Abstract
Relapsed/refractory multiple myeloma represents a major challenge in multiple myeloma therapy. For patients with relapsed/refractory multiple myeloma, we developed a treatment schema of metronomically scheduled drug therapy. We identified 186 patients who had been treated with metronomic therapy between March 2004 and January 2012 with a median follow up of 24.2 months. Median age was 61 years (range 36–83). Median number of prior therapies was 14 (range 1–51). Median number of completed metronomic therapy cycles was 1 (range 1–5), while 45 of 186 (25%) received 2 or more cycles. Responses included complete remission in 11 of 186 patients (6%), very good partial remission in 12 of 186 (7%), partial remission in 65 of 179 (36%), and minimal response in 29 of 186 (16%), for an overall response rate of 63% (117 of 186). Median overall survival and progression-free survival were 11.2 and 3.6 months, respectively. Hematologic toxicity grading was problematic as 146 of 186 (78%) of patients presented with at least grade 2 thrombocytopenia within 90 days prior to starting metronomic therapy. Grade 4 leukopenia, anemia, and/or thrombocytopenia following metronomic therapy occurred in 108 of 186 (58%), 12 of 186 (6%), and 147 of 186 (79%) patients, respectively. Incidence of grade 3–4 neutropenic fever was 4 of 186 (2%). Most patients (177 of 186, 95%) were treated in an outpatient unit and secondary admissions due to regimen-related toxicity occurred in 37 of 186 (20%). Treatment-related mortality was evident in 2 of 186 (1%). In conclusion, metronomic therapy is an effective late salvage treatment in relapsed/refractory multiple myeloma, with a high overall response rate and a favorable toxicity profile.
Introduction
Since the introduction of novel agents, especially in the context of autologous transplantation, overall survival (OS) and progression-free survival (PFS) in multiple myeloma (MM) have been extended to more than seven and four years, respectively.1 It is now recognized that the dominant adverse features for outcome relate to MM genetics, captured by metaphase cytogenetic abnormalities,2 fluorescence in situ hybridization (FISH)-derived amp1q213 and del17p4 and, more recently, gene expression profiling (GEP)-derived high risk.5 While present in a minority of newly diagnosed MM patients, these severe prognostic markers increase with disease progression. Additional challenges arise towards the end stage of MM when many patients further present with cytopenia due either to extensive bone marrow involvement, exhausted hematopoiesis or myelodysplastic syndrome (MDS).
Applied with anti-angiogenic therapeutic intent, thalidomide proved to be a major breakthrough in the treatment of relapsed/refractory MM6 and MM in general.7,8 Based on the concept that low-dose continuous application of certain cytotoxic drugs (e.g. doxorubicin, cis-platinum, cyclophosphamide) can inhibit tumor progression by interrupting neo-angiogenesis without increasing toxicity in experimental models and clinical practice,9–12 we developed a treatment schema of metronomically scheduled drug therapy, especially in the setting of pancytopenia, for relapsed/refractory MM.13 We are now up-dating our experience in 186 patients treated with metronomic therapy between March 2004 and January 2012, with a median follow-up period of 26 months.
Design and Methods
Patients
One hundred and eighty-six patients with relapsed/refractory MM between 31 and 82 years of age received at least one cycle of metronomic therapy at the Myeloma Institute of Research and Therapy of the University of Arkansas for Medical Sciences (Little Rock, AR, USA) between March 2004 and January 2012. All data were collected prior to November 29, 2012. Metronomic therapy was applied off-protocol to provide maximum flexibility in dosing. Patients signed a written informed consent in keeping with institutional and federal guidelines. The institutional review board approved the review of the patients’ data and outcomes toward the generation of this report.
Metronomic therapy
While variations in dosing occurred, the main elements of metronomic therapy consisted of bortezomib: 1.0 or 1.3 mg/m2 on Days 1, 4, 7, 10, 13, 16; thalidomide: 200 mg daily for 16 days; dexamethasone: 12, 20 or 40 mg on Days 1, 4, 7, 10, 13, 16; doxorubicin: 1–3 mg/m2 as continuous 24-h intravenous infusion for 16 days; cisplatin: 1.0–3 mg/m2 (adjusted according to renal function) as continuous 24-h intravenous infusion for 16 days, with or without the addition of the m-TOR inhibitor rapamycin: 3 mg on Day 1, then 1 mg for Days 2–16 (depending on renal function). For patients with a creatinine level of 2.0 mg/dL or over at the initiation of therapy, cisplatin and rapamycin were omitted. When side effects attributed to specific drugs of the regimen developed during the course of treatment, doses were lowered or drug administration was interrupted before resuming the intended completion of 16 days of treatment. In the majority of the cases, infusions were administered through a central venous catheter by using a portable infusion pump. With each cycle, patients received infection prophylaxis with fluconazole 200 mg daily by oral administration (PO), levofloxacin 500 mg PO daily, and acyclovir 400 mg PO twice a day. The use of hematopoietic growth factors varied according to patient condition.
