Abstract
Anti-CD20-containing chemotherapy regimens have become the standard of care for patients with follicular lymphoma needing cytotoxic therapy. Four randomized trials demonstrated a clinical benefit for patients treated with rituximab. However, no long-term follow up (i.e. > 5 years) of these trials is yet available. Between May 2000 and May 2002, 358 newly diagnosed patients with high tumor burden follicular lymphoma were randomized to receive cyclophosphamide, adriamycin, etoposide and prednisolone plus interferon-α2a or a similar chemotherapy-based regimen plus rituximab, and outcome was up-dated. With a median follow up of 8.3 years, addition of rituximab remained significantly associated with prolonged event-free survival (primary end point) (P=0.0004) with a trend towards a benefit for overall survival (P=0.076). The Follicular Lymphoma International Prognostic Index score was strongly associated with outcome for both event-free and overall survival in univariate analysis and its prognostic value remained highly significant after adjusting for other significant covariates in multivariate models (P<0.0001 and P=0.001, respectively). Considering long-term toxicity, the addition of rituximab in the first-line setting was confirmed as safe with regards to development of secondary malignancies. Long-term follow up of patients with follicular lymphoma treated in the FL2000 study confirms the sustained clinical benefit of rituximab without long-term toxicity. This study was registered at ClinicalTrials.gov (Identifier:00136552).
Introduction
Follicular lymphoma (FL) is the second most common subtype of non-Hodgkin’s lymphoma (NHL) and accounts for approximately 20–30% of newly diagnosed cases, behind diffuse large B-cell lymphomas.1,2 A vast majority of patients present with a disseminated disease (i.e. stages III or IV in the Ann Arbor classification). In spite of a high initial chemosensitivity, the course of the disease is invariably characterized by repeated relapses and no cure has been achieved to date.3,4 Although no consensus has been reached on the regimen of chemotherapy (CHOP, CVP, MCP or bendamustine), anti-CD20 antibodies are now systematically combined with chemotherapy in FL since four randomized trials and one meta-analysis have demonstrated superior outcome for rituximab (R)-containing regimen in the first-line setting.5–10 R-chemotherapy has, therefore, become a new standard of care for disseminated FL worldwide and its broad use contributed to the recently described improvement in outcome after decades of disappointing results for this disease.11–13
Given the prolonged overall survival (OS) in patients with FL, progression- or event-free survival (PFS or EFS) have been widely used as surrogate markers for evaluation of OS end point in clinical trials. Nevertheless, long-term follow up of randomized trials is crucial to ascertain whether PFS or EFS improvement actually translates into sustained longer OS, precluding unexpected long-term toxicity or impairment in the use of a second-line regimen. To the best of our knowledge, we report here the longest follow up to date (i.e. > 8 years) of R-chemotherapy in patients with de novo FL enrolled in a randomized trial.
Design and Methods
Patients
Patients aged 18–75 years with grade 1, 2 or 3a FL were eligible for this open-label randomized trial. Inclusion and exclusion criteria, study design and follow-up assessment have been previously described9 and high tumor burden definition is available in the Online Supplementary Appendix. The protocol was approved by local or national ethics review committees, and patients were required to give written informed consent before being included in accordance with the Declaration of Helsinki. The FL2000 trial was registered on the National Institute of Health website (Identifier:00136552).
Treatments
Clinical staging, response evaluation and treatment arms were extensively described in the original publication reporting a 5-year analysis of the study (Online Supplementary Figure S1).9 In line with other studies,14,15 previous results from our co-operative group (GELF86 trial) demonstrated improved disease control associated with the addition of interferon to low-dose anthracycline combination chemotherapy regimen. This combination therapy was thus considered as the control arm of treatment for the present FL2000 trial.16–19 Briefly, patients either in the control ‘CHVP+I’ arm or the experimental ‘R-CHVP+I’ arm received 6 CHVP+I monthly courses consisting of cyclophosphamide (600 mg/m2 intravenously on Day 1), doxorubicin (25 mg/m2 intravenously on Day 1), etoposide (100 mg/m2 intravenously on Day 1) and prednisolone (40 mg/m2 orally from Day 1 to 5) combined to interferon-α2a (4.5 million unit (MU) or 3 MU per injection 3 times a week for patients ≤70 or >70 years, respectively). Patients assigned to the CHVP+I arm received 6 subsequent courses of CHVP every two months whereas patients in the R-CHVP+I arm received 6 infusions of rituximab along with CHVP during the first six months of treatment and no further CHVP course. Patients in both arms received 18 months of interferon-α2a. Clinical examination for patients who completed the treatment was performed every three months for the first year, every six months for five years, and then on a yearly basis. CT scans were performed yearly for five years and then when clinically indicated.
