Abstract
Macrophages reside in tissues infiltrated by chronic lymphocytic leukemia B cells and the extent of infiltration is associated with adverse prognostic factors. We studied blood monocyte population by flow cytometry and whole-genome microarrays. A mixed lymphocyte reaction was performed to evaluate proliferation of T cells in contact with monocytes from patients and normal donors. Migration and gene modulation in normal monocytes cultured with CLL cells were also evaluated. The absolute number of monocytes increased in chronic lymphocytic leukemia patients compared to the number in normal controls (792±86 cells/μL versus 485±46 cells/μL, P=0.003). Higher numbers of non-classical CD14+CD16++ and Tie-2-expressing monocytes were also detected in patients. Furthermore, we performed a gene expression analysis of monocytes in chronic lymphocytic leukemia patients, showing up-regulation of RAP1GAP and down-regulation of tubulins and CDC42EP3, which would be expected to result in impairment of phagocytosis. We also detected gene alterations such as down-regulation of PTGR2, a reductase able to inactivate prostaglandin E2, indicating immunosuppressive activity. Accordingly, the proliferation of T cells in contact with monocytes from patients was inhibited compared to that of cells in contact with monocytes from normal controls. Finally, normal monocytes in vitro increased migration and up-regulated CD16, RAP1GAP, IL-10, IL-8, MMP9 and down-regulated PTGR2 in response to leukemic cells or conditioned media. In conclusion, altered composition and deregulation of genes involved in phagocytosis and inflammation were found in blood monocytes obtained from chronic lymphocytic leukemia patients, suggesting that leukemia-mediated “education” of immune elements may also include the establishment of a skewed phenotype in the monocyte/macrophage population.
Introduction
Tumor-associated macrophages (TAM) play an important role in tumor cell invasion, proliferation and survival. TAM have a phenotype and functions more similar to alternatively activated (M2) macrophages, characterized by the IL-10highIL-12low profile, poor antigen-presenting capacity, immunosuppressive effects and pro-angiogenic properties.1 An increase in the number of TAM has been associated with shortened survival and drug-resistance in patients with hematologic malignancies such as classic Hodgkin’s lymphoma, follicular lymphoma, diffuse large B-cell lymphoma, and multiple myeloma.2–5 Macrophages are also involved in the ingestion of rituximab-opsonized cells via the Fcγ receptor and may have a role in rituximab resistance.6
TAM derive from monocytic precursors circulating in the blood and are recruited into the tumor tissues by chemoattractants. Monocytes can be divided into three subsets: the largest population of circulating human monocytes (≈90%), with high CD14 but no CD16 expression (CD14++CD16−), are called classical monocytes, whereas the minor population is subdivided into the intermediate subset, with low CD16 and high CD14 (CD14++CD16+), and the non-classical subset, with high CD16 and lower CD14 expression (CD14+CD16++).7,8 Moreover, among circulating monocytes, those expressing the angiopoietin receptor Tie2 (Tie-2-expressing monocytes, TEM) are characterized by tumor-promoting properties.9,10
Chronic lymphocytic leukemia (CLL) B cells show prolonged survival in vivo and therefore accumulate in peripheral blood, bone marrow and lymphoid organs of patients. In vitro CLL cell survival was extended in co-cultures with several types of cells such as mesenchymal stromal cells, follicular dendritic cells, endothelial cells and monocytoid cells (defined as nurse-like cells, NLC).11 The amount of NLC in CLL-infiltrated bone marrow was reported to be closely associated with adverse prognostic factors.12
Here, we evaluated the monocytic population in peripheral blood of CLL and normal subjects by flow cytometry. In addition, circulating TEM were measured and compared. We also investigated the ability of leukemic cells to influence the migration and gene expression profile of monocytes.
Design and Methods
Further details are provided in the Online Supplementary Design and Methods.
Patients
After obtaining informed consent in accordance with the Declaration of Helsinki with a protocol approved by the local Institutional Review Board, blood samples were collected from 31 untreated CLL patients fulfilling standard criteria13 and 13 healthy donors (controls).
Flow cytometry
Peripheral blood mononuclear cells (PBMC) from 26 patients and 13 age- and gender-matched healthy controls were stained with anti-CD14 FITC, anti-CD16 APC-Alexa750 (Becton Dickinson, San José, CA, USA) and anti-TIE-2 PE (R&D Systems, Minneapolis, MN, USA). Monocytes were divided into classical (CD14++CD16−), intermediate (CD14++CD16+) and non-classical (CD14+CD16++) subsets. The percentages of Tie2+ cells among CD14+ monocytes (TEM) were determined by gating on the DUMP− channel, as defined in the Online Supplementary Design and Methods.
Gene expression analysis
Large-scale gene expression profiling was performed by hybridizing RNA from highly purified CD14+ monocytes, isolated from five CLL patients and five healthy controls, on a 4×44K Whole Human Genome Microarray (Agilent Technologies, Palo Alto, CA, USA) as previously described.14 The data have been deposited in the NCBI Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/, GSE35179). Expression of the RAP1GAP, ARHGEF12, PTGR2 and PLA2G4A genes was measured by Real-Time Ready Custom Panel 96 and LightCycler 480 Probes Master (Roche Applied Science, Penzberg, Germany). IL-6, IL-10, IL-8, VEGF and MMP9 were amplified by LightCycler 480 SYBR Green I Master (Roche).
