Abstract
The emergence of an oligoclonal humoral response, resulting in the appearance of a different serum M-protein to that observed at diagnosis is a well-recognized event after autologous stem cell transplantation in multiple myeloma in complete response, and it has been considered to be a benign phenomenon. The aim of the present study was to investigate the incidence, biological characteristics and prognostic value of the oligoclonal bands in patients with myeloma who underwent autologous transplantation at our institution in the last 18 years. We proceed with a retrospective systematic review of all serum and urine immunofixation studies performed in the 211 patients with multiple myeloma who underwent melphalan-based autologous transplantation. Oligoclonal bands were observed in 34% of the patients, with a significantly higher prevalence with the use of novel agents versus conventional chemotherapy in induction (63% vs. 22%; P=0.0001). The incidence of oligoclonal bands was most frequent in non-IgG isotype, particularly in light chain only myeloma. The oligoclonal phenomenon was almost exclusive to patients in complete remission compared to other degrees of response (87% vs. 13%; P=0.0001), and lasted for a median of 1.35 years, persisting during follow up in all patients except in those who relapsed. In prognostic terms, the presence of oligoclonality resulted in a significantly longer progression-free and overall survival. Patients with oligoclonal humoral response lasting for more than one year after transplantation had a significantly longer clinical progression-free and overall survival than those with shorter duration (P=0.008 and P=0.0001, respectively), likely reflecting the importance of a robust humoral immune response.
Introduction
Multiple myeloma (MM) is characterized by the production of a monoclonal immunoglobulin of constant isotype and light chain restriction due to the clonal proliferation of neoplastic plasma cells.1 The definition of complete remission (CR) in MM requires the absence of the original monoclonal protein in both serum and urine immunofixation (IFE).2–4 The relapse from CR always occurs with the same monoclonal component observed at diagnosis.5 However, the emergence of an oligoclonal humoral response, resulting in the appearance of a serum M-protein that is different to that observed at diagnosis is a well-recognized event after autologous stem cell transplantation in multiple myeloma (MM), and this has been considered to be a benign phenomenon.5–7 Although initially described as transient,6 there is growing evidence that this oligoclonal humoral response can last for some years.5,7,8 In addition, the appearance of oligoclonal bands has also been reported with higher frequency after treatment with novel drugs,9 such as lenalidomide, thalidomide and bortezomib, than with conventional cytotoxic therapy.10
The optimal treatment in patients with MM under 65 years of age includes induction therapy followed by high-dose therapy with autologous peripheral blood stem cell rescue (ASCT).11 Patients who developed a humoral response resulting in oligoclonal bands seem to have a better prognosis in terms of progression-free survival and overall survival.6,8,9,12 However, reports on this are controversial.7,13,14 The aim of the present study was to investigate the incidence, biological characteristics and prognostic value of the oligoclonal bands in patients with MM who underwent ASCT at our institution in the last 18 years.