Statistical analysis
Further details regarding initial randomization, stratification methods and endpoints are available in the Online Supplementary Appendix. Survival curves were constructed with the Kaplan-Meier method and compared with the log rank test.20 Cox proportional hazards regression model was used to assess the effect of multiple variables on OS and EFS.21 Significant variables at P=0.05 in univariate analyses were incorporated into multivariate models. The hazard function h(t) represents the evolution of the hazard rate over time. The hazard rate is defined as the probability per time unit (i.e. per year) that a case that has survived to the beginning of the respective interval will fail in that interval (Online Supplementary Appendix). All analyses were performed on an intention-to-treat basis. As the FLIPI prognostic score was not published when patients were enrolled in the FL2000 trial,22 its calculation was retrospectively assessed based upon prospectively collected data.
Results
Patients’ characteristics
Between May 2000 and May 2002, 360 patients with high tumor burden FL were randomly assigned to receive either CHVP+I or R-CHVP+I. Two patients were excluded (for consent withdrawal and major inclusion violation) and 358 patients (183 in the CHVP+I group and 175 in the R-CHVP+I group of treatment) were, therefore, analyzed (Figure 1).
Figure 1.
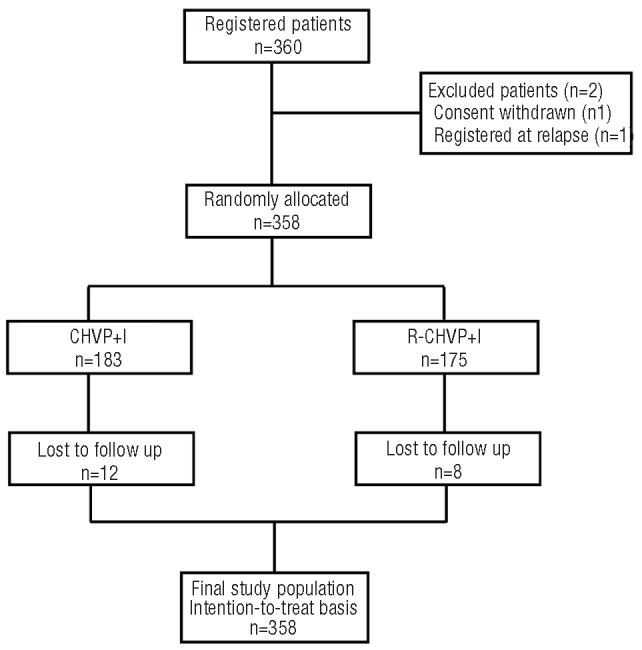
Trial profile of the FL2000 study.
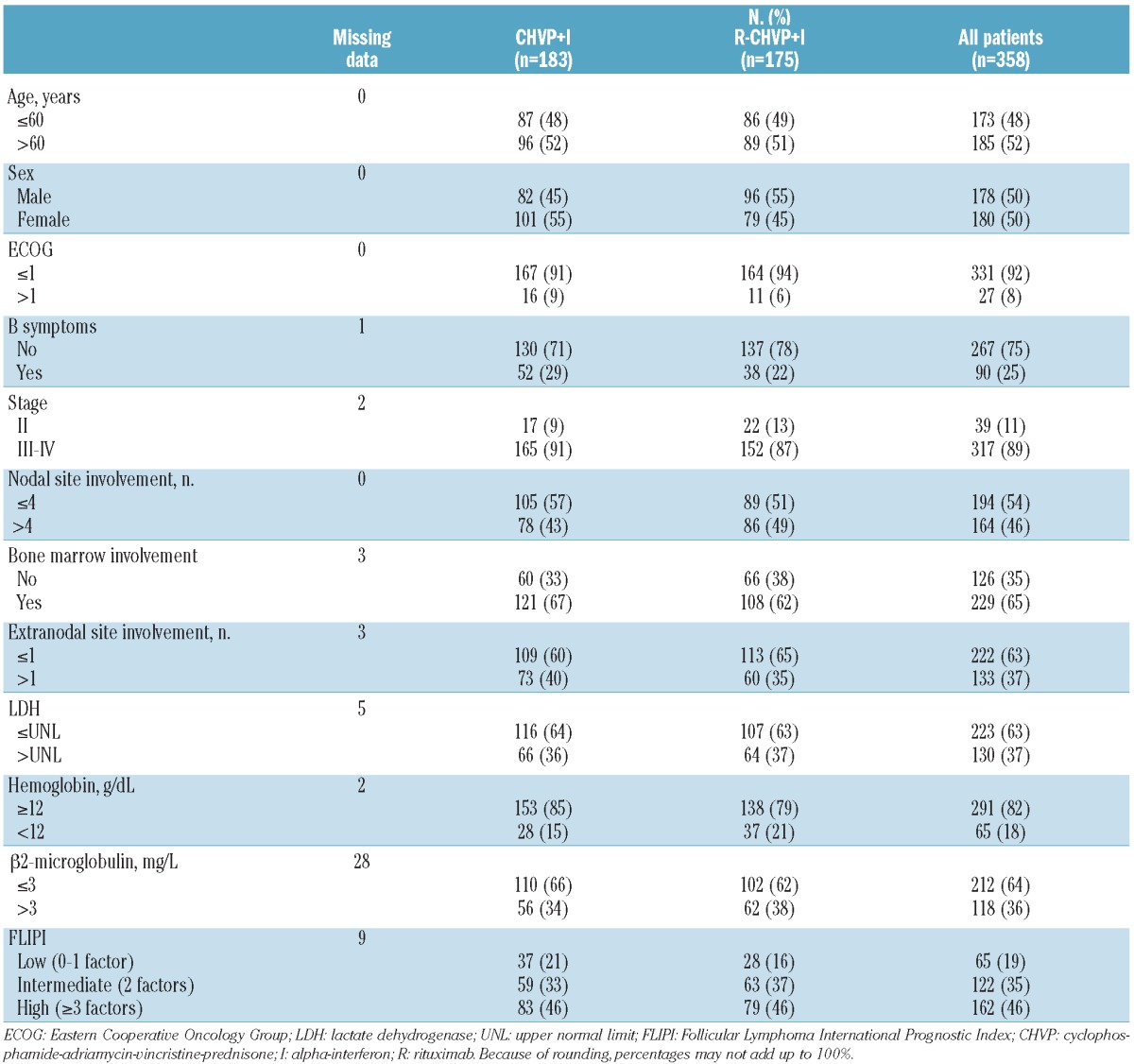
Patients’ characteristics have been previously described.9 Briefly, high-risk patients defined by an FLIPI score of 3 or over adverse factors accounted for 46% of the cohort. Adverse factors of the FLIPI are: hemoglobin < 12 g/dL, lactate dehydrogenase (LDH) > upper normal limit, stage III–IV, more than 4 nodal sites involved or age >60 years. Low- and intermediate-risk patients represented 19% and 35%, respectively. Other parameters of known prognostic significance such as β2-microglobulin or bone marrow involvement were also collected at diagnosis and are summarized in Table 1.