Culture condition of monocytes and nurse-like cells
CD14+ monocytes from healthy donors were cultured in IMDM supplemented with 2% fetal bovine serum and 2 mM L-glutamine. Adherent monocytes were exposed to conditioned media derived from CLL cells (CM CLL, dilution 1:2 with fresh medium) or to direct contact with CLL cells or normal B cells (ratio 1:2). Lastly, NLC were obtained from five CLL patients, as previously described.15
Functional assays
Migration assays were performed on monocytes in 24-well plates containing 5-μm pore size PET inserts (Millipore, Billerica, MA, USA). The migratory cells labeled with 8 μM calcein-AM (Sigma-Aldrich, St. Luis, MO, USA) were quantified by the fluorescence plate reader Infinite200 (Tecan, Männedorf, Switzerland). To evaluate T-cell proliferation, we isolated CD3+ cells from PBMC of three CLL patients and five healthy donors using the Pan-T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Autologous and allogeneic mixed reactions were performed by culturing T cells with adherent monocytes obtained from CLL patients or normal controls for 5 days. Cell proliferation was monitored using the MTT assay (Trevigen, Gaithersburg, MD, USA) or CFSE dilution assay (eBioscience, S Diego, CA, USA).
Solid-phase enzyme-linked immunosorbent assays
The levels of PGE2 (as 13, 14-dihydro-15-keto PGE2), Ang2, IL-6, IL-10, IL-8, VEGF and MMP9 proteins were determined by Quantikine ELISA assays (R&D Systems).
Immunohistochemistry and confocal microscopy
For immunohistochemical analysis, sections from lymph node samples of CLL patients were stained with goat anti-human Tie2 (R&D System) or mouse anti-human CD68 (Novocastra, Leica Microsystems, Wetzlar, Germany) as previously reported.16 For confocal microscopy, cover slips were incubated with anti-Tie2 monoclonal antibody, followed by Alexa-488 conjugated secondary antibody. Cells were counterstained with Alexa 568–conjugated phalloydin (Invitrogen, Portland, OR, USA) as described elsewhere.17
Results
Blood monocyte subsets in chronic lymphocytic leukemia
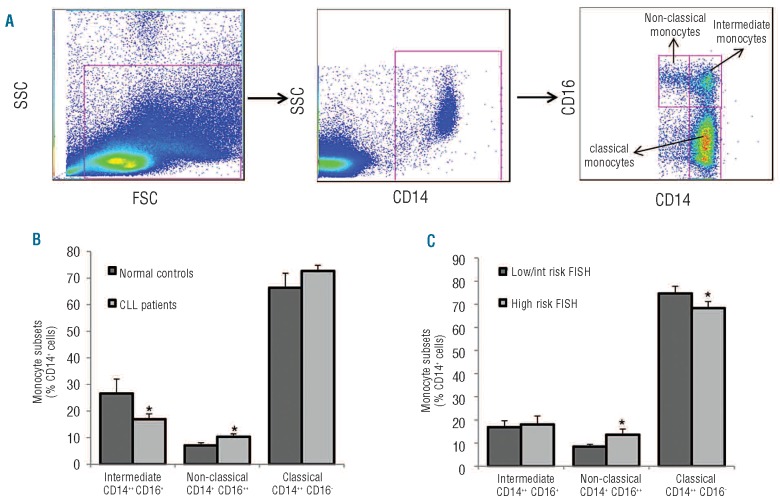
We stained PBMC obtained from 26 untreated CLL patients and 13 healthy donors with anti-CD14 and anti-CD16 monoclonal antibodies and analyzed cells by polychromatic flow cytometry. The clinical and biological characteristics of CLL patients are presented in Online Supplementary Table S1. The blood monocyte population was subdivided on the basis of the expression of CD14, the lipopolysaccharide receptor broadly expressed by human monocytes, and CD16, an Fcγ receptor III, into three subsets: classical monocytes (CD14++, CD16−), non-classical monocytes (CD14+, CD16++) and intermediate monocytes (CD14++, CD16+) (Figure 1A). As expected, the major monocytic subset was represented by classical monocytes, accounting for 66.4%±5.5% and 72.7%±2.1% of total monocytes in normal controls and CLL patients, respectively; intermediate monocytes accounted for 26.6±5.4% and 16.9±1.99%, respectively. The non-classical CD14+CD16++ monocytic subpopulation was significantly larger in CLL patients than in normal controls (10.4±1.1% versus 7.0±1.1%, P=0.04) (Figure 1B).
Figure 1.
Distribution of monocyte subsets in peripheral blood of CLL patients and normal controls. (A) Fresh peripheral blood samples were collected from 26 untreated CLL patients and 13 normal controls. Mononuclear cells were stained with anti-CD14, anti-CD16 monoclonal antibodies and analyzed by polychromatic flow cytometry. Monocytes were defined as CD14+ and then subdivided in classical monocytes (CD14++CD16−), non-classical monocytes (CD14+CD16++) and intermediate monocytes (CD14++CD16+) as indicated. (B) Histograms represent the percentage of classical, intermediate and non-classical monocytes in CLL patients and normal controls. A shift from intermediate (down-modulated) towards non-classical monocytes (increased) is present in CLL compared to normal controls (*P<0.05, Mann-Whitney non-parametric test). (C) When CLL patients were divided into two prognostic subsets on the basis of genomic aberrations detected by FISH analysis (low risk, no abnormalities or deletion 13q, n=16; high risk= deletions 11q, 17p or trisomy 12, n=7), the unfavorable prognostic subgroup had an increased percentage of non-classical monocytes (*P<0.05, Mann-Whitney non-parametric test). Data are presented as mean ± standard error of mean.
We then evaluated whether differential representation of blood monocyte subsets could be detected between CLL patients with distinct clinical, hematologic and biological features at the time of blood sample collection. No significant differences in monocyte subpopulations were observed among CLL prognostic subsets defined by age, gender, Binet stage, white blood cell count, IGHV mutational status, CD38 and ZAP70 expression. The non-classical monocyte subset was slightly larger in CLL patients defined, according to fluorescence in situ hybridization (FISH) abnormalities, as high risk compared to those at low risk (13.6±2.5% versus 8.5±1.0%, P=0.038) (Figure 1C).