Design and Methods
Patients
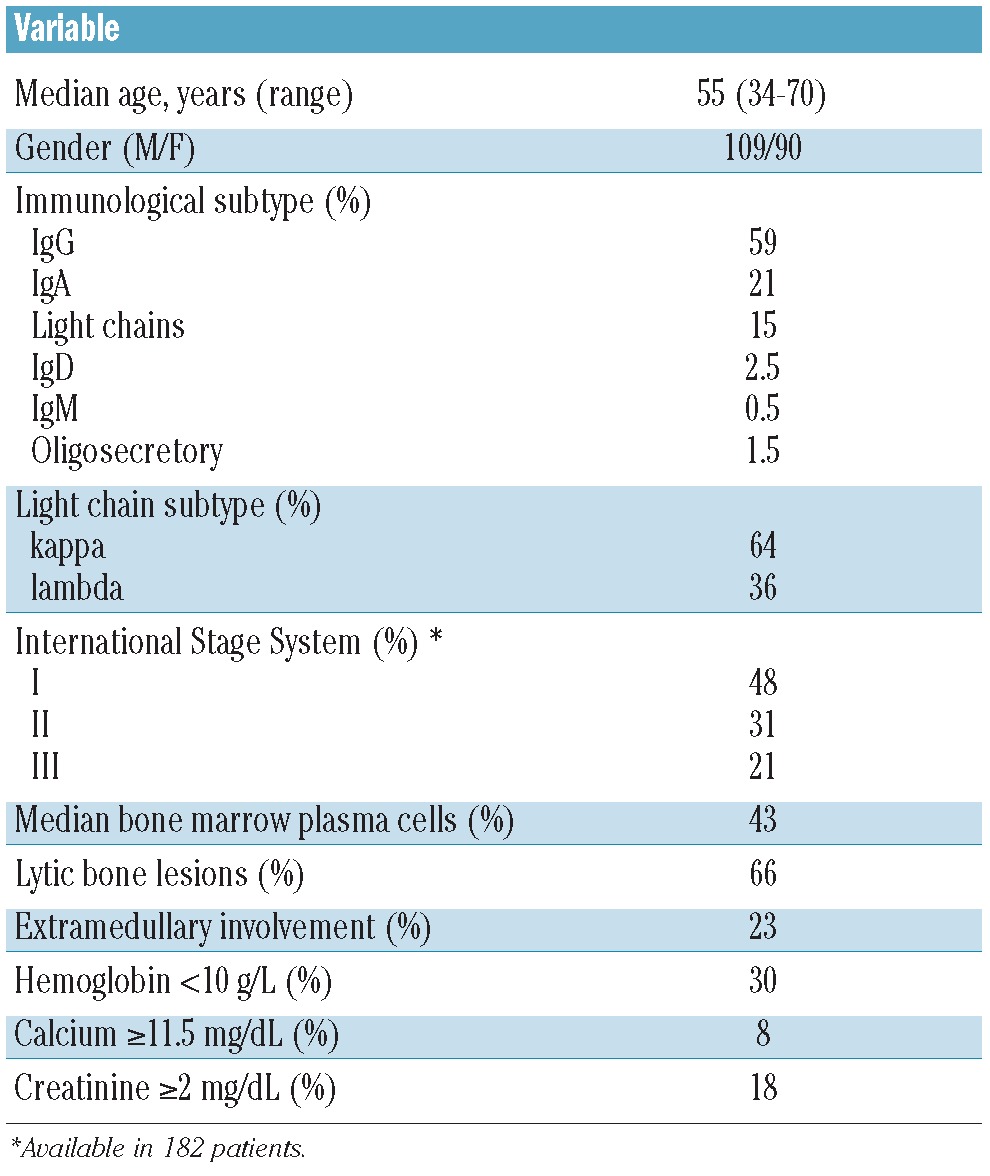
Two hundred and eleven patients underwent melphalan-based ASCT at our institution from March 31st 1994 to December 27th 2011. Of these, 199 patients (109M/90F; median age 55 years, range 34–70) who achieved at least a partial response (PR) after ASCT make up the study population, thus excluding patients with minimal response, stable or progressive disease. Initial baseline demographics, clinical and laboratory data, and information concerning treatment and follow up were collected. Main patients’ characteristics are shown in Table 1. No patient was lost to follow up. The median follow up for alive patients was 4.7 years (range 6 months–18 years). The Ethics Committee of the Hospital Clínic of Barcelona provided institutional review board approval for this study.
Table 1.
Patients’ characteristics.
Oligoclonal bands
A retrospective systematic review of all serum and urine IFE studies was carried out. An oligoclonal humoral response was defined as the presence of a serum and/or urine IFE monoclonal spike that was different from the original myeloma protein either in heavy and/or light chains, as well as a different IFE migration pattern. Response, relapse, and progression were defined according to European Blood and Marrow Transplantation (EBMT) criteria.3 Therefore, CR patients required a negative serum and urine IFE for the original monoclonal myeloma protein and less than 5% bone marrow plasma cells. IFE was performed at three and six months after ASCT, and then usually every six months until confirmed progression or relapse.
Statistical analysis
Differences among the subgroups of patients were compared by using a two-tailed χ2 test, Student’s t-test or non-parametric tests when necessary. The actuarial survival analysis was performed by the Kaplan and Meier method and differences assessed by the log rank test. Progression-free survival (PFS) was defined as survival from ASCT until relapse or death from any cause. Overall survival (OS) was calculated from the time of ASCT. IFE was performed every six months in patients in CR after ASCT. Cox proportional hazards model was used to estimate the risk ratio of events (ORR) with the respective confidence interval (CI) after controlling for prognostic variables in multivariate analysis. Statistical tests were performed with PASW software 18.0 for Windows® (Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
A total of 143 patients (71.9%) received induction with conventional chemotherapy while 54 (27.1%) were initially treated with regimens incorporating novel agents; 2 patients underwent ASCT without previous cytoreductive treatment. The main conventional cytotoxic regimens in the first group were: VBCMP/VBAD(P) (73.4%), VAD (10.5%), cyclophosphamide and dexamethasone (7%) or other combinations (9.1%). In the second group, the induction regimen was based on combinations of glucocorticoids with bortezomib (59%, including 8 patients with VBCMP/VBAD), thalidomide (30%), or with bortezomib plus thalidomide (11%).