Table 1.
Patients’ characteristics.
Long-term patient outcome
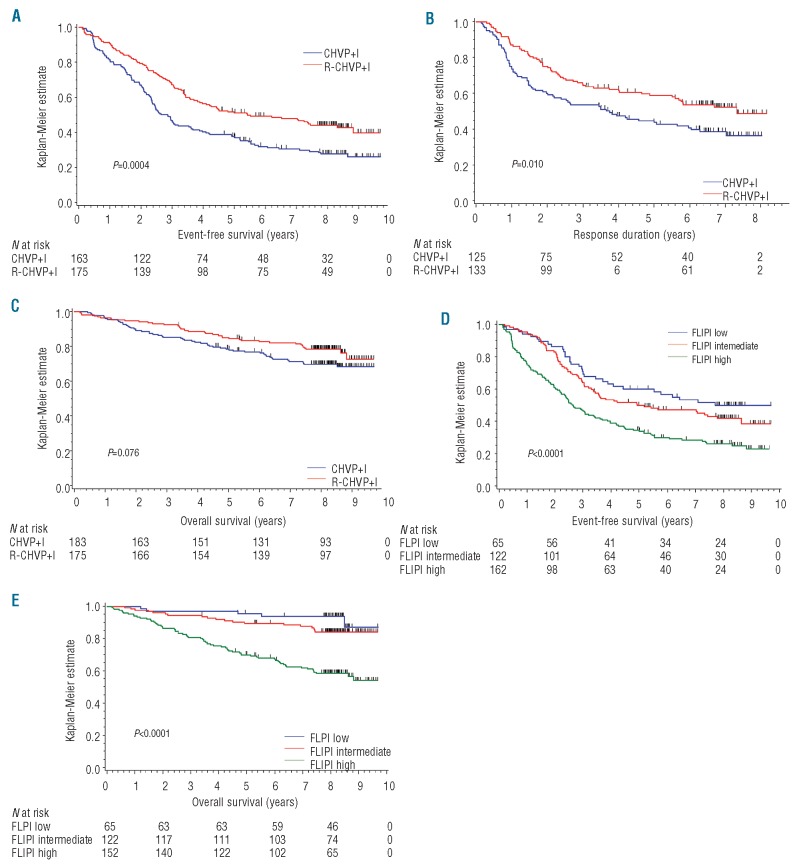
Three hundred and fifty-eight patients were considered on an intention-to-treat basis. Cut-off date was set on 1st February 2010 and the median follow up was 8.3 years (range 3.3–9.6 years). Twenty patients (5.5%) were lost to follow up at that time. Minimum follow up for surviving patients not lost to follow up was 7.7 years. Over these additional three years, and compared with the original publication, OS and EFS rates decreased only slightly: 81.8% at five years (95%CI: 77.8–85.8%) and 74.1% at eight years (95%CI: 69.5–78.7%) for OS and 44.1% (95%CI: 38.9–49.2%) and 35.9% (95%CI: 30.8–41.0%) for EFS respectively at five and eight years. With longer follow up, addition of rituximab remained significantly associated with prolonged EFS and suggested a trend towards longer OS in univariate analysis. Median EFS was, therefore, 2.8 years (95%CI: 2.4–3.6 years) with CHVP+I compared to 5.5 years (95%CI: 3.9–8.8 years) with R-CHVP+I (P=0.0004) and 8-year EFS rates were 27.9% (95%CI: 21.1–34.6%) and 44.1% (95%CI: 36.7–51.7%), respectively (Figure 2A). Interestingly, the addition of rituximab yielded significantly prolonged response duration calculated for patients in complete, complete unconfirmed or partial response from evaluation performed at 18 months (P=0.010) (Figure 2B). Finally, OS at eight years was 69.8% (95%CI: 63.1–76.6%) and 78.6% (95%CI: 72.5–84.7%) in CHVP+I and R-CHVP+I arms, respectively, and the difference between survival distributions approached statistical significance (P=0.076) (Figure 2C). For 82 patients (out of 89 who have died) in whom the cause of death was reported, lymphoma progression was felt to be directly responsible for the death in 50 cases (61%). A summary of 3-, 5- and 8-year EFS, relapse-free survival and OS is available in the Online Supplementary Table S1.