Statistical analysis
Response and toxicity were assessed in 186 patients in accordance with the intent-to-treat principle. Response criteria used were the latest of the International Myeloma Working Group (IMWG).14 Toxicity was estimated using the National Cancer Institute Common Toxicity Criteria version 3.0.15
Overall survival (OS) and progression-free survival (PFS) were measured from the time of initiation of metronomic therapy. Events used in determining OS were death from any cause and for PFS included death from any cause, disease relapse, or disease progression. Kaplan-Meier statistical methods were used for OS and PFS, and the log rank test was used for comparisons. Cox proportional hazards regression modeling was used to determine which base-line parameters significantly affected the aforementioned end points. Logistical regression analysis was used to determine which factors were associated with the achievement of various response levels.
Results
Patients’ characteristics
Patients’ characteristics are listed in Table 1. Median age of the patient population was 61 years (range 36–83); 20% were older than 70 years. There was a high prevalence of abnormal results on standard clinical laboratory tests with known prognostic significance in MM. Furthermore, 84 (45%) patients had abnormal levels of creatinine (≥1.3 mg/dL), a factor that has recently been recognized to have adverse prognostic effect in relapsed/refractory MM patients.16 Metaphase cytogenetic abnormalities (CA) within six months prior to the initiation of metronomic therapy were evident in 111 of 186 (60%). Of