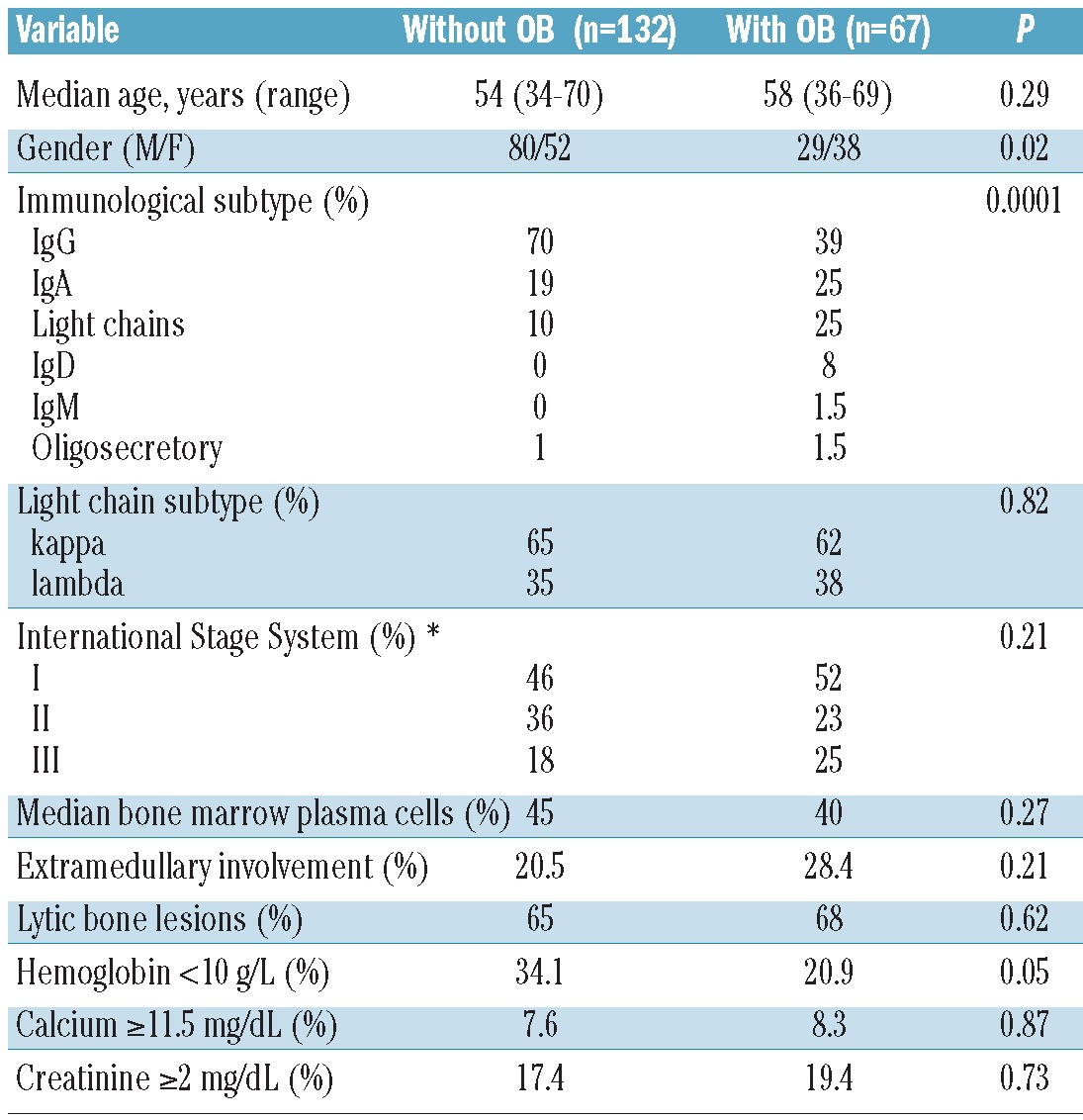
Eighty-seven of the 199 patients (43.7%) achieved CR after ASCT. The median progression-free survival (PFS) and overall survival (OS) after ASCT were 3.2 and 6.6 years, respectively. Oligoclonal bands after ASCT were observed in 34% (67 of 199) of the patients. The characteristics of the patients with or without this humoral response are summarized in Table 2. The incidence of oligoclonal bands was most frequent in non-IgG isotype, particularly in light chains only or Bence Jones MM. As far as induction is concerned, a significantly higher prevalence with the use of novel agents versus conventional chemotherapy in this phase (63% vs. 22%; P=0.0001) was observed. The incidence in the group receiving novel agents in induction was quite similar, irrespective of the use of bortezomib, thalidomide or the combinations of the two agents (62.5% vs. 62.5% vs. 66.7%; P=0.98).