Figure 2.
(A) Kaplan-Meier (KM) estimates of event-free survival (EFS) according to treatment arm (CHVP+I vs. R-CHVP+I). (B) Response duration (RD) according to treatment arm. (C) Overall survival (OS) according to treatment arm. (D) KM estimates of EFS according to FLIPI category. (E) OS according to FLIPI category. FLIPI, Follicular Lymphoma International Prognostic Index. CHVP: cyclophosphamide-adriamycin-vincristine-prednisone; I: alpha-interferon; R: rituximab.
Univariate and multivariate analysis of prognostic factors
Significant variables in univariate analysis for EFS and OS end points (Online Supplementary Table S2) were incorporated in a multivariate model. Only the FLIPI retained statistical significance over β2-microglobulin and ECOG for OS or β2-microglobulin, ECOG, B symptoms, bone marrow involvement and extranodal sites involvement for EFS (Table 2). Eight-year survival for low, intermediate or high FLIPI patients were respectively 49.8%, 41.7% and 26.0% for EFS and 93.7%, 84.0% and 58.5% for OS, and there was a significant difference in survival distributions (log rank P<0.0001 for both EFS and OS), respectively. (Figure 2D and E) FLIPI was also found to be significant for predicting outcome in subgroup analyses by arm of treatment (Online Supplementary Figure S2A-D). Similar results were found when multivariate analysis was performed using FLIPI individual factors instead of the composite score (Online Supplementary Table S3).
Table 2.
Multivariate analysis of baseline characteristics.
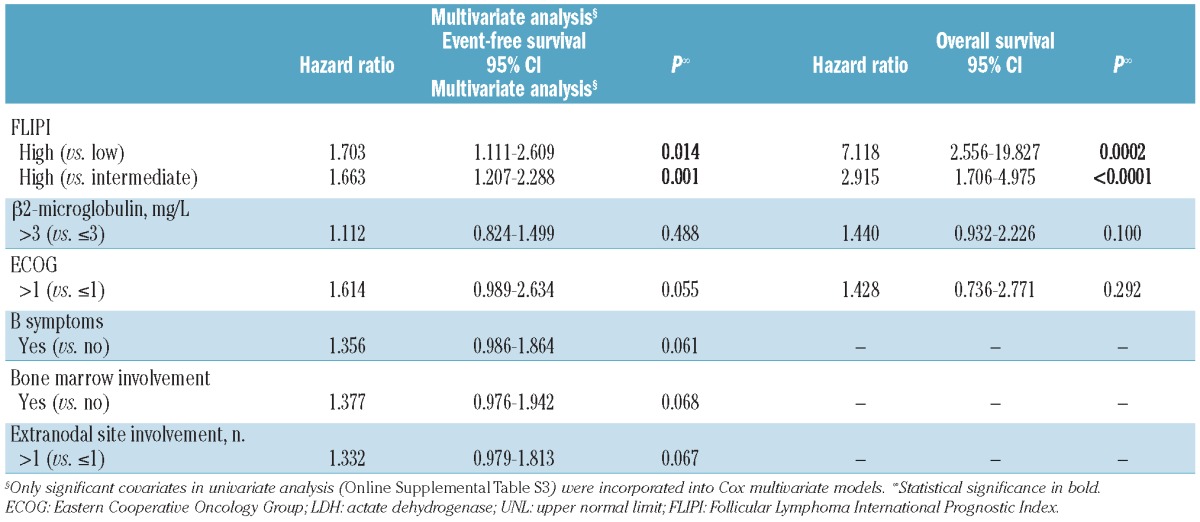
It should be noted that, since multivariate analysis showed FLIPI to be strongly correlated to outcome and the FL2000 trial was not stratified by FLIPI category (because the score was defined after the study began), we conducted an exploratory bivariate analysis in order to avoid sampling fluctuation in treatment groups. After FLIPI adjustment, and although this analysis was not considered as the primary end point of the study, it is worth noting that OS appeared significantly prolonged in the rituximab-containing arm (HR=0.64, 95%C: 0.42–0.96; P=0.033).