In order to evaluate whether CLL patients had an increase of CD14+CD16++ cells, or only a shift in the distribution of specific monocyte subsets, we considered the absolute number of monocytes. CLL patients had a higher absolute number of monocytes compared to normal controls (792±86 cells/μL versus 485±46 cells/μL, P=0.003) as well as higher numbers of classical (568±55/μL versus 313±35/μL, P=0.001) and non-classical monocytes (79±9/μL versus 33±4/μL, P=0.001). In addition, the analysis of absolute numbers of monocytes confirmed the increase of non-classical monocytes in the CLL subset carrying high-risk FISH abnormalities (107±13/μL in high risk versus 65±11/μL in low risk, P=0.023).
Tie2 expression in peripheral blood of patients with chronic lymphocytic leukemia
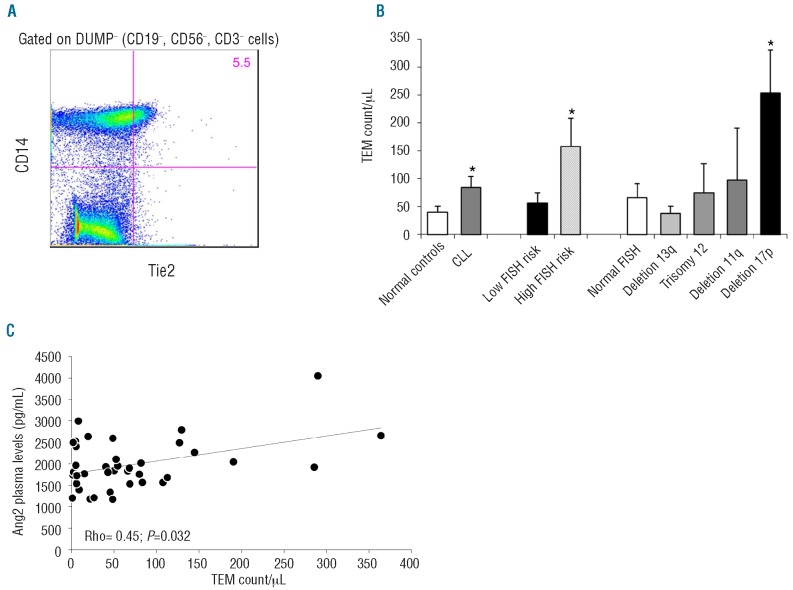
CLL cells were reported to secrete angiopoietin-2 (Ang2) protein, an agonist/antagonist of the Tie2 receptor, and higher levels of Ang2 were detected in CLL patients with adverse clinical outcomes.14,18 We, therefore, evaluated in our cohort the extent of a small subpopulation of circulating monocytes able to express Tie2 receptor (TEM) and showing tumor-promoting properties.9 We stained PBMC from CLL patients and healthy donors with anti-human Tie2 and CD14 monoclonal antibodies (Figure 2A). TEM accounted for 19.6±6.2% and 24.5±4.8% of total monocytes in controls and CLL patients, respectively. Dividing monocytes into subsets, we found that the majority of TEM were within the intermediate subset accounting for 31.7±4.9% in CLL, whereas there were fewer TEM present in the classical (23.8±4.8%) or non-classical (15.1±3.7%) monocyte subsets. No differences in TEM distribution among monocyte subsets were detected between CLL and normal controls.
Figure 2.
Tie2-expressing monocytes (TEM) in CLL patients and normal controls. (A) Flow cytometric analysis shows the percentage of Tie2+ cells among CD14+ monocytes, gated on the DUMP− channel. (B) Histograms represent mean ± SEM of TEM count/μL in CLL patients (n=26) and healthy age-matched controls (n=13) and also within CLL patients divided according to FISH abnormalities. CLL patients had higher numbers of TEM compared to normal controls. Moreover, CLL patients harboring high-risk FISH abnormalities, in particular deletion 17p, had higher numbers of TEM in peripheral blood. (*P<0.05, Mann-Whitney test). (C) TEM count/μL correlates positively with Angiopoietin-2 (Ang2) plasma levels (Rho=0.42, P=0.032, Spearman’s non-parametric test).
Considering the absolute number of circulating TEM, CLL patients had 84.5 TEM/μL compared to 39.9/μL in normal controls (P=0.02) (Figure 2B). CLL patients with adverse FISH aberrations had more TEM than patients with low-risk FISH lesions (157.9±50.2/μL versus 57.1±17.9/μL, P=0.04). The mean number of TEM in CLL patients harboring the 17p deletion was 253.5/μL compared to 62.9/μL in patients without the deletion (P=0.01). No significant differences were observed among other CLL prognostic subsets defined by age, gender, Binet stage, white blood cell count, IGHV mutational status, CD38 or ZAP70 expression.
It was recently demonstrated that Ang2 has a role in regulating the tumor-promoting functions and migration of TEM.9,10 We investigated the association between Ang2 plasma levels and TEM number in peripheral blood. Ang2 levels were detected in plasma samples collected at the time of flow cytometric analyses and ranged from 1170 pg/mL to 4052 pg/mL. A significant positive correlation was found between Ang2 plasma levels and number of circulating TEM (Spearman’s rho=0.42, P=0.032) (Figure 2C).