Table 2.
Characteristics of the patients according to the presence or absence of oligoclonal bands (OB).
The oligoclonal phenomenon was almost exclusive to patients in CR compared to other degrees of response. Thus, 58 patients were in CR while the remaining 9 only achieved VGPR or PR (87% vs. 13%; P=0.0001). Four patients with IgA and 5 with Bence Jones MM were the only ones in less than CR (6 VGPR, 3 PR) showing a transient co-existence of the original M-protein with IgG serum oligoclonal bands. The humoral response of these 9 patients lasted for a median of only seven months (range 2.6–14.6 months), with a median PFS and OS in this small subset of only one and three years, respectively.
The median number of different isotypes accounting for oligoclonal humoral response was 2 (range 1–6). Humoral oligoclonal response lasted for a median of 1.35 years (range 3 months – >11 years). Oligoclonal bands were more frequently observed in serum than in urine (75.4% vs. 31.6%) and the most usually involved serum heavy-chain was IgG (73%), with almost the same kappa/lambda distribution. Kappa light-chain was the predominant isotype in the urine (60%).
In the overall series, the disappearance of the oligoclonal bands preceded serological relapse in all cases, except in two settings. First, 6 patients who progressed with extramedullary disease with soft-tissue plasmacytomas without significant bone marrow or serum M-protein increase had a transient persistence of the oligoclonal bands (median 1.5 months, range 1–4) that finally disappeared. Second, 6 patients with light chain only MM, had an increase in the original light chain in the urine at the time of relapse, transiently co-existing with serum oligoclonal bands (median 2 months, range 1–3).
The presence of oligoclonal bands after ASCT resulted in a significantly longer PFS (P=0.004) (Figure 1). This translated into a significantly longer OS in patients with this humoral oligoclonal response (median: not reached vs. 5.58 years; P=0.003) (Figure 2). Patients with oligoclonal humoral response lasting for more than one year after ASCT had a significantly longer clinical PFS and OS than those with shorter duration (P=0.008 and P=0.0001, respectively). Interestingly, the estimated OS of patients with oligoclonal bands lasting for more than one year was 70% at ten years. In contrast, the PFS and OS of patients with oligoclonal bands lasting for less than one year were similar to those who never developed this phenomena. In patients in CR after ASCT, the presence or absence of oligoclonal humoral response was not associated with a significant prolongation of PFS or OS, even when the duration of CR and oligoclonal responses was studied as time-dependant covariates.
Figure 1.
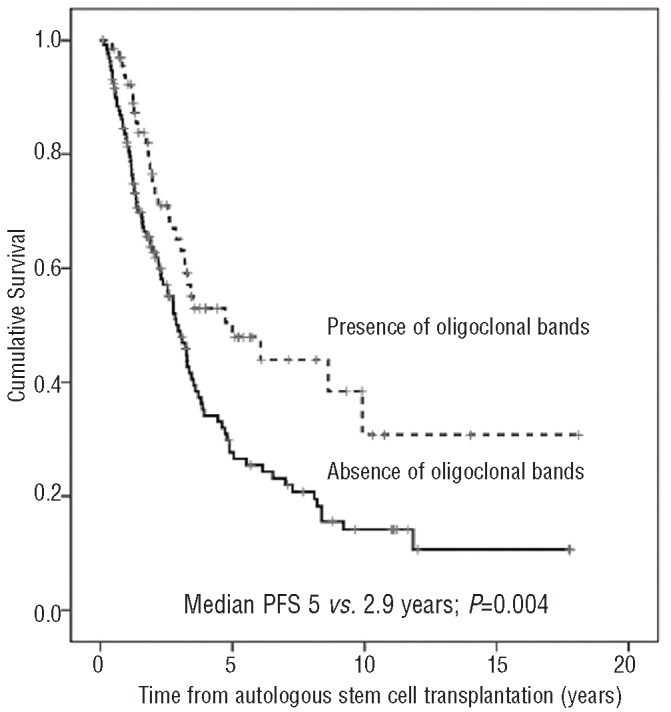
Progression-free survival after autologous stem cell transplantation according to the presence of oligoclonal bands.
Figure 2.