Effect of rituximab addition across base-line parameters
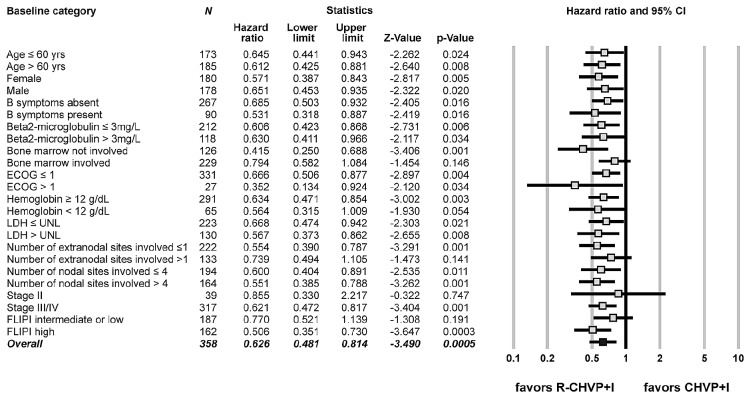
For exploratory purposes, Cox regression analyses were performed to evaluate the effect of addition of rituximab on EFS improvement according to all base-line parameters (Figure 3). Due to a relatively low death rate in each subgroup category, effect on OS could not be assessed. The addition of rituximab to the CHVP+I chemotherapy regimen was significantly associated with a prolonged EFS across almost all categories except for patients presenting with bone marrow involvement or involvement of more than one extranodal site at diagnosis. In this cohort, patients with a high-risk disease according to the FLIPI benefited the most from the addition of rituximab whereas statistical significance was not reached for patients with a low/intermediate FLIPI score or with a limited stage II disease.
Figure 3.
Forest plot representation of the effect of the addition of rituximab on event-free survival according to baseline categories. ECOG, Eastern Cooperative Oncology Group; LDH: lactate dehydrogenase; UNL: upper normal limit; FLIPI: Follicular Lymphoma International Prognostic Index; CHVP: cyclophosphamide-adriamycin-vincristine-prednisone; I: alpha-interferon; R: rituximab.
Prolonged benefit of rituximab addition in terms of EFS
As suggested by analyzing the slopes of the EFS curves (Figure 2A) and confirmed by showing the hazard function (Figure 4A), the number of events per year is not constant in those patients with FL but follows a bimodal evolution over time with a slight increase in rates of events after the end of therapy (i.e. at 18 months) followed by a steady decrease over the years until Year 6. However, no plateau was observed in this long-term follow-up cohort of patients.
Figure 4.
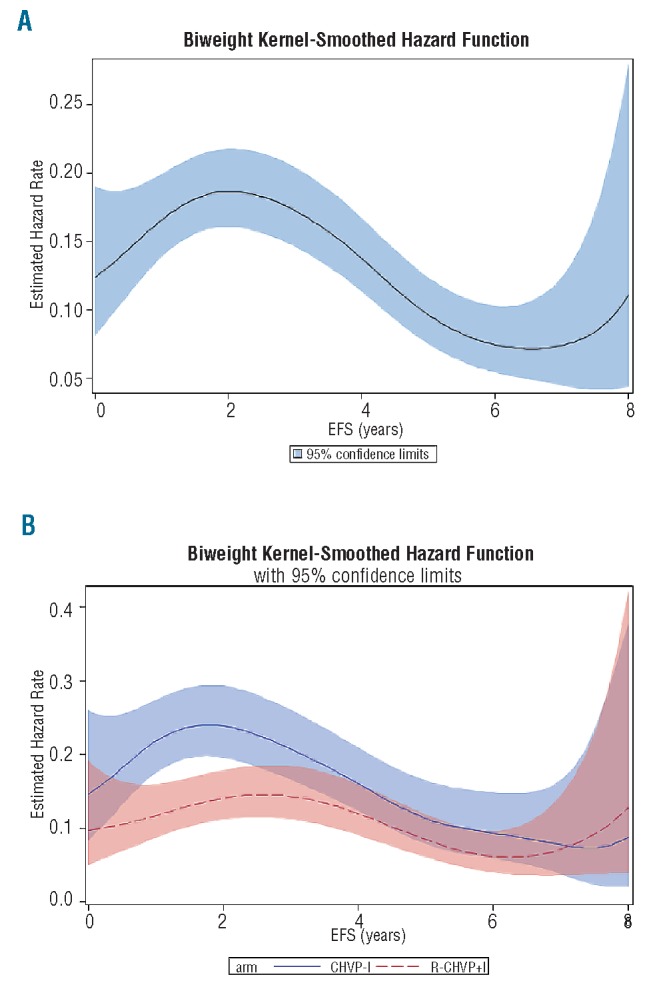
Biweight Kernel smoothed estimates of event hazard rates. (A) For the whole cohort. (B) According to treatment arm. Hazard rate functions represent the rate of events per year.
Interestingly, splitting hazard functions according to treatment arm (Figure 4B) shows the curves cross after only seven years, suggesting that corresponding EFS curves do not run strictly parallel before that time. Therefore, the duration of the benefit conferred by the addition of rituximab to the chemotherapy regimen appears to culminate during the three first years after treatment but might extend over seven years. However, a firm conclusion cannot be drawn after three years as the upper limit of the 95% confidence interval for patients treated with rituximab intersects the lower limit for patients in the chemotherapy arm of treatment.