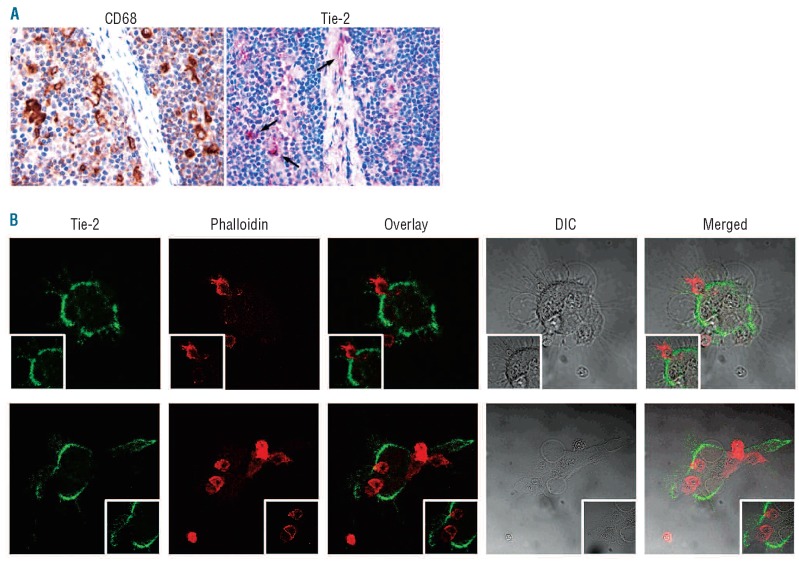
Immunohistochemical analysis for the markers CD68 and Tie2 performed on serial lymph node sections confirmed that a fraction of NLC intermingling with CLL cells expressed Tie2 (Figure 3A). These Tie2+ NLC had a predominantly peri-vascular distribution (Figure 3A). Concordantly, we found that a subset of adherent NLC generated in vitro from circulating monocytes of five CLL patients expressed Tie2 receptor (Figure 3B).
Figure 3.
Tie2 staining on nurse-like cells (NLC). (A) Tie-2 expressing NLC (red signal) populate lymph node CLL infiltrates. Immunohistochemistry, Strept-ABC method, original magnifications ×200 and ×400. (B) Representative images of NLC cultures (n=5) showing intense surface staining for the Tie2 receptor. Live CLL lymphocytes are counterstained using phalloidin.
Gene-expression profiling of chronic lymphocytic leukemia monocytes
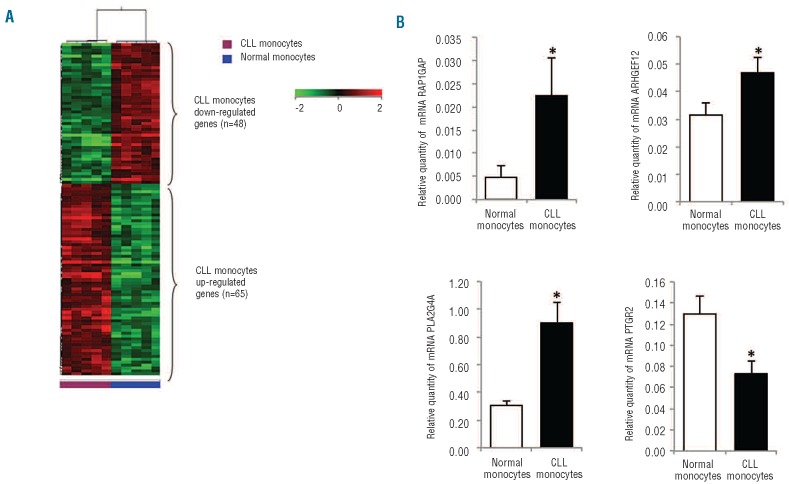
Blood monocytes were isolated from five healthy donors and five previously untreated CLL patients. The clinical and biological characteristics of the CLL patients are shown in Online Supplementary Table S2. Gene expression profiles of the monocyte population were obtained and analyzed using unsupervised and supervised learning. Although analyzed cells were not part of the malignant clone, in unsupervised hierarchical clustering analysis, the gene expression profiles of normal monocytes were clearly distinguishable from those of monocytes obtained from CLL patients. Supervised analysis identified 65 genes significantly up-regulated and 48 genes down-regulated in CLL monocytes compared with monocytes from normal controls [fold-change (FC) ≥2, P<0.05] (Figure 4A and Online Supplementary Table S3). Classifying differentially expressed genes into cellular pathways, we found that up-regulated genes were involved in the Wnt signaling pathway, apoptosis, VEGF signaling pathway, and angiogenesis, while down-regulated ones were involved in cytoskeletal regulation, oxidative stress response and inflammation signaling. In particular, in monocytes from CLL patients we found a 6.5-fold up-regulation of RAP1GAP, able to enhance the intrinsic GTPase activity of RAP1 to hydrolyze the bound GTP to GDP and inhibit its downstream function. RAP1 GTPase has been reported to be involved in both Fcγ-receptor and complement-receptor phagocytosis.19 Conversely, we found a 12- and 3-fold down-regulation of tubulins TUBB3 and TUBB2 as well as a 2-fold decrement of CDC42EP3, a Rho GTPase effector protein involved in actin assembly at nascent phagosomes and internalization of IgG-opsonized particles.20 Functionally, these modifications would be expected to result in impaired phagocytosis.
Figure 4.
Gene expression profiles of CLL-derived monocytes. (A) Heat map depicts differentially expressed genes between CLL-derived monocytes and normal monocytes. The data are represented in a grid format in which each column represents a case and each row a single gene. Normalized intensity signals are depicted in the pseudo color scale as indicated. (B) Histograms represent the mRNA relative quantity of RAP1GAP, ARHGEF12, PLA2G4A and PTGR2 evaluated by RT-PCR in monocytes obtained from 12 CLL patients and 13 healthy donors. (* P<0.05, Mann-Whitney test).
