Abstract
Despite therapeutic advances multiple myeloma remains largely incurable, and novel therapeutic concepts are needed. The Hsp90-chaperone is a reasonable therapeutic target, because it maintains oncogenic signaling of multiple deregulated pathways. However, in contrast to promising preclinical results, only limited clinical efficacy has been achieved through pharmacological Hsp90 inhibition. Because Hsp70 has been described to interact functionally with the Hsp90-complex, we analyzed the suitability of Hsp72 and Hsp73 as potential additional target sites. Expression of Hsp72 and Hsp73 in myeloma cells was analyzed by immunohistochemical staining and western blotting. Short interfering RNA-mediated knockdown or pharmacological inhibition of Hsp72 and Hsp73 was performed to evaluate the role of these proteins in myeloma cell survival and for Hsp90-chaperone function. Furthermore, the role of PI3K-dependent signaling in constitutive and inducible Hsp70 expression was investigated using short interfering RNA-mediated and pharmacological PI3K inhibition. Hsp72 and Hsp73 were frequently overexpressed in multiple myeloma. Knockdown of Hsp72 and/or Hsp73 or treatment with VER-155008 induced apoptosis of myeloma cells. Hsp72/Hsp73 inhibition decreased protein levels of Hsp90-chaperone clients affecting multiple oncogenic signaling pathways, and acted synergistically with the Hsp90 inhibitor NVP-AUY922 in the induction of death of myeloma cells. Inhibition of the PI3K/Akt/GSK3β pathway with short interfering RNA or PI103 decreased expression of the heat shock transcription factor 1 and down-regulated constitutive and inducible Hsp70 expression. Treatment of myeloma cells with a combination of NVP-AUY922 and PI103 resulted in additive to synergistic cytotoxicity. In conclusion, Hsp72 and Hsp73 sustain Hsp90-chaperone function and critically contribute to the survival of myeloma cells. Translation of Hsp70 inhibition into the clinic is therefore highly desirable. Treatment with PI3K inhibitors might represent an alternative therapeutic strategy to target Hsp70.
Introduction
Multiple myeloma (MM) is a malignant disease of the terminally differentiated B cell (plasma cell).1–3 Although the therapeutic arsenal has been enlarged by the introduction of novel agents such as bortezomib and lenalidomide, MM presently remains incurable.3–4 Further progress is, therefore, required from new therapeutic concepts based on greater knowledge of MM pathobiology.3,5
The heat shock proteins Hsp90 and Hsp70 are different multi-protein complexes, which have been shown to interact jointly to act as molecular chaperones. The Hsp90-chaperone complex mediates the accurate conformation, stability and activity of many proteins, including key components of deregulated signaling pathways in tumor cells.6,7 It has recently been shown that Hsp90 is frequently over-expressed in MM, sustains oncogenic deregulation of survival pathways, and critically contributes to malignant growth.8 Pharmacological Hsp90 inhibition has, therefore, been investigated as a promising novel therapeutic strategy in MM.8–11 However, despite promising preclinical results, only limited clinical efficacy was achieved by monotherapy with the Hsp90 inhibitor tanespimycin.12 This suggests that combination approaches may need to be developed to successfully translate the therapeutic concept of Hsp90-chaperone inhibition into the clinic.
The Hsp70 family comprises a total of eight members of which the inducible Hsp72 and the constitutively expressed Hsp73 are the major isoforms. Hsp70 family members play an essential role in the substrate-loading phase of the Hsp90-chaperone. In non-tumor tissues expression of Hsp72 is rather low, but it increases greatly under conditions of cellular stress.13 In contrast, constitutive over-expression of both Hsp70 isoforms has been observed in cancer cells.14 Interestingly, a strong up-regulation of Hsp72 has been reported after pharmacological Hsp90 inhibition, also in MM cells.10,11,15 Furthermore, it has recently been shown that dual silencing of Hsp72 and Hsp73 in cell lines derived from solid tumors led to degradation of Hsp90 client proteins and to tumor-specific growth inhibition.16 Taken together these data suggest that Hsp72 and Hsp73 may mitigate Hsp90 blockade-mediated cytotoxicity in cancer cells, and thus contribute to drug resistance. However, the precise role of Hsp72 and Hsp73 in MM remains to be elucidated. We, therefore, decided to investigate the expression, function and regulation of both Hsp70 in MM.
Design and Methods
Immunohistochemical analyses
Immunohistochemical and immunofluorescence analyses were performed according to previously published methods,8 and are described in full in the Online Supplementary Design and Methods.
Cell culture
The culture conditions of primary MM cells, MM cell lines and primary bone marrow stromal cells (BMSC) have been described in detail elsewhere.11,17,18
Transfection of INA-6 and MM.1S cells with short-interfering RNA expression plasmids
PSUPER-derived short interfering (si) RNA expression constructs were designed according to previously described guidelines.19 The sequences of the sense oligonucleotides of the most effective siRNA are provided in the Online Supplementary Design and Methods. The protocol for transient transfection and purification of transfected cells has been described elsewhere.19 Western blot analysis of the respective target in selected cells was performed after 72 h (pSU/Hsp72 or pSU/Hsp73) or after 120 h (pSU/PI3Kp110α) to verify their efficiency and specificity.
Western blot analysis
The western blotting procedures were performed as described elsewhere.18 Details are provided in the Online Supplementary Design and Methods.
Viability assays
Apoptotic and viable cell fractions were assessed by annexin V-FITC/propidium iodide (PI) staining (Bender MedSystems, Vienna, Austria) as previously described.18 The AlamarBlue assay (MorphoSys, Oxford, UK) was performed according to the manufacturer’s instructions to measure drug effects on metabolic activity of MM cells. Both assays are described in the Online Supplementary Design and Methods.
Drug combination analyses
MM cell lines INA-6 and MM.1S were treated with combinations of NVP-AUY922/VER-155008 or NVP-AUY922/PI103 in accordance with the guidelines discussed by Chou20 using the AlamarBlue assay to quantify viability and CalcuSyn (software Version 2.1; Biosoft, Cambridge, UK) to calculate correlation coefficients for the dose-effect curves. The experimental design is described in full in the Online Supplementary Design and Methods.
Reagents
The catalogue numbers and sources of the reagents used in this study are listed in the Online Supplementary Design and Methods.
Statistical analysis
Statistical analyses were performed using a two-tailed unpaired Student’s t test. P-values < 0.05 were considered statistically significant.