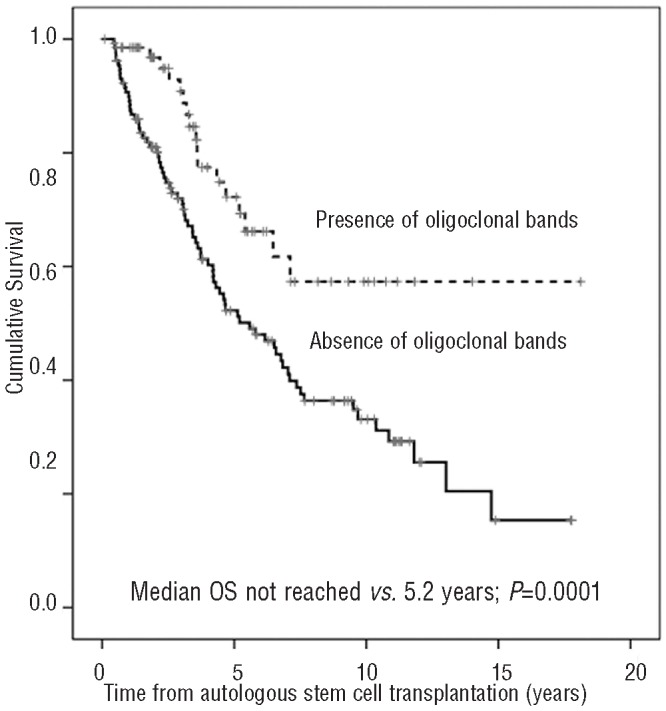
Overall survival after autologous stem-cell transplantation according to the presence of oligoclonal bands.
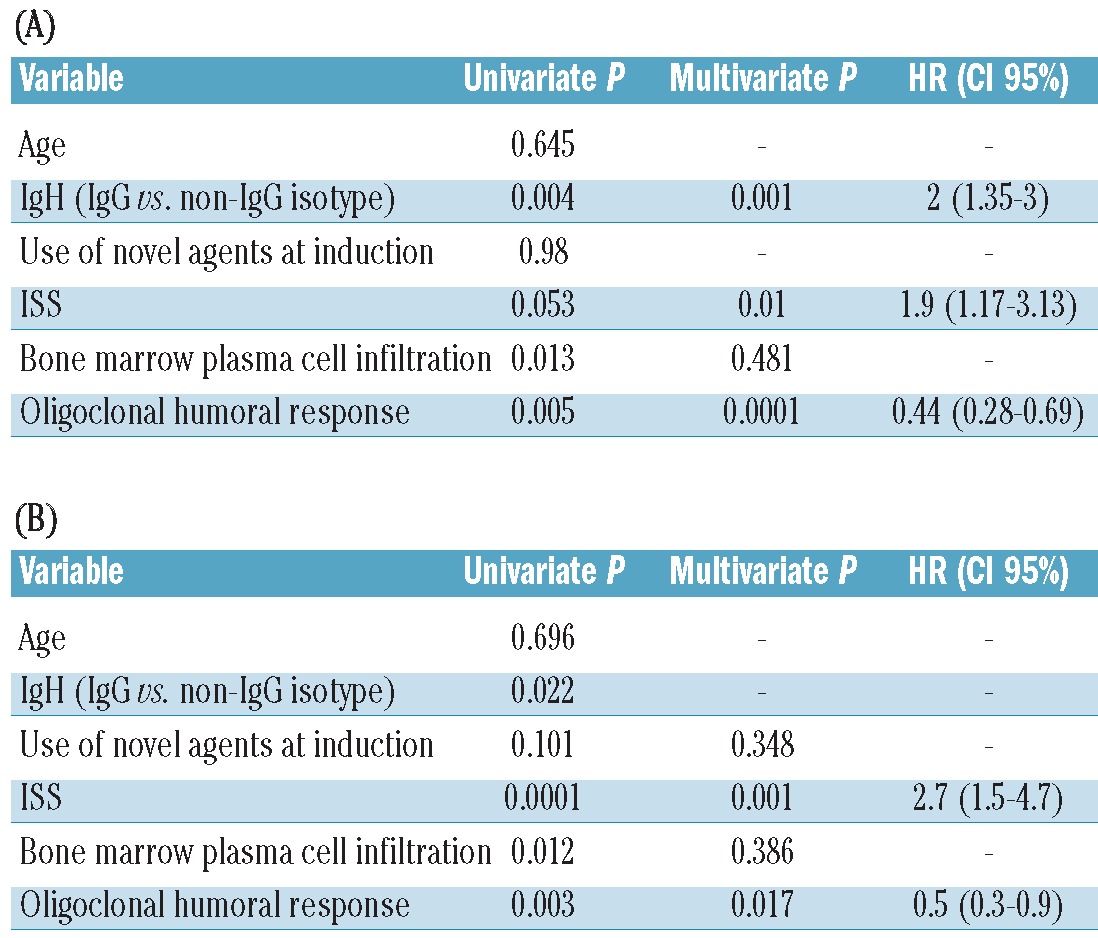
Results of the univariate and multivariate analysis are summarized in Table 3. For PFS, a model including oligoclonal response, ISS and heavy chain isotype (IgG vs. non-IgG patients) was able to predict longer PFS. As far as OS is concerned, only the ISS and oligoclonal response remained at a significant level.