Furthermore, there was a 3.8-fold down-regulation of the chemokine (C-C motif) ligand 5 (CCL5) involved in M1 polarization and inflammation response. CLL monocytes up-regulated the leukemia-associated Rho guanine nucleotide exchange factor 12 (ARHGEF12/LARG) (FC=2.61) and the lysophosphatidic acid receptor 6 (LPAR6/P2Y5) (FC=3.95), able to activate RhoA GTPase involved in cell migration and Raf/ERK signaling.21,22 Among up-regulated genes in CLL-derived monocytes, we also found Toll-like receptor 4 (TLR4) as well as Lipin-2 (LIP2) and -3 (LIP3), two phosphatidic acid (PA) phosphatases (PAP). Lipins hydrolyze PA to yield diacylglycerol (DAG), which activates cytosolic group IVA phospholipase A2 (PLA2G4A). PLA2G4A was also found to be 4-fold up-regulated in CLL monocytes compared to normal monocytes. Functionally, these modifications would be expected to result in mobilization of free arachidonic acid, the precursor of inflammatory mediators, eicosanoids.23,24 Finally, we also found down-regulation of PTGR2 (FC=−3.2), involved in inactivation of PGE2 as well as reduced expression of dual specificity phosphatase 3 (DUSP3/VHR) (FC=−2.0) and cyclin A1 (CCNA1) (FC=−6.2), indicating a reduction in cycling monocytes (Online Supplementary Table S3).25,26
We validated the up-regulation of RAP1GAP, ARHGEF12 and PLA2G4A and the down-regulation of PTGR2 by RT-PCR in monocytes obtained from 12 CLL patients and 13 healthy donors (Figure 4B). Furthermore, we quantified PGE2 metabolites in conditioned media (CM) collected from CLL-derived (n=6) and normal (n=8) monocytes after 24 h of culture and in plasma samples from CLL patients (n=16) and healthy donors (n=8). The levels of PGE2 metabolites were lower in samples from CLL patients than in those from normal controls (CM, 18±5 versus 41±13 pg/mL; plasma, 144±15 versus 333±143 pg/mL, P<0.05, Online Supplementary Figure S1).
Chronic lymphocytic leukemia-derived monocytes suppress T-cell proliferation
The results of gene expression profiling analysis suggest that monocytes in CLL patients may have immunosuppressive properties that are absent or less marked in normal monocytes. We, therefore, compared the effect of CLL-derived monocytes and normal monocytes on proliferation of autologous T cells. We found that autologous T cells proliferated less when co-cultured with monocytes from CLL patients than with monocytes from healthy donors (n=3 for both, P<0.05, Online Supplementary Figure S2). A 30% reduction in T-cell proliferation was detected in CLL by a MTT assay, in which metabolically active cells convert MTT into formazan, and confirmed by CFSE dilution assays. To exclude that the immunosuppression was due to impairment of CLL-derived T lymphocytes, we also performed an allogeneic lymphocyte reaction. Normal T cells showed reduced proliferation when cultured on monocytes collected from CLL patients compared to monocytes from healthy donors (n=3 for both, P<0.05, Online Supplementary Figure S2).
Chronic lymphocytic leukemia cells increase CD16 and RAP1GAP, but decrease PTGR2 expression on monocytes
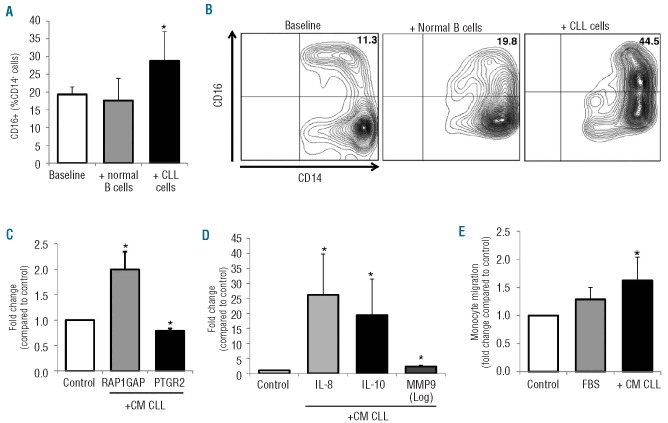
To determine whether tumor-derived factors may cause the observed increase in CD16 and RAPGAP1 expression and the decrease of PTGR2, monocytes from healthy donors were cultured with CLL-derived conditioned media (CM CLL) or in direct contact with CLL cells or normal B cells. In addition, we evaluated the expression of cytokines involved in inflammation (IL-6 and IL-10), angiogenesis (IL-8 and VEGF) and invasiveness (MMP9). Compared to supernatants from control cells, supernatants from CLL cells induced a slight increase in CD16 expression on normal monocytes, as measured by flow cytometry (n=5, 24 h, %CD16+CD14+, 3.3±1.0% in culture controls versus 5.3±1.2% in CM-treated monocytes, P=0.043). As shown in Figure 5A-B, when monocytes were cultured in contact with CLL cells, we detected a greater increase in CD16 expression (32.3±5.7%) than in the baseline condition (18.0±1.6%) or than when the monocytes were treated with normal B cells (19.3±4.0%) (n=8, P=0.012).
Figure 5.
In vitro CLL-induced modifications of the monocytic population. CD14+ monocytes collected from healthy donors were cultured in the presence of CLL-derived conditioned media (CM CLL) or in direct contact with CLL cells or normal B cells. (A) Histograms represent the percentage of CD16+ cells in the monocytic population collected from healthy donors (n=8) measured by flow cytometry at baseline and after 24 h-culture with normal B cells or with CLL cells. Data are reported as mean±SEM of three independent experiments (*P=0.012, Wilcoxon’s test). (B) Counter plots of a representative sample showing an increase of CD16+ cells among CD14+ monocytes after CLL contact. (C-D) mRNA levels were measured by RT-PCR on normal monocytes (n=8) collected after 24 h of treatment with CM CLL and represented as fold change compared to control medium. CLL-derived soluble factors up-regulate the expression of RAP1GAP, IL-10, IL-8 and MMP9, whereas they down-regulate PTGR2. All *P<0.05 compared to untreated controls (Wilcoxon’s test). (E) CM-CLL stimulates migration of normal monocytes. Monocytes were induced to migrate through a 5 μm-pore size PET membrane for 2 h in response to Iscove’s modified Dulbecco’s medium (IMDM) (negative control) or 10% fetal bovine serum (FBS) (positive control), and with addition of CM-CLL. Histograms represent mean±SEM of three independent experiments. Data are normalized on IMDM controls and represented as fold change. *P<0.05 compared to IMDM control (Wilcoxon’s test).