Results
Hsp72 and Hsp73 proteins are frequently overexpressed in multiple myeloma cells, but not in normal plasma or B cells
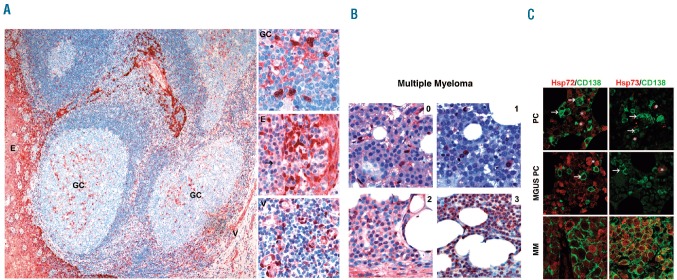
We initially established immunohistochemical staining protocols for Hsp72 and Hsp73 in lymph node (Figure 1A) and bone marrow biopsies (Figure 1B,C) to investigate the respective in situ protein expression levels in human plasma cells. Staining with isoform-specific antibodies against Hsp72 and Hsp73 or with a pan-HSP70 antibody (recognizing both isoforms) was visualized using either ACE as a chromogenic substrate (Figure 1A,B) or with fluorescent dyes in co-immunofluorescence studies with the plasma cell marker CD138 (Figure 1C). Normal plasma cells did not have detectable levels of either Hsp72 or Hsp73 (Figure 1A,C). A similar result was found for the majority of plasma cells from the bone marrow of patients with monoclonal gammopathy of undetermined significance, in whom at most there was weak expression of Hsp72 and Hsp73 in a few plasma cells (Figure 1C). In contrast, we observed strong plasma cell expression of Hsp72 and Hsp73 in a large proportion of the MM cases investigated (Figure 1B,C). Staining with the pan-Hsp70 antibody yielded positive signals in 67% (37/55) of the MM samples. This result was in good agreement with staining with isoform-specific antibodies: 38% of samples (21/55) stained positive for Hsp72 and 60% (33/55) were immunopositive for Hsp73. Comparative analysis of the expression patterns revealed that 81% (17/21) of the Hsp72-immunopositive MM samples also stained positive for Hsp73, whereas 52% (17/33) of the Hsp73-immunopositive specimens displayed detectable Hsp72 signals. Of note, all extramedullary (n=6) and seven out of eight morphologically anaplastic MM cases stained strongly positive for Hsp72 and/or Hsp73. A detailed list of the individual staining patterns of the MM samples used in this study is provided in Online Supplementary Table S1). In another approach, the levels of Hsp72/73 expression in six MM cell lines were compared to those in normal B cells obtained from the peripheral blood of two healthy donors. Western blot analysis showed strong overexpression of Hsp72 in four out of the six MM cell lines and of Hsp73 in all six MM cell lines (Online Supplementary Figure S1).
Figure 1.
Hsp72 and Hsp73 proteins are strongly expressed in MM cells as opposed to normal plasma cells (PC) or PC from patients with monoclonal gammopathy of undertermined significance (MGUS). (A) Immunohistochemical staining of Hsp72 in the normal human tonsil is shown. The overview depicts Hsp72-positive epithelial cells (E), scattered Hsp72-positive cells within the reactive germinal center (GC) and Hsp72-positive endothelial cells of small vessels (V). The insets show the corresponding regions with positive cells at higher magnification. Hsp72-negative normal PC (arrow) are interspersed between the Hsp72-positive epithelial cells. (B) Immunohistochemical staining of Hsp72 in bone marrow biopsies from MM patients representing different scoring groups (0–3; details in the Online Supplementary Design and Methods section). Of note, in the HSP72-immunonegative sample (1) stromal and endothelial cells displayed nuclear Hsp72-positivity, and thus served as a positive control. (C) Immunofluorescence analyses of Hsp72 and Hsp73 protein expression in bone marrow biopsies. Merged immunofluorescence images (CD138 and either Hsp72 or Hsp73) of bone marrow biopsies either from a patient without a plasma cell disorder (PC), from a patient with MGUS and a patient with MM. Of note, Hsp72 was strongly expressed in myeloid precursor cells (*) and Hsp73 was expressed in a few megakaryocytes (*).
Hsp72/Hsp73 inhibition efficiently induces apoptosis in multiple myeloma cells
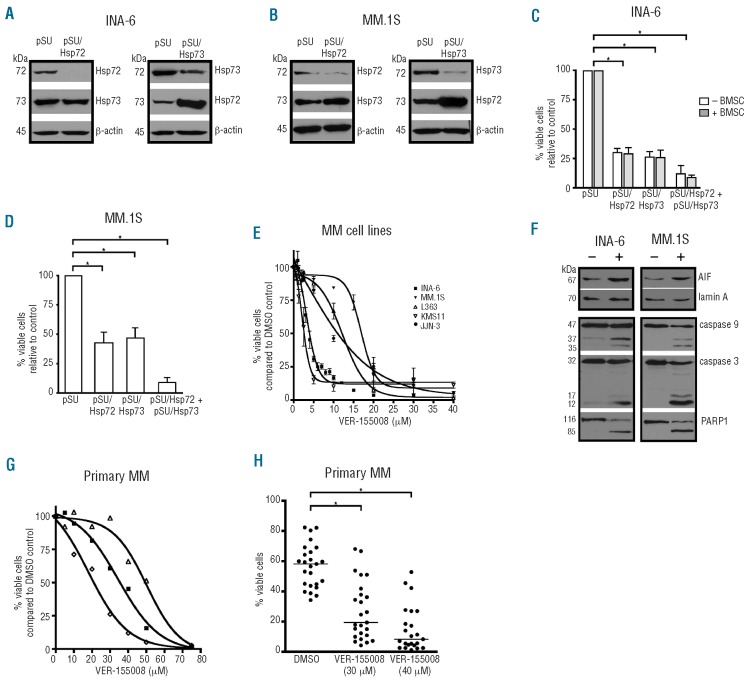
To investigate the respective role of Hsp72 and Hsp73 in MM, isoform-specific siRNA sequences against both Hsp70, which share a high degree of amino acid homology (86%), were selected, and pSUPER-based siRNA expression plasmids constructed. INA-6 and MM.1S cells were transiently transfected with these vectors to selectively deplete Hsp72, Hsp73 or both. Western blot analysis showed that the siRNA against Hsp72 caused down-regulation of Hsp72 protein without reducing Hsp73 levels and vice versa, confirming that both siRNA are highly specific for their respective target (Figure 2A,B). Interestingly, in both cell lines the level of Hsp72 protein increased substantially after siRNA-mediated down-regulation of Hsp73, indicating a specific compensatory mechanism between the two Hsp70 (Figure 2A, B). In order to study the effects of Hsp72/Hsp73 knockdown on cell survival, INA-6 and MM.1S cells were assayed for apoptosis 4 days after transfection (Figure 2C, D). Hsp72 knockdown reduced the survival rate to 30% in INA-6 cells, and to 50% in MM.1S cells, whereas depletion of Hsp73 led to the survival of INA-6 and MM.1S cells being 26% and 47%, respectively, that of the appropriate mock-transfected controls. Concomitant down-regulation of Hsp72 and Hsp73 proteins induced large-scale apoptosis with survival rates of just 5% in INA-6 and 9% in MM.1S cells (Figure 2C,D). Next, we investigated whether cells from the bone marrow microenvironment can protect INA-6 cells from apoptosis induced by knockdown of Hsp72 and Hsp73. INA-6 cells transfected with siRNA expression constructs against either Hsp72 or Hsp73 or against both, were co-cultured with BMSC and cell death assessed 4 days post-transfection. The siRNA-mediated down-regulation of Hsp72 and Hsp73 proteins effectively decreased survival even in the presence of BMSC (Figure 2C). In order to complement these analyses with a pharmacological approach we tested the effects of the novel small molecule Hsp72/Hsp73 inhibitor VER-155008 on MM cell survival. Cells of five MM cell lines (INA-6, MM.1S, L363, KMS11 and JJN-3) were incubated with different concentrations of VER-155008 for 72 h prior to viability analysis with annexin V-FITC/PI staining. Treatment with VER-155008 decreased cell viability through induction of apoptosis in all MM cell lines. The dose-effect curves showed a near complete demise of viable cells at concentrations between 10 and 30 μM, although the sensitivity towards VER-155008 differed between cell lines (Figure 2E). The highest sensitivity was observed for INA-6 and KMS11 cells (IC50: 2.5 and 4 μM, respectively), whereas JJN-3, L363 and MM.1S cells were somewhat less affected (IC50: 11, 12 and 17 μM, respectively).