Table 3.
Univariate and multivariate analysis of factors associated to (A) progression-free survival and (B) overall survival.
Discussion
The existence of an oligoclonal humoral response, detectable in serum and more rarely in urine, is a well-recognized phenomenon.15 It can be found during the development of the B-cell response during childhood and in different clinical settings.16 Even a localized production in the cerebrospinal fluid is a common finding in multiple sclerosis.17 There is evidence of this type of response in the serum of patients with systemic infections, autoimmune disorders, immunosupression in the context of organ transplantation, and also after allogeneic and autologous stem cell transplantation.18–19 It seems that, in the context of ASCT for MM, the emergence of these oligoclonal immunoglobulins can be a consequence of a strong immune reconstitution.
Since this phenomena was first recognized,6 a number of studies on the issue have been reported. The EBMT group emphasized the fact that the presence of monoclonal immunoglobulins in the absence of the original myeloma protein was consistent with CR3 and that the characterization of serum and urine immunoglobulins with the recognition of oligoclonal bands is crucial in the response evaluation in MM. Although this has been known for more than two decades, few studies on this issue were performed; most studies have only been carried out in recent years. What creates some confusion is that the same phenomenon has been described under different names: oligoclonal or abnormal protein bands (APB),5,6 apparent isotype class switching,6 atypical serum immunofixation patterns (ASIPs),9 or even more recently as secondary monoclonal gammopathies of undetermined significance (MGUS).12,13
There is also a wide range of incidence of the oligoclonal humoral response among different series, ranging from 7% to 73%.5–9,12,13 In the present study it was 34%. One reason for this discrepancy is the denominator of this percentage. It is a phenomenon more frequently observed in patients after ASCT than conventional chemotherapy10,12 and this could explain the low percentage reported in the Mayo Clinic series (7%) where almost two-thirds of the patients had not received ASCT,12 or the higher rate of 42% in our previous report in which only patients after ASCT or allogeneic SCT in CR were included.7 Another factor to take into consideration is the use of novel drugs, i.e. thalidomide, lenalidomide and bortezomib. Therefore, we had previously reported a significant difference when these agents were used versus conventional chemotherapy (60% vs. 11%) in patients in CR after induction not candidates for ASCT.10 This fact is confirmed in the present series including only patients eligible for ASCT; patients who received any of these drugs during induction show a significantly higher rate (63% vs. 22%) of oligoclonal humoral response. With the continuous improvement in the CR rate and the worldwide use of new drugs in the treatment of patients with MM,20 the prevalence of oligoclonal bands will likely increase. An alternating pattern of different oligoclonal bands was very frequent in our patients. Otherwise, it has been noted that oligoclonal bands can occur in patients not in CR. In contrast to the Mayo report12 in which 82% of the patients with oligoclonal bands were not in CR, we only identified 9 patients (13%) with co-existing oligoclonal and the original M-spike. Interestingly, all our patients had IgA or Bence Jones MM, and had a short PFS and OS.