Furthermore, we found that CLL-derived soluble factors were able to induce up-regulation of RAP1GAP (n=7, P=0.018) and down-regulation of PTGR2 (n=8, P=0.012) on normal monocytes (Figure 5C). Normal monocytes also had up-regulated IL-10, IL-8 and MMP9 expression in response to CM CLL (all P<0.05) (Figure 5D). No significant variations were found compared to unstimulated controls for IL-6 and VEGF expression levels. Moreover, we collected supernatants from monocyte cultures and the release of cytokines was measured by ELISA. CLL stimulation induced normal monocytes to release increased levels of IL-6 and IL-10 (from 7.9 to 27.3 pg/mL for IL-6 and from 0.39 to 1.43 pg/mL for IL-10, P=0.043 both) (data not shown). Release of VEGF was unaffected by stimulation. Lastly, we evaluated whether CLL may stimulate migration of monocytes. Purified CD14+ monocytes were induced to migrate through a 5 μm-pore size PET membrane for 2 h. We found that CM CLL increased the migration of monocytes (P<0.05 versus control medium) (Figure 5E).
Discussion
The purpose of our study was to improve knowledge about in vivo functional and phenotypic deregulation of monocytes in CLL patients. We found that CLL patients had a higher absolute number of monocytes compared to normal controls. In addition, we detected a significant increase in non-classical monocytes, characterized by a low level of CD14 together with high CD16 (CD14+CD16++). This modification seems to be more prominent in CLL cases with adverse genomic aberrations. However, prospective studies on larger series are necessary to confirm this observation and to evaluate the association with other established prognostic factors and clinical behavior. Of note, we demonstrated that CLL cell contact in vitro can induce the up-regulation of CD16 expression on normal monocytes compared to the baseline condition or to contact with normal B cells. Non-classical monocytes exhibit patrolling behavior in vivo, are weak phagocytes, do not produce reactive oxygen species or cytokines in response to cell-surface Toll-like receptors and react strongly to nucleic acid and viruses, but poorly to lipopolysaccharide.27 In healthy individuals, non-classical monocytes have a gene expression profile closely related to that of intermediate monocytes, but with higher expression of several genes involved in cytoskeletal rearrangement.7 Moreover, the number of CD16+ monocytes is increased in patients with solid tumors and correlates with poor prognosis and a higher density of TAM.28,29 This subpopulation may represent more mature monocytes able to adhere to vascular endothelium and migrate into tumor-involved areas.
The full spectrum of monocyte functions, and in particular the specific functions of different monocyte subsets, is not completely known but comprises anti-inflammatory properties, tissue repair and angiogenesis. Monocytes form a heterogeneous population of cells and include TEM,30 which account for 2% to 7% of blood mononuclear cells in healthy donors. In human cancer patients, TEM were observed in the blood and, intriguingly, within tumors, but hardly detected in non-neoplastic tissues.30 We found increased numbers of TEM in the peripheral blood of CLL patients and in particular in cases with high-risk genomic aberrations. Moreover, higher numbers of circulating TEM were present in CLL characterized by increased plasma levels of Tie2 agonist/antagonist Ang2. CLL cells are able to secrete Ang2 in infiltrated tissues and higher plasma Ang2 levels are present in CLL patients with adverse clinical outcome and with increased tissue vascularization.18 Here, we first found Tie2+ NLC in CLL-infiltrated lymph nodes, mainly in a peri-vascular distribution. We argue that the production of Ang2 by leukemic cells in infiltrated-tissue may mediate not only a pro-angiogenic effect but also the recruitment into tissue of the TEM subpopulation. TEM have a profound pro-angiogenic activity, a tumor-promoting M2-like phenotype, suppress T-cell activation and promote regulatory T-cell expansion via an IL-10-dependent mechanism.9 Our results indicate a possible role of this subset of monocytes/macrophages in CLL-mediated mechanisms of angiogenesis and immunosuppression.
Overall, the increased frequencies of CD14+CD16++ and Tie2-expressing monocytes are additional abnormalities of circulating monocyte populations in CLL patients besides the higher number of CD14+CD16− HLA-DRlow/neg immuno-suppressive monocytes recently reported by Gustafson et al.31 These alterations seem to be associated with more aggressive disease.
Microarray-gene expression profiling is a useful tool for unraveling the complexity of altered molecular mechanisms in tumor cells and also in non-malignant components of cancer-bearing patients. Analysis of the differentially expressed genes in monocytes from CLL patients compared to normal controls demonstrated a number of abnormalities in specific pathways. The most up-regulated gene in NLC is that encoding the GTPase-activating protein RAP1GAP, able to enhance the intrinsic GTPase activity of RAP1 to hydrolyze the bound GTP to GDP and as a consequence inhibit RAP1 downstream functions. The major biological functions of RAP1 are Ras-mediated ERK activation and integrin-dependent cellular functions such as immunological synapse formation, cell adhesion, macrophage phagocytosis and chemokine-induced migration of leukocytes. In particular, RAP1 activation is required for both Fcγ receptor-dependent and β2-integrin-mediated phagocytosis.19 Inhibition of RAP1 activity by RAP1GAP expression was reported to induce severe impairment of the ability of macrophages to ingest opsonized particles. This abnormality, together with the down-regulation of tubulins and CDC42EP3, a CDC42 Rho GTPase effector protein, would be expected to result in impairment in cytoskeleton rearrangement, cell spreading and phagocytosis. Immunomodulatory drugs rapidly regulate cytoskeleton reorganization in monocytes and T cells through selective activation of Rho family GTPases, inducing increase in F-actin formation, microtubule stabilization and synapse formation.32 In the light of our findings, the normalization of leukemia-derived impairment of actin cytoskeleton in both T cells and monocytes by immunomodulatory drugs could be a key mechanism of immune activation described in CLL patients treated with immunomodulatory drugs. Furthermore, impairment of IgG-mediated phagocytosis may be considered a potential mechanism of rituximab-resistance in CLL patients.6 Further studies are necessary to unravel these issues.