Figure 2.
Inhibition of Hsp72 and Hsp73 by siRNA-mediated knockdown or by pharmacological inhibition strongly induces apoptosis of MM cells. Hsp72/Hsp73 inhibition experiments using either siRNA (A-D) or the pharmacological inhibitor VER-155008 (E-H). INA-6 or MM.1S cells were transiently transfected with pSUPER-based siRNA expression vectors to selectively knock down Hsp72 or Hsp73, and analyzed by western blotting (after 72 h) and by annexin V-FITC/PI staining (after 96 h). An exemplary staining for β-actin is shown as a loading control. In (C) and (D) the means and the standard deviations based on at least seven independent experiments are shown. (E) Dose-effect curves with human MM cell lines treated with VER-155008 for 3 days. (F) Western blot analyses of MM cells treated with 15 μM VER-155008: staining of the nuclear cell fraction for the apoptosis-inducing factor (AIF) or for lamin A as a loading control after 6 h (top panels); staining of whole cell lysates for caspase 3, caspase 9 or PARP1 after 24 h (lower panels). (G and H) Primary MM cells (n=25) were co-cultured with BMSC and treated with VER-155008 for 3 days prior to annexin V-FITC/PI staining. (G) Dose-effect curves for VER-155008 for three primary MM samples. (H) Survival of primary MM samples after treatment with 30 μM (n=25) or 40 μM (n=23) VER-155008. (*) indicates statistical significance (P<0.05).
In order to investigate apoptotic pathways that have previously been associated with Hsp70 function, we next analyzed nuclear translocation of the apoptosis-inducing factor (AIF) and cleavage of pro-caspases 9 and 3 upon treatment of INA-6 or MM.1S cells with 15 μM VER-155008. Western blot analyses showed substantial accumulation of AIF in the nucleus after 6 h of drug treatment, as well as increased cleavage of pro-caspases 9 and 3, and of the caspase substrate poly (ADP-ribose) polymerase 1 (PARP 1) (Figure 2F). Next, we evaluated the effects of VER-155008 on the survival of primary MM cells in vitro (Figure 2G,H). Primary MM cells (n=25), freshly isolated from bone marrow aspirates, were kept in co-culture with primary BMSC and treated with VER-155008 for 3 days prior to viability analyses with annexin V-FITC/PI staining. Dose-effect curves for VER-155008 in three primary MM samples showed that these had weaker sensitivity (IC50: 20, 35 and 50 μM) than MM cell lines (Figure 2G). Based on these results, two concentrations (30 and 40 μM) were chosen to further evaluate the effectiveness of VER-155008 in a larger number of primary MM samples (Figure 2H). Survival of primary MM samples was assessed with annexin V-FITC/PI staining after 3 days of treatment with 30 μM (n=25) or 40 μM (n=23) of VER-155008. These concentrations led to strong induction of apoptosis in the majority of primary MM samples. A comparison of the median viability of VER-155008-treated and DMSO-treated MM cells showed decreases of 67% (30 μM VER-155008) and 90% (40 μM VER-155008) in the drug-treated sets (Figure 2H). Whereas at a concentration of 30 μM VER-155008, 10/25 MM samples retained sizeable quantities of viable cells (>30%), such differential responses were largely abolished at 40 μM. Thus, in primary MM cells too, pharmacological inhibition of Hsp72/Hsp73 activity induced apoptosis even in the presence of BMSC.
The Hsp90-chaperone function in myeloma cells is critically dependent on Hsp72 and Hsp73
Although it is well established that Hsp70 acts as a structural co-chaperone within the Hsp90-multi-chaperone complex, it has recently been shown that dual silencing of the major Hsp70 isoforms Hsp72 and Hsp73 in cancer cells inhibited Hsp90 function and induced tumor-specific apoptosis.16 We, therefore, investigated a potential structural and functional interaction between Hsp72/Hsp73 and Hsp90 in MM cells. Our in situ expression analyses based on immunohistochemical staining revealed that Hsp90 was overexpressed in 73% (40/55) of primary MM samples, whereas 67% (37/55) stained immunopositive for Hsp72/73. Eighty-six percent (32/37) of the Hsp72/73-positive MM samples, and 44% (8/18) of the Hsp72/73-negative MM samples also stained for Hsp90 (P<0.001 or P=0.001, respectively; χ2 test). Thus, both Hsp70 isoforms were significantly co-expressed with Hsp90. Furthermore, immunofluorescent confocal analysis of INA-6 and MM.1S cells as well primary myeloma cells from bone marrow biopsies of MM patients showed partial co-localization of Hsp72/Hsp73 and Hsp90 in situ (Online Supplementary Figure S2A). In addition, immunoprecipitation studies using INA-6 cells showed binding of Hsp90 to both Hsp72 and Hsp73, indicating a direct structural interaction of Hsp70 and Hsp90 in MM (Online Supplementary Figure S2B).