Another intriguing issue about oligoclonal bands concerns their origin. A first hypothesis was that these immunoglobulins could result from an altered immunoglobulin production by the malignant plasma cell clone. However, this hypothesis was not supported by immunophenotyic and immunohistochemistry studies,6 and their non-malignant nature was confirmed by molecular studies with DNA sequencing immunoglobulin variable genes, showing that no myeloma clonal-related cells were found in post-transplantation samples in spite of the appearance of new serum M-components.21 An increase in polyclonal B cells in the bone marrow has been reported in these patients.22 On the other hand, in patients in CR after induction therapy, the disappearance of the oligoclonal bands preceded the serological and clinical relapse.5 This finding was confirmed in the present series in which, in almost all patients, the oligoclonal bands vanished immediately before or at the time of the reappearance of the original myeloma protein, suggesting a clonal competition among malignant plasma cells and polyclonal B lymphocytes. Only two exceptions were noted: patients who relapsed with extramedullary plasmacytomas or with only Bence Jones myeloma. In extramedullary progression, the persistence of an oligoclonal band can reflect the control of medullary disease through immune mechanisms, but with an extramedullary plasma cell escape in soft tissues.23 As far as light chain (Bence Jones) myeloma is concerned, the different kinetics of light chains and intact immunoglobulin are likely responsible for the early urine relapse recognized by the persistence of the oligoclonal band in serum. This is likely due to a significantly longer half-life of the serum intact oligoclonal immunoglobulin.24
With regards to the prognostic significance, our results showed that PFS of patients with oligoclonal bands after ASCT was a median two years longer than those without this humoral response. Furthermore, in our patients, this difference was translated into a significantly longer OS. Contradictory results on prognosis have been reported in previous studies.6,7,9,12,14 Differences in sample size, induction treatment, population heterogeneity, and, in particular, duration of follow up could be responsible for the discordant results. In any event, the presence of oligoclonal bands should be considered to be a characteristic of patients in CR rather than an independent prognostic marker.
The duration of oligoclonal bands is also a controversial issue. Thus, from the original Arkansas series and the Mayo Clinic experience, it seems to be limited to several months.12 In our experience, they lasted for a median of 1–2 years, persisting during follow up in all patients except in those who relapsed. The fact that all our patients underwent ASCT could explain the longer duration of oligoclonal response due to a deeper malignant clone suppression and/or stronger immunological reconstitution. We also found that oligoclonal response lasting more than one year was associated with a significantly longer OS. Some aspects still to be explored but that were beyond the scope of our historical series include the possible associations of oligoclonal bands with some myeloma features. For example, the possible relationship between the cytogenetic status and oligoclonal response has not been explored in our study; however, in the Mayo experience, no association was found.12 Antigen specificity of oligoclonal bands with potential anti-tumor activity25 or associated oligoclonal cellular immune response26 are other new potential targets for research.
In summary, the emergence of oligoclonal bands after ASCT is usually observed in patients in CR and has prognostic impact. This phenomenon likely reflects a robust humoral immune response and consequently an immune system reconstitution. The duration of this humoral response is also associated with significantly longer survival.
Acknowledgments
We would like to thank Esther Bladé for her technical support in this research.
Footnotes
Funding
This work has been supported in part by grant RD12/0036/0046 from Instituto de Salud Carlos III; and a “Josep Font” Grant from Hospital Clínic de Barcelona.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18): 4701–5 [DOI] [PubMed] [Google Scholar]
- 3.Bladé J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT: European Group for Blood and Marrow Transplant. Br J Haematol.1998;102: (5)1115–23 [DOI] [PubMed] [Google Scholar]
- 4.Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9): 1467–73 [DOI] [PubMed] [Google Scholar]
- 5.Fernández de Larrea C, Cibeira MT, Elena M, Arostegui JI, Rosiñol L, Rovira M, et al. Abnormal serum free light chain ratio in patients with multiple myeloma in complete remission has strong association with the presence of oligoclonal bands: implications for stringent complete remission definition. Blood. 2009;114(24):4954–6 [DOI] [PubMed] [Google Scholar]
- 6.Zent CS, Wilson CS, Tricot G, Jagannath S, Siegel D, Desikan KR, et al. Oligoclonal protein bands and Ig isotype switching in multiple myeloma treated with high-dose therapy and hematopoietic cell transplantation. Blood. 1998;91(9):3518–23 [PubMed] [Google Scholar]
