having an additional platelet/hemoglobin response. The exact mechanism by which deferasirox treatment leads to hematologic responses remains to be determined. One possible hypothesis is that as deferasirox facilitates the reduction in iron stores, erythropoietin production is up-regulated, resulting in a rise in hemoglobin.22 A further possibility is that deferasirox chelation decreases reactive oxygen species generation in the marrow microenvironment, leading to greater genomic stability, as has been suggested in patients with myelodysplastic syndromes.23 Based on the serum ferritin data in this analysis, hematologic responses were observed in patients with greater reductions in serum ferritin levels, suggesting that hematologic response might be dependent, at least partially, on reductions in levels of body iron overload. Interestingly, non-responders had received a larger number of transfusions in the year prior to study entry and included a higher proportion of patients categorized with severe AA, indicating more severe disease and iron overload status. It is possible that these patients would require a longer period of treatment to achieve hematologic response. Further studies are still required.
In conclusion, this analysis provides further evidence supporting previous observations in case reports that deferasirox treatment over 1 year may improve hematologic parameters in some iron-overloaded patients with AA.8–10 These findings add to the evidence in patients with AA and other bone marrow failure conditions, such as myelodysplastic syndromes.15–21 There are some limitations to these post hoc analyses. Since patients had been diagnosed with AA for a considerable time prior to the study (as demonstrated by a mean transfusion duration of approximately 5 years), details of the methodology used for diagnosis is not discussed in this paper. We also acknowledge that to determine the effect of iron chelation alone on hematologic response it is necessary to assess patients without concomitant medication; however, this may not be in line with the current treatment standards. This study is also limited by its post hoc nature and, therefore, we acknowledge that further prospective, controlled studies are needed to confirm these findings and clarify the mechanisms of hematologic improvement with deferasirox.
Acknowledgments
We thank Rebecca Helson, PhD (Mudskipper Business Ltd), for medical editorial assistance with this manuscript.
Footnotes
The online version of this paper has a Supplementary Appendix.
Funding
This study was sponsored by Novartis Pharma AG. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
Full details of EPIC investigators and participating centers are also available online.
References
- 1.Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147(1):43–70 [DOI] [PubMed] [Google Scholar]
- 2.AlKhouri N, Ericson SG. Aplastic anemia: review of etiology and treatment. Hosp Physician. 1999;35:46–52 [Google Scholar]
- 3.Lee JW. Iron chelation therapy in the myelodysplastic syndromes and aplastic anemia: a review of experience in South Korea. Int J Hematol. 2008;88(1):16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J-W, Yoon S-S, Shen ZX, Ganser A, Hsu H-C, Habr D, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: A subgroup analysis of 116 patients from the EPIC trial. Blood. 2010; 116(14):2448–54 [DOI] [PubMed] [Google Scholar]
- 5.Min Y, Cheong J, Kim H, Lee K, Yoon S, Lee J, et al. A multi-center, open label study evaluating the efficacy of iron chelation therapy with deferasirox in transfusional iron overload patients with myelodysplastic syndromes or aplastic anemia using quantitative R2 MRI. Leuk Res. 2009;33(1 Suppl):S118–S119 (Abstract P102). [Google Scholar]
- 6.Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Cappellini MD, et al. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol. 2008; 80(2):168–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SJ, Han CW. Complete hematopoietic recovery after continuous iron chelation therapy in a patient with severe aplastic anemia with secondary hemochromatosis. J Korean Med Sci. 2008;23(2):320–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliva EN, Ronco F, Marino A, Alati C, Pratico G, Nobile F. Iron chelation therapy associated with improvement of hematopoiesis in transfusion-dependent patients. Transfusion. 2010;50(7):1568–70 [DOI] [PubMed] [Google Scholar]