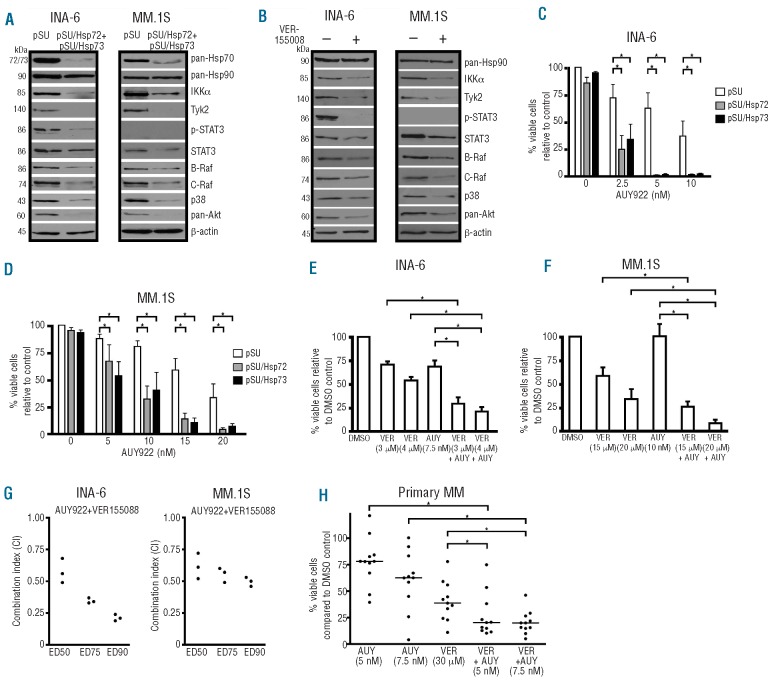
In order to investigate a potential functional interplay between the Hsp70 isoforms and Hsp90 in INA-6 and MM.1S cells, the levels of expression of several well-defined Hsp90 client proteins were probed by western blotting after Hsp72/Hsp73 inhibition (Figure 3A,B). Hsp72 and Hsp73 were either knocked down in concert (Figure 3A) or were pharmacologically inhibited with VER-155008 (Figure 3B) and then 48 h after transfection or 24 h after treatment with VER-155008 (i.e. before the onset of large-scale apoptosis) cells were harvested and the levels of Hsp90 client proteins determined. To avoid any misinterpretation through potential effects mediated by active caspases, cell cultures were supplemented with 50 μM of the pan-caspase inhibitor z-VAD-fmk. Whereas Hsp90 expression levels remained unaffected, substantial decreases of the Hsp90 clients B- and C-Raf, Akt, p38, IKKα and Tyk2 were observed after Hsp72/73 depletion in INA-6 and MM.1S cells. Of note, INA-6 cells, which are dependent on the IL-6R/STAT3 signaling pathway, showed a strong decrease of phosphorylated STAT3 and a partial decrease of total STAT3 protein after Hsp72/73 inhibition; the latter effect was also observed in phospho-STAT3-negative MM.1S cells. These data demonstrate that Hsp72 and Hsp73 are crucial for the stability of many Hsp90-dependent protein kinases, thereby indicating their critical contribution to the full activity of the Hsp90-multi-chaperone complex in MM.
Figure 3.
Inhibition of Hsp72 and Hsp73 by siRNA-mediated knockdown or pharmacologically with VER-155008 leads to degradation of Hsp90-chaperone client proteins and enhances apoptosis induced by the Hsp90 inhibitor NVP-AUY922 in MM cells. (A and B) Western blot analyses of Hsp90 client proteins 48 h post-transfection with siRNA against Hsp72 and Hsp73 (A) or after 24 h treatment with the Hsp70 inhibitor VER-155008 (15 μM) (B). Exemplary β-actin staining is shown as a loading control. (C-H) Experiments with combined inhibition of Hsp70/Hsp90 in MM cells are shown. (C and D) Hsp72/Hsp73 knockdown in INA-6 or MM.1S cells combined with the Hsp90 inhibitor NVP-AUY922. The drug was added 36 h post-transfection to purified transfected cells and cell death was determined after another 24 h by annex-in V/PI staining. Means and standard deviations are based on five independent experiments. (E and F) Concomitant treatment with VER-155008 and NVP-AUY922 for either 3 (INA-6) or 4 days (MM.1S) prior to cell death assessment with annexin V/PI (three independent experiments). (G) Combination analyses (three independent experiments) for NVP-AUY922 and VER-155008 with the combination index (CI) determined according to the method of Chou (fixed ratio design, simultaneous drug addition, 3 days of drug treatment). Results obtained for three different effect levels are shown. (H) Primary samples from 11 MM patients in co-culture with BMSC were treated for 3 days with either NVP-AUY922, VER-155008 or a combination of both. Viability was assessed by annexin V-FITC/PI and calculated with respect to the cognate DMSO controls. Where indicated (*), there was a significant (P<0.05) reduction in viability induced by the combination treatments.
Concomitant Hsp72/Hsp73 inhibition significantly enhances apoptosis induced by the Hsp90 inhibitor NVP-AUY922 in MM.1S and INA-6 cells
It has been previously demonstrated that the in vitro survival of MM cells is dependent on Hsp90 function.8–11 We, therefore, evaluated survival of MM cells after combined targeting of both Hsp72/Hsp73 and Hsp90. INA-6 (Figure 3C) or MM.1S (Figure 3D) cells were transfected either with empty pSUPER vector as a mock control or with pSUPER/Hsp72 or pSUPER/Hsp73 siRNA expression plasmids, and 36 h after transfection were treated with different concentrations of the novel Hsp90 inhibitor NVP-AUY922 for an additional 24 h before viability was assessed. At that time point and at the concentrations chosen neither the Hsp90 inhibitor alone, nor the Hsp72 or Hsp73 siRNA displayed their full cytotoxic effects, as shown by the limited proportion of apoptotic cells in the control settings. However, combined inhibition of either Hsp72 or Hsp73 and Hsp90 led to robust apoptotic responses in both MM cell lines, and these responses were strongly augmented with rising concentrations of NVP-AUY922. In an alternative assessment of combined Hsp90/Hsp70 blockade with a purely pharmacological approach we treated MM cells with NVP-AUY922 and the new Hsp70 inhibitor VER-155008 (Figure 3E-H). Based on the dose-effect curves for the single drugs (for VER-155008 see Figure 2E; for NVP-AUY922 data not shown) concentrations were chosen that did not exceed EC50 levels. An exception from this rule was made for the more VER-155008-insensitive MM cell line MM.1S, which was also tested with the high concentration of 20 μM VER-155008 (see Figure 2E). INA-6 (Figure 3E) and MM.1S (Figure 3F) cells were treated either with DMSO, VER-155008, NVP-AUY922 or with a combination of VER-155008 and NVP-AUY922 for 3 days prior to viability analyses by annexin V-FITC/PI staining. Concomitant Hsp72/Hsp73 inhibition with VER-155008 significantly enhanced the induction of apoptosis by sub-effective concentrations of NVP-AUY922 (Figure 3E,F). In order to quantify the effects of the VER-155008/NVP-AUY922 combination better, we used the AlamarBlue metabolic assay to determine the effects of the drug combination on the viability of MM cells according to the method of Chou and Talalay (see Design and Methods section). Combination indices had values below 1 for all effect levels calculated, indicating synergy of the drug combination in both MM cell lines (Figure 3G). We then tested the drug combination with freshly isolated primary MM cells (n=11) co-cultured with primary BMSC using fixed concentrations of NVP-AUY922 (5 and 10 nM) and VER-155008 (30 μM), and their respective combinations (Figure 2H). Viable cell fractions were assessed by annexin V-FITC/PI staining after 3 days and compared to DMSO-treated control cells. Although treatment with 30 μM VER-155008 already caused strong apoptotic effects on its own, the combination with sub-effective concentrations of NVP-AUY922 still significantly enhanced the overall rate of MM cell death. These results suggest that combining Hsp90 and Hsp70 inhibition could be an effective therapeutic strategy for the treatment of MM.