- 7.Hovenga S, de Wolf JT, Guikema JE, Klip H, Smit JW, Smith Sibinga CT, et al. Autologous stem cell transplantation in multiple myeloma after VAD and EDAP courses: a high incidence of oligoclonal serum Igs post transplantation. Bone Marrow Transplant. 2000;25(7):723–8 [DOI] [PubMed] [Google Scholar]
- 8.Hall SL, Tate J, Gill D, Mollee P. Significance of abnormal protein bands in patients with multiple myeloma following autologous stem cell transplantation. Clin Biochem Rev. 2009;30(3):113–8 [PMC free article] [PubMed] [Google Scholar]
- 9.Mark T, Jayabalan D, Coleman M, Pearse RN, Wang YL, Lent R, et al. Atypical serum immunofixation patterns frequently emerge in immunomodulatory therapy and are associated with a high degree of response in multiple myeloma. Br J Haematol. 2008;143 (5):654–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández de Larrea C, Tovar N, Cibeira MT, Arostegui JI, Rosiñol L, Elena M, et al. Emergence of oligoclonal bands in patients with multiple myeloma in complete remission after induction chemotherapy: association with the use of novel agents. Haematologica. 2011;96(1):171–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bladé J, Rosiñol L, Cibeira MT, Rovira M, Carreras E. Hematopoietic stem cell transplantation for multiple myeloma beyond 2010. Blood. 2010;115(18):3655–63 [DOI] [PubMed] [Google Scholar]
- 12.Wadhera RK, Kyle RA, Larson DR, Dispenzieri A, Kumar S, Lazarus HM, Rajkumar SV. Incidence, clinical course, and prognosis of secondary monoclonal gammopathy of undetermined significance in patients with multiple myeloma. Blood. 2011;118(11):2985–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manson GV, Campagnaro E, Balog A, Kaplan D, Sommers SR, Fu P, et al. Secondary MGUS after autologous hematopoietic progenitor cell transplantation in plasma cell myeloma: a matter of undetermined significance. Bone Marrow Transplant. 2012;47(9):1212–6 [DOI] [PubMed] [Google Scholar]
- 14.Byrne E, Giles C, Andrews J, Rahemtulla A, Naresh KN. Lack of correlation between emergence of an abnormal protein band or of oligoclonal bands and survival in patients with multiple myeloma achieving complete remission following autologous stem cell transplantation. Haematologica. 2011;96 (4):e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitus AJ, Stein R, Rappeport JM, Antin JH, Weinstein J, Alper CA, Smith BR. Monoclonal and oligoclonal gammopathy after bone marrow transplantation. Blood. 1989;74:2764–8 [PubMed] [Google Scholar]
- 16.Small TN, Keever CA, Weiner-Fedus S, Heller G, O’Reilly RJ, Flomenberg N. B-cell differentiation following autologous, conventional, or T-cell depleted bone marrow transplantation: A recapitulation of normal B-cell ontogeny. Blood. 1990;76(8):1647–56 [PubMed] [Google Scholar]
- 17.Krumbholz M, Derfuss T, Hohlfeld R, Meinl E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol. 2012;8(11):613–23 [DOI] [PubMed] [Google Scholar]
- 18.Brito-Zerón P, Retamozo S, Gandía M, Akasbi M, Pérez-de-Lis M, Diaz-Lagares C, et al. Monoclonal gammopathy related to Sjögren syndrome: a key marker of disease prognosis and outcomes. J Autoimmun. 2012;39(1–2):43–8 [DOI] [PubMed] [Google Scholar]
- 19.Gerritsen EJ, Van Tol MJ, Lankester AC, van der Weijden-Ragas CP, Jol-van der Zijde CM, Oudeman-Gruber NJ, et al. Immunoglobulin levels and monoclonal gammopathies in children after bone marrow transplantation. Blood. 1993;82(11): 3493–502 [PubMed] [Google Scholar]
- 20.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guikema JE, Vellenga E, Veeneman JM, Hovenga S, Bakkus MH, Klip H, Bos NA. Multiple myeloma related cells in patients undergoing autologous peripheral blood stem cell transplantation. Br J Haematol. 104:(4)748–54 [DOI] [PubMed] [Google Scholar]
- 22.Byrne E, Naresh KN, Giles C, Rahemtulia A. Excess bone marrow B-cells in patients with multiple myeloma achieving complete remission after autologous stem cell transplant is a biomarker for improved survival. Br J Haematol. 2011;155(4):509–11 [DOI] [PubMed] [Google Scholar]
- 23.Bladé J, Fernández de Larrea C, Rosiñol L, Cibeira MT, Jiménez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29(28):3805–12 [DOI] [PubMed] [Google Scholar]
- 24.Pratt G, Mead GP, Godfrey KR, Hu Y, Evans MD, Chappell MJ, et al. The tumor kinetics of multiple myeloma following autologous stem cell transplantation as assessed by measuring serum-free light chains. Leuk Lymphoma. 2006;47(1):21–8 [DOI] [PubMed] [Google Scholar]
- 25.Rahlff J, Trusch M, Haag F, Bacher U, Horst A, Schlüter H, Binder M. Antigen specificity of oligoclonal abnormal protein bands in multiple myeloma after allogenic stem cell transplantation. Cancer Immunol Immunother. 2012,61(10):1639–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blotta S, Tassone P, Prabhala RH, Tagliaferri P, Cervi D, Amin S, et al. Identification of novel antigens with induced immune response in monoclonal gammopathy of undetermined significance. Blood. 2009; 114(15):3276–84 [DOI] [PMC free article] [PubMed] [Google Scholar]