- 9.Koh KN, Park M, Kim BE, Im HJ, Seo JJ. Restoration of hematopoiesis after iron chelation therapy with deferasirox in 2 children with severe aplastic anemia. J Pediatr Hematol Oncol. 2010;32(8):611–4 [DOI] [PubMed] [Google Scholar]
- 10.Lee JW, Jang PS, Chung NG, Cho B, Jeong DC, Kim HK. Iron chelation therapy with deferasirox results in recovery of hematopoiesis in a child with aplastic anemia. Pediatr Hematol Oncol. 2011;28(8): 718–20 [DOI] [PubMed] [Google Scholar]
- 11.Hartmann J, Sinzig U, Wulf G, Truemper LH, Braulke F, Konietschke F, et al. Evidence for a suppression of the colony forming capacity of erythroid progenitors by iron overload in patients with MDS. Blood. 2008;112: (Abstract 2694).(11) [Google Scholar]
- 12.Guariglia R, Martorelli MC, Villani O, Pietrantuono G, Mansueto G, D'Auria F, et al. Positive effects on hematopoiesis in patients with myelodysplastic syndrome receiving deferasirox as oral iron chelation therapy: A brief review. Leuk Res. 2011;35 (5):566–70 [DOI] [PubMed] [Google Scholar]
- 13.Cappellini MD, Porter JB, El-Beshlawy A, Li C-K, Seymour JF, Elalfy M, et al. Tailoring iron chelation by iron intake and serum ferritin trends: the prospective multicenter EPIC study of deferasirox in 1744 patients with various transfusion-dependent anemias. Haematologica. 2010;95(4):557–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camitta BM. What is the definition of cure for aplastic anemia? Acta Haematol. 2000; 103(1):16–8 [DOI] [PubMed] [Google Scholar]
- 15.Capalbo S, Spinosa G, Franzese MG, Palumbo G. Early deferasirox treatment in a patient with myelodysplastic syndrome results in a long-term reduction in transfusion requirements. Acta Haematol. 2009; 121(1):19–20 [DOI] [PubMed] [Google Scholar]
- 16.Jensen PD, Heickendorff L, Pedersen B, Bendix-Hansen K, Jensen FT, Christensen T, et al. The effect of iron chelation on haemopoiesis in MDS patients with transfusional iron overload. Br J Haematol. 1996; 94(2):288–99 [DOI] [PubMed] [Google Scholar]
- 17.Messa E, Cilloni D, Messa F, Arruga F, Roetto A, Saglio G. Deferasirox treatment improved the hemoglobin level and decreased transfusion requirements in four patients with the myelodysplastic syndrome and primary myelofibrosis. Acta Haematol. 2008;120(2):70–4 [DOI] [PubMed] [Google Scholar]
- 18.Breccia M, Loglisci G, Salaroli A, Cannella L, Santopietro M, Alimena G. Deferasirox treatment interruption in a transfusion-requiring myelodysplastic patient led to loss of erythroid response. Acta Haematol. 2010;124(1):46–8 [DOI] [PubMed] [Google Scholar]
- 19.Okabe H, Suzuki T, Omori T, Mori M, Uehara E, Hatano K, et al. Hematopoietic recovery after administration of deferasirox for transfusional iron overload in a case of myelodysplastic syndrome. Rinsho Ketsueki. 2009;50(11):1626–9 [PubMed] [Google Scholar]
- 20.Gattermann N, Finelli C, Della Porta M, Fenaux P, Stadler M, Guerci-Bresler A, et al. Hematologic responses with deferasirox therapy in transfusion-dependent myelodysplastic syndromes patients. Haematologica. 2012;97(9):1364–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.List AF, Baer MR, Steensma DP, Raza A, Esposito J, Martinez-Lopez N, et al. Deferasirox reduces serum ferritin and labile plasma iron in RBC transfusion-dependent patients with myelodysplastic syndrome. J Clin Oncol. 2012;30(17):2134–9 [DOI] [PubMed] [Google Scholar]
- 22.Vreugdenhil G, Smeets M, Feelders RA, van Eijk HG. Iron chelators may enhance erythropoiesis by increasing iron delivery to haematopoietic tissue and erythropoietin response in iron-loading anaemia. Acta Haematol. 1993;89(2):57–60 [DOI] [PubMed] [Google Scholar]
- 23.Gattermann N, Rachmilewitz EA. Iron overload in MDS-pathophysiology, diagnosis, and complications. Ann Hematol. 2011; 90(1):1–10 [DOI] [PubMed] [Google Scholar]