The PI3K/Akt/GSK3β signaling pathway stabilizes the expression of heat shock transcription factor 1, thereby controlling constitutive and inducible expression of Hsp70 in multiple myeloma cells
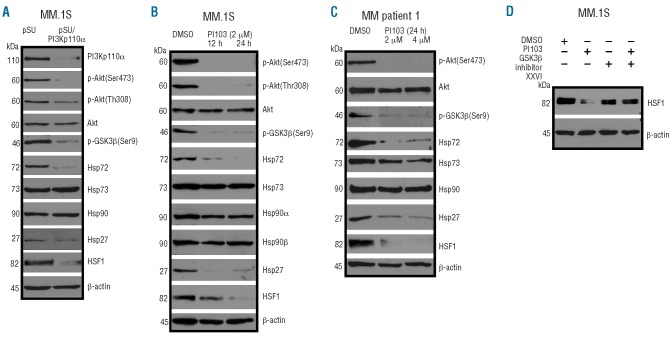
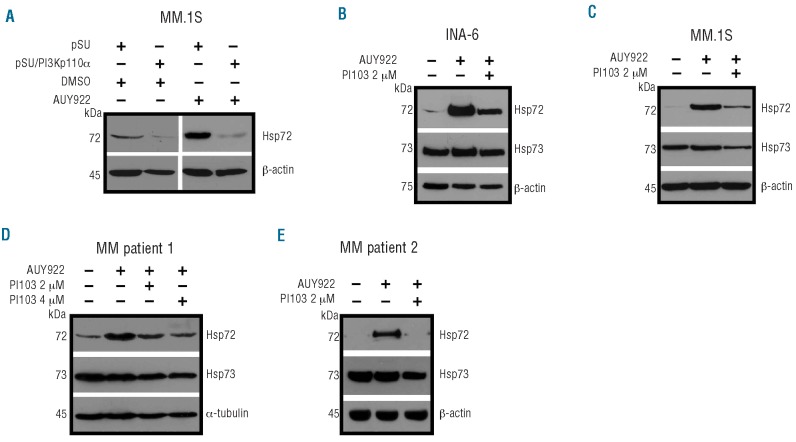
Deregulated oncogenic signaling is considered to be a hallmark of the pathogenesis of MM. We, therefore, explored the potential regulation of Hsp70 by different signaling pathways including the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway, which is frequently activated in MM and contributes to tumor cell survival.21–23 Two different experimental approaches were pursued to inhibit PI3K-mediated signaling: pSUPER-based siRNA-mediated knockdown of the p110α subunit of PI3K (Figure 4A) and treatment with the small molecule class I PI3K inhibitor PI103, which exhibits activity against the PI3K isoforms p110α, p110β, p110γ, and p110δ (Figure 4B).24,25 MM.1S cells, which display strong constitutive activation of the PI3K/Akt pathway, were transfected with either pSUPER (mock control) or with pSUPER/PI3Kp110α, purified, and harvested 5 days after electroporation for western blotting (Figure 4A). Alternatively, MM.1S cells, as well as primary MM cells, were treated with PI103 for 24 h prior to harvesting (Figure 4B,C). Both approaches yielded strong inhibition of PI3K downstream signaling, as shown by drastically reduced levels of phosphorylated Akt and phosphorylated glycogen synthase kinase 3β (GSK3β) (Figure 4A-C). Whereas the levels of Hsp90 expression remained virtually unaffected, and just slight decreases in the amount of Hsp73 in the case of treatment with PI103 were observed, the constitutive levels of Hsp72 were always strongly down-regulated. In order to investigate the underlying mechanism of this observation, we analyzed expression of HSF1, which has been shown to regulate different heat shock proteins during physiological stress responses, and found substantial down-regulation of HSF1 upon PI3K inhibition (Figure 4A-C). PI3K inhibition also caused strong decreases in the level of Hsp27, another HSF1-regulated heat shock protein (Figure 4). Because GSK3β has been reported to have a role in the stability of HSF126–28 we tested whether dephosphorylated (i.e. active) GSK3β might be involved in the down-regulation of HSF1. MM.1S were treated with either PI103, the GSK3β inhibitor XXVI, or a combination of both. Interestingly, the decrease in HSF1 after treatment with PI103 was largely prevented by concomitant inhibition of GSK3β, suggesting that GSK3β might indeed play a role as a negative regulator of HSF1 protein expression in MM cells (Figure 4D). It has previously been reported that pharmacological inhibition of Hsp90 causes a strong induction of Hsp70 expression, suggesting a potential drug resistance mechanism. Therefore, we next evaluated whether HSF1-dependent induction of Hsp70 upon pharmacological Hsp90 inhibition is controlled by PI3K in MM cells (Figure 5). siRNA-mediated knockdown of PI3Kp110α in MM.1S cells efficiently prevented strong Hsp72 induction upon treatment with the Hsp90 inhibitor NVP-AUY922 (Figure 5A). Accordingly, a similar block of Hsp72 up-regulation was observed upon treatment of INA-6 cells (Figure 5B), MM.1S cells (Figure 5C) or primary MM cells (Figure 5D,E) with PI103. These data strongly suggest that the PI3K/Akt/GSK3β signaling axis crucially controls HSF1 activity and thereby regulates constitutive as well as inducible expression of Hsp70 in MM cells.
Figure 4.
Inhibition of PI3K/Akt/GSK3β signaling causes degradation of HSF1 protein, and subsequently leads to down-regulation of constitutive Hsp70 expression. (A-C) Western blot analyses of constitutive protein expression of HSF1 and different heat shock proteins after PI3Kp110α knockdown in MM.1S cells 5 days post-transfection (A) or after treatment of MM.1S cells (B) or primary MM cells obtained from a patient with plasma cell leukemia (C) with the PI3K inhibitor PI103 for 24 h. (D) Determination of HSF1 expression after treatment of MM.1S cells with 4 μM PI103, 5 μM of the GSP3β inhibitor XXVI, or a combination of both. Staining of β-actin served as a loading control.
Figure 5.
Induction of Hsp70 expression upon Hsp90 inhibition is dependent on PI3K activity in MM cells. Western blot analyses of inducible Hsp70 expression upon pharmacological Hsp90 inhibition with or without concomitant PI3K inhibition in the cell lines INA-6 and MM.1S, and in primary MM cells. (A) MM.1S cells were transfected with either pSUPER or with pSUPER/PI3Kp110α, purified, and at 5 days post-transfection incubated for another 5 h with either DMSO or NVP-AUY922. INA-6 (B) or MM.1S (C) cells were incubated for 12 h with the PI3 kinase inhibitor PI103, and then subjected to treatment with 10 nM of the Hsp90 inhibitor NVP-AUY922 for another 5 h. Primary MM cells derived from a patient with plasma cell leukemia (D) or from a patient with intramedullary MM (E) were treated with DMSO or with PI103 for 12 h prior to addition of 10 nM AUY922 for another 5 h. Staining of β-actin served as a loading control.