Long-term toxicity
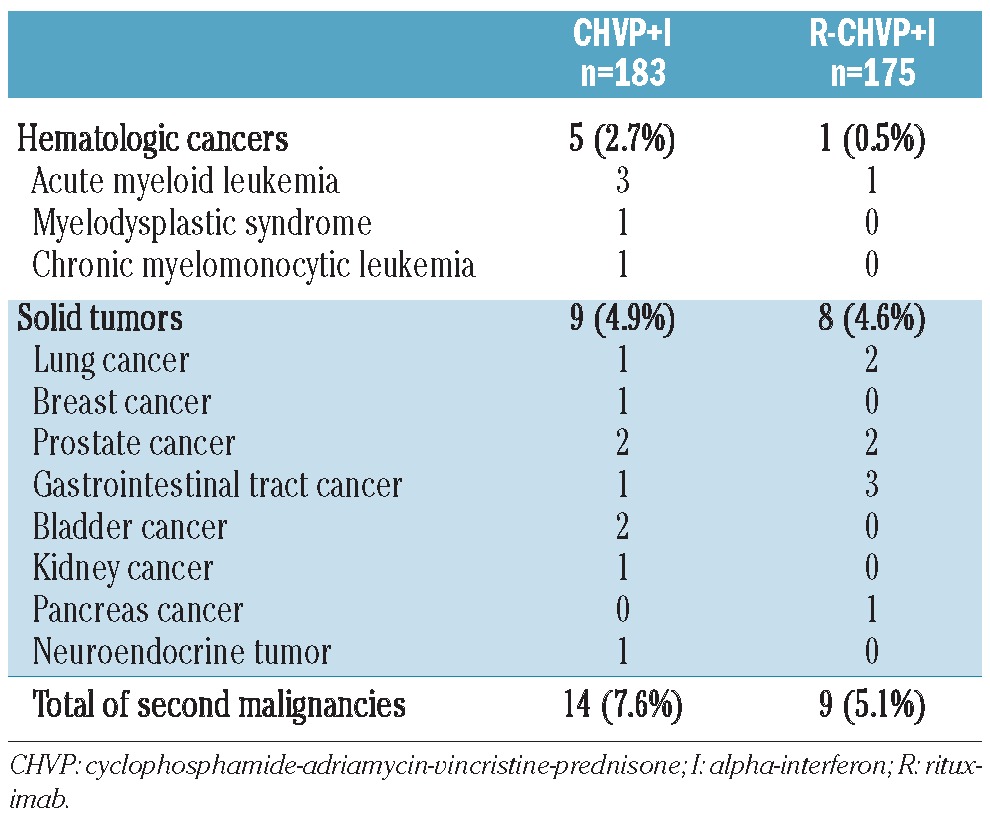
Acute and subacute toxicities have been previously reported. After a median follow up of 8.3 years, 23 (6.4%) secondary malignancies occurred: 14 (7.6%) in the CHVP+I and 9 (5.1%) in the R-CHVP+I group of treatment (P=0.26). Table 3 lists the type of malignancies encountered according to treatment. Of note, 5 hematologic malignancies occurred in the conventional arm of treatment whereas only one patient developed such a condition in the experimental R-containing group (P=0.11). Among those patients, 3 had received autologous stem cell transplant at FL relapse.
Table 3.
Secondary malignancies.
Furthermore, one patient randomized in the R-containing arm of treatment and another treated with rituximab only at relapse later developed neurological symptoms and had radiological findings potentially compatible with progressive multifocal leukoencephalopathy (PML) but without JC virus testing.
Discussion
Four published randomized trials, including the previous report of the present FL2000 trial with a shorter follow up, and a meta-analysis contributed to establish the addition of rituximab as the new standard of care for de novo FL.5–10 However, long-term follow up of randomized trials are mandatory to ascertain whether improvement in progression- or event-free survival actually translates into sustained longer overall survival and whether the experimental treatment does not yield unexpected long-term toxicity or impairment in the use of a second-line regimen. In the present study, we report the long-term follow up of patients enrolled in the FL2000 study, in which the addition of rituximab was compared with the previous standard regimen of our co-operative group (CHVP+I) for patients with de novo FL. With a median follow up of 8.3 years, the longest reported among the four randomized trials, this study demonstrates a sustained clinical benefit for patients with FL treated with a rituximab-containing regimen.6–10 In line with previously published data by Marcus et al.8,10 or Hiddemann et al.7 with extended follow up we found a trend towards a significant improvement in OS in univariate analysis (P=0.076) which became significant when sampling fluctuations due to the absence of initial FLIPI stratification (published after completion of the trial) were taken into account (HR 0.64, 95%CI: 0.42–0.96; P=0.033). Borderline significance in univariate analysis might be explained by the favorable outcome of patients treated in the standard CHVP+I arm. As a matter of fact, the 4-year Kaplan-Meier estimate for OS in this standard group without rituximab (83%, 95%CI: 77–88%) was identical to the 4-year OS reported in the experimental R-CVP arm by Marcus et al. (83%, 95%CI: 77–89%). Other randomized trials also failed to directly demonstrate a translation from improved outcome in terms of EFS to a prolonged OS.23 However, recent cross-trial comparisons indicated steady progress in OS for patients with FL in part due to improved front-line therapies.11–13 This clearly suggests that phase III trials with a PFS or EFS end point, such as the FL2000 study, were likely underpowered to demonstrate a significant OS prolongation. Apart from the efficacy of anthracycline-based chemotherapy and immunomodulatory effects of interferon in the conventional arm of treatment, salvage regimens contained rituximab for a vast majority of anti-CD20 naïve patients (approx. three-quarters (73%) as recently reported in FL2000 patients with treatment failure after first-line therapy) and also probably accounted for the limited difference in OS.24 Concerning prognosis and within the limits of patient numbers, all potential prognostic factors were overridden by the strong predictive power of the FLIPI in the present cohort.