Our gene expression analysis of CLL monocytes showed up-regulation of TLR4 and protein kinase C-ɛ (PRKCE) as well as up-regulation of two lipins (LPIN2 and LPIN3), metabolic enzymes that possess PAP activity, hydrolyzing PA to yield DAG. DAG regulates the activation of the cytosolic PLA2G4A, which also showed a 4-fold up-regulation in NLC. PLA2G4A induces the mobilization of free arachidonic acid to produce eicosanoids.33 These abnormalities could imply activation of NFκB signaling pathways and production of pro-inflammatory mediators. On the other hand, TLR4 activation in TAM was reported to enhance tumor growth and to mediate production of angiogenic factors.34 Moreover, PLA2G4A was up-regulated during M2 polarization of macrophages and contributed to cytotoxic T-cell immunosuppression.35 A possible explanation could be the generation of specific immunosuppressive eicosanoids such as PGE2.36 In agreement with this hypothesis, we also found down-regulation of PTGR2, which catalyzes the reaction for inactivating PGE2, with a consequent decreased level of PGE2 metabolites secreted by CLL-derived monocytes and also in CLL plasma samples. PGE2 suppresses multiple immune functions; for example, it inhibits proliferation and stimulation of T cells and represses macrophage activation, it promotes IL-10 and reduces IL-12 expression, it inhibits the maturation of dendritic cells, induces Th2 responses, and also exerts a suppressive effect on B-cell function.25 The aberration identified by gene expression profiling would be expected to cause increased accumulation, compared to the normal condition, of PGE2 due to reduced activity of degradation and inactivation in NLC. Together with leukemic production of PGE2 by constitutive expression of cyclo-oxygenase-2,37 this abnormality could contribute to immunosuppression in CLL patients. We demonstrated that monocytes collected from CLL patients had immunosuppressive properties inducing the inhibition of T-cell proliferation. Further studies are necessary to unravel the functional role of increased PGE2 production in CLL monocytes.
Lastly, we found that CLL cells were able to attract monocytes and to induce the up-regulation of some tumor-promoting factors. In particular, RAP1GAP up-regulation and PTGR2 down-regulation were induced in normal monocytes when cultured with CLL-derived CM. Moreover, CLL stimulation of normal monocytes also implied the up-regulation of IL-10, a Th2-derived cytokine able to inhibit the inflammatory response, the up-regulation of IL-8, a pro-angiogenic CXC chemokine, and the increase of MMP9, a proteolytic proenzyme involved in degradation of the extracellular matrix.25
CLL patients have leukemic cells expressing high levels of immune-suppressing factors, low levels of adhesion and co-stimulatory molecules, lower numbers of natural killer cells, and deregulated T cells with an impairment of immunological synapse formation. The alterations described in our study further contribute to characterizing the complexity of factors potentially involved in the acquired immune deficiency of CLL patients. In conclusion, we detected altered composition and deregulation of genes involved in phagocytosis and inflammation in blood monocytes obtained from CLL patients, suggesting that CLL-mediated “education” of immune elements may also include the establishment of a skewed phenotype in the monocyte/macrophage population.
Acknowledgments
This work was supported by grants from: Associazione Italiana per la Ricerca sul Cancro (AIRC IG10621-R.Mar. and IG8590-S.D.), Milan, Italy; Programma di Ricerca di Interesse Nazionale (PRIN) 2008, Ministero dell’Università e della Ricerca (MIUR), Rome, Italy.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55 [DOI] [PubMed] [Google Scholar]
- 2.Cai QC, Liao H, Lin SX, Xia Y, Wang XX, Gao Y, et al. High expression of tumor-infiltrating macrophages correlates with poor prognosis in patients with diffuse large B-cell lymphoma. Med Oncol. 2012;29(4): 2317–22 [DOI] [PubMed] [Google Scholar]
- 3.Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood. 2005;106(6):2169–74 [DOI] [PubMed] [Google Scholar]
- 4.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362(10):875–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009; 114(17):3625–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor RP, Lindorfer MA. Antigenic modulation and rituximab resistance. Semin Hematol. 2010;47(2):124–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118(5):e16–31 [DOI] [PubMed] [Google Scholar]
- 8.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–80 [DOI] [PubMed] [Google Scholar]
- 9.Coffelt SB, Chen YY, Muthana M, Welford AF, Tal AO, Scholz A, et al. Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. J Immunol. 2011;186(7):4183–90 [DOI] [PubMed] [Google Scholar]
- 10.Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010; 70(13):5270–80 [DOI] [PubMed] [Google Scholar]
- 11.Burger JA. Nurture versus nature: the microenvironment in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:96–103 [DOI] [PubMed] [Google Scholar]
- 12.Zucchetto A, Benedetti D, Tripodo C, Bomben R, Dal Bo M, Marconi D, et al. CD38/CD31, the CCL3 and CCL4 chemokines, and CD49d/vascular cell adhesion molecule-1 are interchained by sequential events sustaining chronic lymphocytic leukemia cell survival. Cancer Res. 2009; 69(9):4001–9 [DOI] [PubMed] [Google Scholar]