Combined pharmacological PI3K and Hsp90 inhibition leads to strongly enhanced apoptotic effects in INA-6 and MM.1S cells
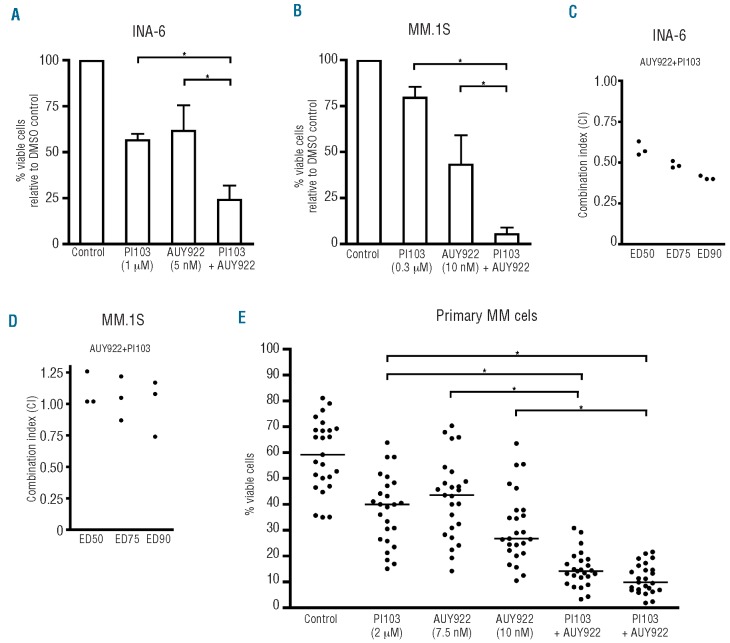
Concomitant inhibition of Hsp70 and Hsp90 could potentially be an exciting therapeutic strategy. However, specific pharmacological Hsp70 inhibitors with clinical potential are currently lacking. Because we observed PI3K-dependent regulation of HSF1/Hsp70 (Figures 4 and 5), we next evaluated whether combined PI3K and Hsp90 inhibition might represent a suitable approach to induce MM cell death (Figure 6). Based on the dose-effect curves of NVP-AUY922 and PI103 (data not shown) mildly effective concentrations of these drugs were chosen for combination experiments. Specifically, INA-6 cells were incubated with 5 nM NVP-AUY922 or with 1 μM PI103 or with both drugs combined (Figure 6A), and MM.1s cells were exposed to 10 nM NVP-AUY922 or 0.3 μM PI103 or their combination (Figure 6B). Combination treatment resulted in significantly enhanced apoptosis compared to the respective single drug treatments. Thus, concomitant exposure to NVP-AUY922 and PI103 decreased the viability of INA-6 cells to 25%, and that of MM.1s cells to just 5% (Figure 6A, B). Combination index analysis revealed synergistic activity of the two drugs in INA-6 cells, and additive effects in MM.1S cells (Figure 6C,D). Finally, we tested apoptosis induction in freshly purified primary MM cells (n=25) co-cultured with BMSC after 72 h of treatment with either NVP-AUY922 (7.5 μM and 10 μM), PI103 (2 μM) or their combination (Figure 6E). Compared to the respective DMSO-treated controls, drug treatments reduced MM cell viability to median values of 67% (PI103; 2 μM), 75% (NVP-AUY922; 7.5 μM) and 42% (NVP-AUY922; 10 μM). However, treatment with both drugs strongly decreased cellular viability to between 10–15%, suggesting a rationale for the development of a therapeutic regimen based on the inhibition of PI3K and Hsp90.
Figure 6.
Combined pharmacological PI3K and Hsp90 inhibition leads to enhanced apoptotic effects in MM cells. (A and B) Viability analyses by annexin V-FITC/PI staining upon treatment of INA-6 or MM.1S cells with fixed concentrations of either PI103, AUY922 or a combination of both drugs for 3 days. Three independent experiments were conducted for each MM cell line. (C and D) Combination analyses (three independent experiments) for PI103 and AUY922 with combination index (CI) determination according to the method of Chou (fixed ratio design, simultaneous drug addition, 3 days of drug treatment). (E) Primary MM samples obtained from the peripheral blood of a patient with plasma cell leukemia or isolated from bone marrow aspirates from 24 MM patients were co-cultured with primary BMSC and treated as indicated for 72 h prior to staining with annexin V-FITC/PI. (*) indicates statistically significant differences (P<0.05).
Discussion
In the present study, we show that the major Hsp70 isoforms, Hsp72 and Hsp73, contribute critically to the patho-biology of MM. Expression analyses revealed frequent elevation of Hsp72 and Hsp73 protein levels in primary MM cells and in MM cell lines, compared to the levels in either non-malignant plasma cells or normal B cells. Our finding is in good agreement with the observation in other studies showing deregulated expression of Hsp70 isoforms in various tumor entities such as endometrial cancer, osteosarcoma and renal cell carcinoma.14 We found that all MM samples from extramedullary manifestations and seven out of eight MM samples with anaplastic morphology were strongly immunopositive for Hsp72/Hsp73. This suggests that elevated Hsp70 levels could be associated with a progressed disease state. However, further correlation studies evaluating larger, clinically well-defined panels of MM patients are needed to clarify the potential relevance of elevated Hsp70 expression for prognosis or progression.
Upon selective knockdown of either Hsp72 or Hsp73 we observed a strong induction of apoptosis in MM cells. This lethal effect appeared more rapidly and was more pronounced if both Hsp70 isoforms were concomitantly silenced, suggesting that both Hsp70 contribute to MM cell survival. Reports regarding the importance of the Hsp72 or Hsp73 isoform for cellular survival do not give a consistent picture and the degree of cell death induction might well be dependent on the respective cellular system. Thus, in cancer models of solid tumors it was recently found that the combined knockdown of both Hsp72 and Hsp73 was required to induce cell death.16 In line with the Hsp72/Hsp73 knockdown results, treatment with the novel Hsp72/Hsp73 inhibitor VER-15500829 induced MM cell apoptosis. In addition to a role in down-regulation of Hsp90 client proteins (see below), the induction of apoptosis of MM cells through Hsp70 blockade was also shown to involve translocation of AIF into the nucleus and cleavage of pro-caspases 9 and 3. Both processes are reportedly Hsp90-independent because binding of the respective proteins to Hsp70 prevents their activity.16,30,31
We observed that Hsp72 and Hsp73 were co-expressed and co-localized with Hsp90 in MM cell lines as well as in primary samples in situ indicating that both multi-chaperone complexes jointly interact in MM. The dynamics of this interaction has recently been illustrated showing that upon Hsp73 knockdown its job in the Hsp90-complex can functionally be fulfilled by Hsp72.16 In line with these observations, we found that Hsp73 knockdown in MM cells caused a strong increase of the inducible isoform Hsp72 (whereas no such change was seen for Hsp73 after Hsp72 knockdown), suggesting a potential compensatory capacity of Hsp72 for rescuing or maintaining Hsp90-chaperone function in MM cells.