All categories of patients according to base-line parameters benefited from the addition of rituximab, but insufficient power may preclude any firm conclusion for patients with low/intermediate FLIPI, limited stage disease, bone marrow involvement or patients with more than one extra-nodal site involvement. As seen with the marginal improvement of OS for the whole cohort, a highly potent control arm of treatment might explain the lack of power to detect significant EFS prolongation in unplanned subgroup analyses.
Interestingly, benefit of rituximab-containing first-line therapy was significantly prolonged over three years when EFS was considered and might extend over seven years. Apart from that, hazard function analysis demonstrated a steady decrease of the events hazard rate after 2–3 years following randomization in both arms (well suggested by the break of slopes on EFS representations). However, no plateau could be detected indicating that, even with this long-term follow up, no sign of cure appeared for patients with high tumor burden FL.
Altogether, the present study provides interesting information, such as the high predictive power of the FLIPI in this cohort or the prolonged benefit in terms of EFS from the addition of rituximab.
As far as long-term toxicity is concerned, the addition of rituximab in a first-line setting was confirmed as safe with regards to development of secondary malignancies. Besides, although statistical significance was not reached, fewer hematologic diseases were observed in the R-containing arm of treatment. On the one hand, it raises the possibility that a better control of the disease could postpone the use of therapy (such as mitoxantrone, fludarabine or autologous stem cell transplant conditioning regimen) at risk of secondary myeloid disorder in the relapse setting, but on the other hand, it could also be associated to the reduced course of cytotoxic therapy in the rituximab arm.17,25,26 Nonetheless, it must be noted that no overall difference in the incidence of secondary malignancies was observed between the two arms of treatment. Even though progressive multifocal leukoencephalopathy is exceptional, its association with the use of rituximab is well documented.27 In the present study, there were only 2 possible cases after rituximab-containing treatment. Thus, we provide evidence in the light of this extended follow up that no long-term toxic side effects have emerged and, in particular, no increase in secondary malignancies.
To conclude, this 8-year follow up of the FL2000 study confirms a sustained benefit of a rituximab-containing regimen for patients with FL in EFS and FLIPI-adjusted OS. After eight years, approximately one-half of the patients who responded to therapy in the experimental arm remain free of lymphoma progression and overall survival for this high tumor burden population is quite favorable. These data indicate that the optimal management of patients with FL is likely to result in a significant improvement in long-term outcome.
Acknowledgments
The authors would like to thank Roselyne Delepine, Denitza Muller, and Delphine Germain for patient data monitoring and Marion Fournier for statistical expertise. We are indebted to Dr Veronique Bachy for her assistance in reviewing the manuscript. We also thank all clinicians and clinical research associates from FL2000 GELA-GOELAMS study participating centers.
Footnotes
The online version of this paper has a Supplementary Appendix.