- 13.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12): 5446–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maffei R, Fiorcari S, Bulgarelli J, Martinelli S, Castelli I, Deaglio S, et al. Physical contact with endothelial cells through beta1- and beta2- integrins rescues chronic lymphocytic leukemia cells from spontaneous and drug-induced apoptosis and induces a peculiar gene expression profile in leukemic cells. Haematologica. 2012;97(6):952–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deaglio S, Vaisitti T, Bergui L, Bonello L, Horenstein AL, Tamagnone L, et al. CD38 and CD100 lead a network of surface receptors relaying positive signals for B-CLL growth and survival. Blood. 2005;105(8): 3042–50 [DOI] [PubMed] [Google Scholar]
- 16.Tripodo C, Gri G, Piccaluga PP, Frossi B, Guarnotta C, Piconese S, et al. Mast cells and Th17 cells contribute to the lymphoma-associated pro-inflammatory microenvironment of angioimmunoblastic T-cell lymphoma. Am J Pathol. 2010;177(2):792–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serra S, Horenstein AL, Vaisitti T, Brusa D, Rossi D, Laurenti L, et al. CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood. 2011;118(23):6141–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maffei R, Martinelli S, Santachiara R, Rossi D, Guarnotta C, Sozzi E, et al. Angiopoietin-2 plasma dosage predicts time to first treatment and overall survival in chronic lymphocytic leukemia. Blood. 2010;116(4):584–92 [DOI] [PubMed] [Google Scholar]
- 19.Chung J, Serezani CH, Huang SK, Stern JN, Keskin DB, Jagirdar R, et al. Rap1 activation is required for Fc gamma receptor-dependent phagocytosis. J Immunol. 2008;181(8): 5501–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch DS, Pirone DM, Burbelo PD. A new family of Cdc42 effector proteins, CEPs, function in fibroblast and epithelial cell shape changes. J Biol Chem. 2001;276(2): 875–83 [DOI] [PubMed] [Google Scholar]
- 21.Ishii S, Noguchi K, Yanagida K. Non-Edg family lysophosphatidic acid (LPA) receptors. Prostaglandins Other Lipid Mediat. 2009;89(3–4):57–65 [DOI] [PubMed] [Google Scholar]
- 22.Yamada T, Ohoka Y, Kogo M, Inagaki S. Physical and functional interactions of the lysophosphatidic acid receptors with PDZ domain-containing Rho guanine nucleotide exchange factors (RhoGEFs). J Biol Chem. 2005;280(19):19358–63 [DOI] [PubMed] [Google Scholar]
- 23.Grkovich A, Armando A, Quehenberger O, Dennis EA. TLR-4 mediated group IVA phospholipase A(2) activation is phosphatidic acid phosphohydrolase 1 and protein kinase C dependent. Biochim Biophys Acta. 2009;1791(10):975–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valdearcos M, Esquinas E, Meana C, Gil-de-Gomez L, Guijas C, Balsinde J, et al. Subcellular localization and role of lipin-1 in human macrophages. J Immunol. 2011;186 (10):6004–13 [DOI] [PubMed] [Google Scholar]
- 25.Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol. 2006;16(1):38–52 [DOI] [PubMed] [Google Scholar]
- 26.Rahmouni S, Cerignoli F, Alonso A, Tsutji T, Henkens R, Zhu C, et al. Loss of the VHR dual-specific phosphatase causes cell-cycle arrest and senescence. Nat Cell Biol. 2006;8(5):524–31 [DOI] [PubMed] [Google Scholar]
- 27.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33(3):375–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saleh MN, Goldman SJ, LoBuglio AF, Beall AC, Sabio H, McCord MC, et al. CD16+ monocytes in patients with cancer: spontaneous elevation and pharmacologic induction by recombinant human macrophage colony-stimulating factor. Blood. 1995;85 (10):2910–7 [PubMed] [Google Scholar]
- 29.Subimerb C, Pinlaor S, Lulitanond V, Khuntikeo N, Okada S, McGrath MS, et al. Circulating CD14(+) CD16(+) monocyte levels predict tissue invasive character of cholangiocarcinoma. Clin Exp Immunol. 2010;161(3):471–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109(12):5276–85 [DOI] [PubMed] [Google Scholar]
- 31.Gustafson MP, Abraham RS, Lin Y, Wu W, Gastineau DA, Zent CS, et al. Association of an increased frequency of CD14+ HLA-DR lo/neg monocytes with decreased time to progression in chronic lymphocytic leukaemia (CLL). Br J Haematol. 2012;156 (5):674–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y, Li J, Ferguson GD, Mercurio F, Khambatta G, Morrison L, et al. Immunomodulatory drugs reorganize cytoskeleton by modulating Rho GTPases. Blood. 2009;114(2):338–45 [DOI] [PubMed] [Google Scholar]
- 33.Giannattasio G, Lai Y, Granata F, Mounier CM, Nallan L, Oslund R, et al. Expression of phospholipases A2 in primary human lung macrophages: role of cytosolic phospholipase A2-alpha in arachidonic acid release and platelet activating factor synthesis. Biochim Biophys Acta. 2009;1791(2):92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CH, Wu CL, Shiau AL. Toll-like receptor 4 signaling promotes tumor growth. J Immunother. 2010;33(1):73–82 [DOI] [PubMed] [Google Scholar]
- 35.Van Ginderachter JA, Meerschaut S, Liu Y, Brys L, De Groeve K, Hassanzadeh Ghassabeh G, et al. Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands reverse CTL suppression by alternatively activated (M2) macrophages in cancer. Blood. 2006;108(2):525–35 [DOI] [PubMed] [Google Scholar]
- 36.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82 [DOI] [PubMed] [Google Scholar]
- 37.Ryan EP, Pollock SJ, Kaur K, Felgar RE, Bernstein SH, Chiorazzi N, et al. Constitutive and activation-inducible cyclooxygenase-2 expression enhances survival of chronic lymphocytic leukemia B cells. Clin Immunol. 2006;120(1):76–90 [DOI] [PubMed] [Google Scholar]