The Hsp90-chaperone has been identified as a promising therapeutic target, because selective blockade of Hsp90 leads to degradation of multiple oncogenic proteins, and thereby causes concomitant disruption of several oncogenic signaling pathways.6,7,32 We demonstrated that either knockdown or pharmacological inhibition of Hsp72/Hsp73 strongly attenuated the expression levels of several well-characterized Hsp90 client proteins, although Hsp90 expression levels were unaffected. Notably, many of these clients act as signaling proteins, and thus a number of signaling pathways, such as the Ras/Raf/MAPK, JAK/STAT3, PI3K/Akt and IKK/NFκB pathways, which have been reported to contribute to the malignant growth of MM cells, were simultaneously inhibited upon Hsp72/73 knockdown. In accordance with our findings in MM, similar effects of Hsp72/Hsp73 knockdown have recently been described in cells representing solid cancers corroborating the hypothesis that the Hsp90-chaperone function is largely dependent on Hsp72/Hsp73 in cancer cells.16
Targeting Hsp90 using pharmacological inhibitors has proven successful in preclinical MM models.8–11 However, only limited clinical efficacy was achieved by monotherapy with the Hsp90 inhibitor tanespimycin suggesting that combinations of therapeutic agents will be required to translate the concept of Hsp90-chaperone inhibition into the clinic.12 With the assumption that the loss of functional Hsp90 might be compensated for, at least in part, by Hsp70, we observed that the pro-apoptotic effect of the novel pharmacological Hsp90 inhibitor NVP-AUY922 was enhanced by concomitant Hsp72 or Hsp73 knockdown in MM cells. Moreover, we collected experimental evidence of a synergistic mode of action of the combined pharmacological inhibition of Hsp90 and Hsp70 with NVP-AUY922 and VER-155008. In contrast to Hsp90 inhibition as monotherapy, the combined blockade of Hsp70 and Hsp90 might, therefore, have the potential to increase the clinical efficacy of anti-Hsp-directed therapies in MM. Although many efforts have been made to develop specific Hsp70 inhibitors for a potential therapeutic application,33,34 none of these inhibitors – including VER-155008 – has so far been tested in clinical trials. Thus, further attempts to improve the pharmaceutical development of clinically suitable Hsp70 inhibitors are certainly warranted.35,36
Hsp90 inhibition consistently leads to up-regulation of Hsp72, both in vitro as well as in vivo. Because this induction effect may attenuate the efficacy of Hsp90 blockade, preventing Hsp72 up-regulation might represent a promising therapeutic strategy. Accordingly, it was recently shown that treatment of MM cells with small compounds, such as KNK437, which inhibits Hsp72 up-regulation, enhanced the pro-apoptotic efficacy of the Hsp90 inhibitor 17-AAG.15 However, the precise mechanism of action of such inhibitory compounds is largely unknown, and little is understood about the mechanisms governing Hsp72 regulation in MM. Using an siRNA against the p110α subunit of PI3K or the pharmacological PI3K inhibitor PI103, which exhibits activity against all p110 isoforms of PI3K,24,25 we observed that blockade of the PI3K/Akt/GSK3β signaling pathway led to decreased levels of constitutive expression of the proteins, HSF1, Hsp72 and Hsp27. Importantly, we found that Hsp72 induction after pharmacological Hsp90 inhibition was largely prevented by concomitant PI3K inhibition. Thus, our data provide experimental evidence that constitutive as well as inducible Hsp72 expression is regulated by PI3K-dependent HSF1 activity in MM cells. These findings are supported by those of previous studies showing an involvement of PI3K/Akt in the regulation of Hsp70,37,38 and indicating that GSK3β may act as a negative regulator of HSF1 activity.26,27 In accordance with this latter hypothesis, we observed that PI103-mediated down-regulation of HSF1 was largely prevented by concomitant treatment with the GSK3β inhibitor XXVI.
The PI3K/Akt pathway has drawn considerable attention as a promising therapeutic target in MM.21–23 It is, therefore, conceptually intriguing that PI3K inhibition might serve as a useful strategy to target the HSF1/Hsp72 axis. This is of immediate clinical relevance because – in contrast to the situation with Hsp70 – the development of therapeutically suitable PI3 kinase inhibitors is quite advanced. In support of this concept, we found that combined inhibition of PI3K and Hsp90 resulted in synergistic to additive cell death induction in MM cells. Because Akt is a bona fide Hsp90 client protein, an additional rationale for this particular inhibitor combination is that oncogenic Akt would be tackled in a two-pronged approach: down-regulation of total Akt levels by Hsp90 blockade and attenuation of Akt activation through inhibition of PI3K. The latter concept has recently been supported by the observation that the alkylphospholipid perifosine (which interferes with Akt activity) in combination with the HSP90 inhibitor 17-DMAG synergistically enhances MM cell cytotoxicity.39
Taken together, our data demonstrate that Hsp70 isoforms are critically involved in the regulation of Hsp90-chaperone function and contribute to tumor cell survival in MM. Improving the clinical efficacy of Hsp90 inhibition by concomitant targeting of Hsp70, potentially achieved via PI3K inhibition, could therefore represent a new therapeutic strategy for MM patients.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work was supported in parts by grants from the Deutsche Krebshilfe (107715), and from the Deutsche Forschungsgemeinschaft (SFB TR17 and KFO 216).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Chng WJ, Glebov O, Bergsagel PL, Kuehl WM. Genetic events in the pathogenesis of multiple myeloma. Best Pract Res Clin Haematol. 2007;20(4):571–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–60 [DOI] [PubMed] [Google Scholar]
- 4.Laubach JP, Mitsiades CS, Mahindra A, Schlossman RL, Hideshima T, Chauhan D, et al. Novel therapies in the treatment of multiple myeloma. J Natl Compr Canc Netw. 2009;7(9):947–60 [DOI] [PubMed] [Google Scholar]
- 5.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7(8):585–98 [DOI] [PubMed] [Google Scholar]
- 6.Workman P. Combinatorial attack on multistep oncogenesis by inhibiting the Hsp90 molecular chaperone. Cancer Lett. 2004;206 (2):149–57 [DOI] [PubMed] [Google Scholar]