Funding
This study was supported by a grant from the French Government through the Hospices Civils de Lyon (PHRC 2000-081) and by La Ligue Nationale Contre le Cancer. Roche Pharma France supplied the rituximab free of charge.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Groves FD, Linet MS, Travis LB, Devesa SS. Cancer surveillance series: non-Hodgkin’s lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst. 2000;92(15):1240–51 [DOI] [PubMed] [Google Scholar]
- 2.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006; 107(1):265–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horning SJ. Natural history of and therapy for the indolent non-Hodgkin’s lymphomas. Semin Oncol. 1993;20(5 Suppl 5):75–88 [PubMed] [Google Scholar]
- 4.Johnson PW, Rohatiner AZ, Whelan JS, Price CG, Love S, Lim J, et al. Patterns of survival in patients with recurrent follicular lymphoma: a 20-year study from a single center. J Clin Oncol. 1995;13(1):140–7 [DOI] [PubMed] [Google Scholar]
- 5.Schulz H, Bohlius JF, Trelle S, Skoetz N, Reiser M, Kober T, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2007;99(9):706–14 [DOI] [PubMed] [Google Scholar]
- 6.Herold M, Haas A, Srock S, Neser S, Al-Ali KH, Neubauer A, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J Clin Oncol. 2007;25(15):1986–92 [DOI] [PubMed] [Google Scholar]
- 7.Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106 (12):3725–32 [DOI] [PubMed] [Google Scholar]
- 8.Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105(4):1417–23 [DOI] [PubMed] [Google Scholar]
- 9.Salles G, Mounier N, de Guibert S, Morschhauser F, Doyen C, Rossi JF, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMS FL2000 study. Blood. 2008;112(13):4824–31 [DOI] [PubMed] [Google Scholar]
- 10.Marcus R, Imrie K, Solal-Celigny P, Catalano JV, Dmoszynska A, Raposo JC, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579–86 [DOI] [PubMed] [Google Scholar]
- 11.Lister TA. Improved survival for patients with follicular lymphoma. J Clin Oncol. 2005;23(22):4830–1 [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Fayad L, Cabanillas F, Hagemeister FB, Ayers GD, Hess M, et al. Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at The University of Texas M.D. Anderson Cancer Center. J Clin Oncol. 2006;24(10):1582–9 [DOI] [PubMed] [Google Scholar]
- 13.Swenson WT, Wooldridge JE, Lynch CF, Forman-Hoffman VL, Chrischilles E, Link BK. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol. 2005;23(22):5019–26 [DOI] [PubMed] [Google Scholar]
- 14.Arranz R, Garcia-Alfonso P, Sobrino P, Zamora P, Carrion R, Garcia-Larana J, et al. Role of interferon alfa-2b in the induction and maintenance treatment of low-grade non-Hodgkin’s lymphoma: results from a prospective, multicenter trial with double randomization. J Clin Oncol. 1998;16(4): 1538–46 [DOI] [PubMed] [Google Scholar]
- 15.Rohatiner AZ, Gregory WM, Peterson B, Borden E, Solal-Celigny P, Hagenbeek A, et al. Meta-analysis to evaluate the role of interferon in follicular lymphoma. J Clin Oncol. 2005;23(10):2215–23 [DOI] [PubMed] [Google Scholar]
- 16.Bachy E, Brice P, Delarue R, Brousse N, Haioun C, Le Gouill S, et al. Long-term follow-up of patients with newly diagnosed follicular lymphoma in the prerituximab era: effect of response quality on survival--A study from the groupe d’etude des lymphomes de l’adulte. J Clin Oncol. 2010;28(5): 822–9 [DOI] [PubMed] [Google Scholar]
- 17.Sebban C, Brice P, Delarue R, Haioun C, Souleau B, Mounier N, et al. Impact of rituximab and/or high-dose therapy with auto-transplant at time of relapse in patients with follicular lymphoma: a GELA study. J Clin Oncol. 2008;26(21):3614–20 [DOI] [PubMed] [Google Scholar]
- 18.Solal-Celigny P, Lepage E, Brousse N, Reyes F, Haioun C, Leporrier M, et al. Recombinant interferon alfa-2b combined with a regimen containing doxorubicin in patients with advanced follicular lymphoma. Groupe d’Etude des Lymphomes de l’Adulte N Engl J Med. 1993;329(22):1608–14 [DOI] [PubMed] [Google Scholar]
- 19.Solal-Celigny P, Lepage E, Brousse N, Tendler CL, Brice P, Haioun C, et al. Doxorubicin-containing regimen with or without interferon alfa-2b for advanced follicular lymphomas: final analysis of survival and toxicity in the Groupe d’Etude des Lymphomes Folliculaires 86 Trial. J Clin Oncol. 1998;16(7):2332–8 [DOI] [PubMed] [Google Scholar]
- 20.Kaplan E, Meier P. Non parametric estimation from incomplete observations J Am Stat Assoc. 1958;53:457–81 [Google Scholar]
- 21.Cox DR. Regression models and life tables. J Royal Stat Soc Series B. 1972;34:187–220 [Google Scholar]
- 22.Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258–65 [DOI] [PubMed] [Google Scholar]
- 23.Hochster H, Weller E, Gascoyne RD, Habermann TM, Gordon LI, Ryan T, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. J Clin Oncol. 2009;27(10):1607–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Gouill S, De Guibert S, Planche L, Brice P, Dupuis J, Cartron G, et al. Impact of the use of autologous stem cell transplantation at first relapse both in naive and previously rituximab exposed follicular lymphoma patients treated in the GELA/GOELAMS FL2000 study. Haematologica. 2011;96(8): 1128–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carney DA, Westerman DA, Tam CS, Milner A, Prince HM, Kenealy M, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following fludarabine combination chemotherapy. Leukemia. 2010;24(12):2056–62 [DOI] [PubMed] [Google Scholar]
- 26.Gyan E, Foussard C, Bertrand P, Michenet P, Le Gouill S, Berthou C, et al. High-dose therapy followed by autologous purged stem cell transplantation and doxorubicin-based chemotherapy in patients with advanced follicular lymphoma: a randomized multicenter study by the GOELAMS with final results after a median follow-up of 9 years. Blood. 2009;113(5):995–1001 [DOI] [PubMed] [Google Scholar]
- 27.Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113(20):4834–40 [DOI] [PMC free article] [PubMed] [Google Scholar]