- 7.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–72 [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee M, Jain S, Stühmer T, Andrulis M, Ungethüm U, Kuban RJ, et al. STAT3 and MAPK signaling maintain overexpression of heat shock proteins 90alpha and beta in multiple myeloma cells, which critically contribute to tumor-cell survival. Blood. 2007;109(2):720–8 [DOI] [PubMed] [Google Scholar]
- 9.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Kung AL, Davies FE, et al. Antimyeloma activity of heat shock protein-90 inhibition. Blood. 2006;107(3):1092–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stühmer T, Zöllinger A, Siegmund D, Chatterjee M, Grella E, Knop S, et al. Signalling profile and antitumour activity of the novel Hsp90 inhibitor NVP-AUY922 in multiple myeloma. Leukemia. 2008;22(8): 1604–12 [DOI] [PubMed] [Google Scholar]
- 11.Stühmer T, Chatterjee M, Grella E, Seggewiss R, Langer C, Müller S, et al. Antimyeloma activity of the novel 2-aminoth-ienopyrimidine Hsp90 inhibitor NVP-BEP800. Br J Haematol. 2009;147(3):319–27 [DOI] [PubMed] [Google Scholar]
- 12.Richardson PG, Chanan-Khan AA, Alsina M, Albitar M, Berman D, Messina M, et al. Tanespimycin monotherapy in relapsed multiple myeloma: results of a phase 1 dose-escalation study. Br J Haematol. 2010;150(4): 438–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581(19):3702–10 [DOI] [PubMed] [Google Scholar]
- 14.Sherman M, Multhoff G. Heat shock proteins in cancer. Ann NY Acad Sci. 2007;1113: 192–201 [DOI] [PubMed] [Google Scholar]
- 15.Davenport EL, Zeisig A, Aronson LI, Moore HE, Hockley S, Gonzalez D, et al. Targeting heat shock protein 72 enhances Hsp90 inhibitor-induced apoptosis in myeloma. Leukemia. 2010;24(10):1804–7 [DOI] [PubMed] [Google Scholar]
- 16.Powers MV, Clarke PA, Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2008;14(3):250–62 [DOI] [PubMed] [Google Scholar]
- 17.Stühmer T, Arts J, Chatterjee M, Borawski J, Wolff A, King P, et al. Preclinical antimyeloma activity of the novel HDAC-inhibitor JNJ-26481585. Br J Haematol. 2010;149 (4):529–36 [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee M, Hönemann D, Lentzsch S, Bommert K, Sers C, Herrmann P, et al. In the presence of bone marrow stromal cells human multiple myeloma cells become independent of the IL-6/gp130/STAT3 pathway. Blood. 2002;100(9):3311–8 [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee M, Stühmer T, Herrmann P, Bommert K, Dörken B, Bargou RC. Combined disruption of both the MEK/ERK and the IL-6R/STAT3 pathways is required to induce apoptosis of multiple myeloma cells in the presence of bone marrow stromal cells. Blood. 2004;104(12):3712–21 [DOI] [PubMed] [Google Scholar]
- 20.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81 [DOI] [PubMed] [Google Scholar]
- 21.Tu Y, Gardner A, Lichtenstein A. The phosphatidylinositol 3-kinase/AKT kinase pathway in multiple myeloma plasma cells: roles in cytokine-dependent survival and proliferative responses. Cancer Res. 2000;60(23): 6763–70 [PubMed] [Google Scholar]
- 22.Zöllinger A, Stühmer T, Chatterjee M, Gattenlöhner S, Haralambieva E, Müller-Hermelink HK, et al. Combined functional and molecular analysis of tumor cell signaling defines 2 distinct myeloma subgroups: Akt-dependent and Akt-independent multiple myeloma. Blood. 2008;112(8):3403–11 [DOI] [PubMed] [Google Scholar]
- 23.Ikeda H, Hideshima T, Fulciniti M, Perrone G, Miura N, Yasui H, et al. PI3K/p110{delta} is a novel therapeutic target in multiple myeloma. Blood. 2010;116(9):1460–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408(3):297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raynaud FI, Eccles S, Clarke PA, Hayes A, Nutley B, Alix S, et al. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007;67(12):5840–50 [DOI] [PubMed] [Google Scholar]
- 26.Xavier IJ, Mercier PA, McLoughlin CM, Ali A, Woodgett JR, Ovsenek N. Glycogen synthase kinase 3beta negatively regulates both DNA-binding and transcriptional activities of heat shock factor 1. J Biol Chem. 2000;275(37):29147–52 [DOI] [PubMed] [Google Scholar]
- 27.Khaleque MA, Bharti A, Sawyer D, Gong J, Benjamin IJ, Stevenson MA, et al. Induction of heat shock proteins by heregulin beta1 leads to protection from apoptosis and anchorage-independent growth. Oncogene. 2005;24(43):6564–73 [DOI] [PubMed] [Google Scholar]
- 28.Calderwood SK, Xie Y, Wang X, Khaleque MA, Chou SD, Murshid A, et al. Signal transduction pathways leading to heat shock transcription. Sign Transduct Insights. 2010;2:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massey AJ, Williamson DS, Browne H, Murray JB, Dokurno P, Shaw T, et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother Pharmacol. 2010;66(3):535–45 [DOI] [PubMed] [Google Scholar]
- 30.Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3(9):839–43 [DOI] [PubMed] [Google Scholar]
- 31.Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5(22):2592–601 [DOI] [PubMed] [Google Scholar]
- 32.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–16 [DOI] [PubMed] [Google Scholar]
- 33.Huryn DM, Brodsky JL, Brummond KM, Chambers PG, Eyer B, Ireland AW, et al. Chemical methodology as a source of small-molecule checkpoint inhibitors and heat shock protein 70 (Hsp70) modulators. Proc Natl Acad Sci USA. 2011;108(17):6757–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braunstein MJ, Scott SS, Scott CM, Behrman S, Walter P, Wipf P, et al. Antimyeloma effects of the heat shock protein 70 molecular chaperone inhibitor MAL3–101. J Oncol. 2011;2011:232037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powers MV, Clarke PA, Workman P. Death by chaperone: HSP90, HSP70 or both? Cell Cycle. 2009;8(4):518–26 [DOI] [PubMed] [Google Scholar]
- 36.Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem. 2010;53(12):4585–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calderwood SK, Stevenson MA, Hahn GM. Heat stress stimulates inositol trisphosphate release and phosphorylation of phosphoinositides in CHO and Balb C 3T3 cells. J Cell Physiol. 1987;130(3):369–76 [DOI] [PubMed] [Google Scholar]
- 38.Rafiee P, Theriot ME, Nelson VM, Heidemann J, Kanaa Y, Horowitz SA, et al. Human esophageal microvascular endothelial cells respond to acidic pH stress by PI3K/AKT and p38 MAPK-regulated induction of Hsp70 and Hsp27. Am J Physiol Cell Physiol. 2006;291(5):C931–45 [DOI] [PubMed] [Google Scholar]
- 39.Huston A, Leleu X, Jia X, Moreau AS, Ngo HT, Runnels J, et al. Targeting Akt and heat shock protein 90 produces synergistic multiple myeloma cell cytotoxicity in the bone marrow microenvironment. Clin Cancer Res. 2008;14(3):865–74 [DOI] [PubMed] [Google Scholar]