the 186 patients, 71 had gene expression profiling (GEP) within six months prior to metronomic therapy; 36 of 71 (51%) and 44 of 71 (65%) of them were classified as high risk according to the 70-gene (GEP70)5 and 80-gene (GEP80)17 risk models, respectively. Extra-medullary disease (EMD) was apparent via positron emission tomography (PET) just prior to metronomic therapy initiation in 11 of 94 patients (12%).
Table 1.
Patients’ characteristics.*
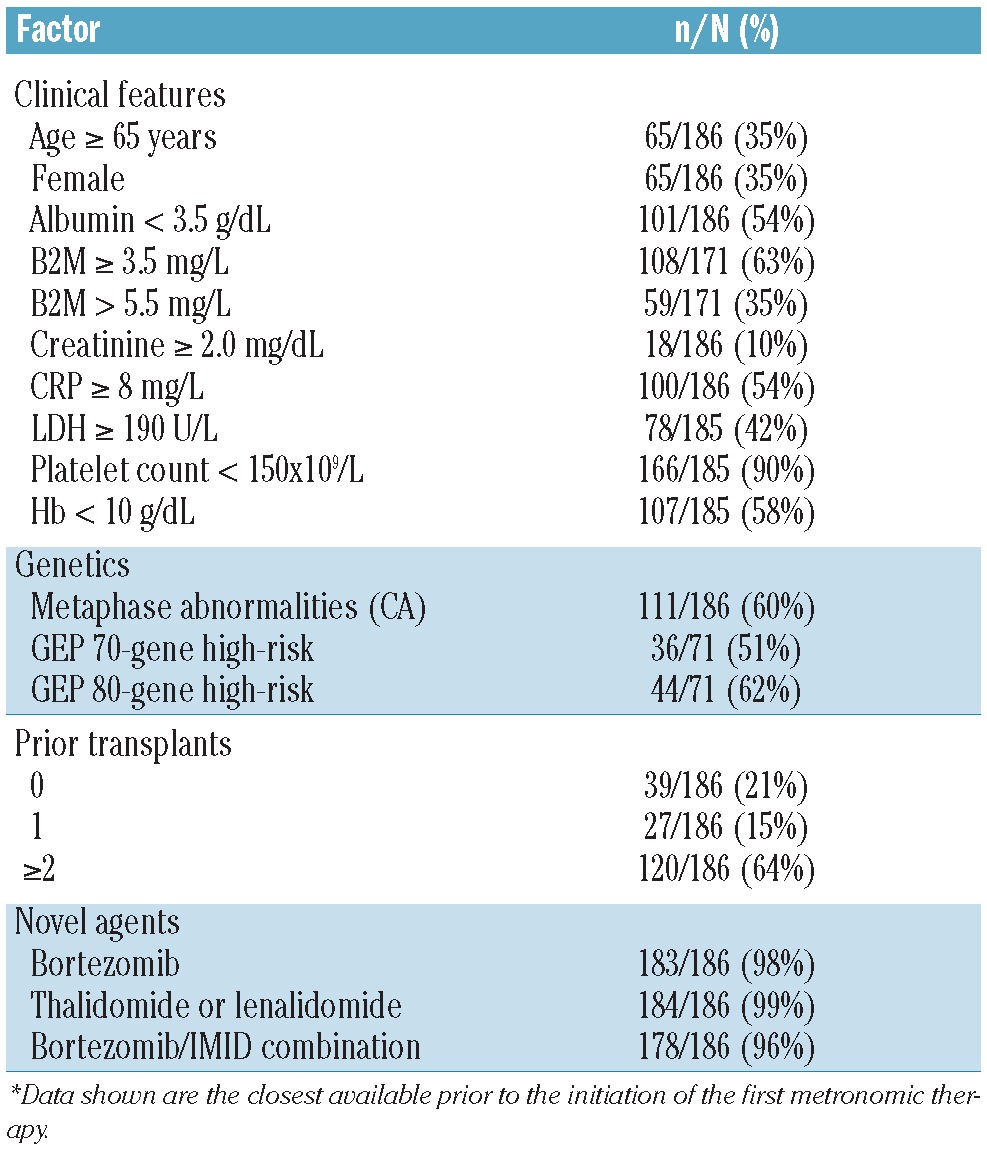
Median number of prior therapies was 14 (range 1–51), where participation in Total Therapy (TT) protocols was considered one line of treatment. At least one prior autologous transplantation had been applied on or off protocol in 148 of 186 (79%) patients. More than 90% of patients had been exposed to novel agents such as bortezomib, thalidomide and lenalidomide, often in combination (Table 1).
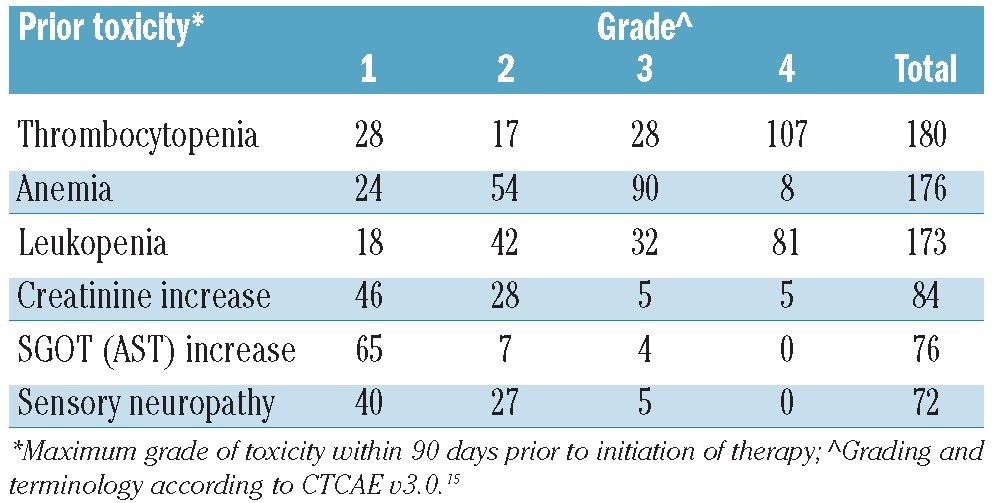
The incidence of cytopenia prior to the initiation of metronomic therapy was relatively high (Table 2). These cytopenias were due to extensive bone marrow infiltration of more than 70% in 40 out of 176 evaluable patients (23%). Cytotoxicity from chemotherapy, exhausted hematopoiesis and/or concurrent clinical myelodysplastic syndrome (MDS) was also considered as contributing to cytopenias. The most frequently occurring grade 2 or over pre-existing non-hematologic toxicities were elevated creatinine greater than 1.3 mg/dL in 84 patients (45%), elevated liver enzymes (SGOT>41 IU/L) in 76 (41%), and sensory neuropathy in 72 (39%) with 32 (17%) showing grade 3 leukopenia (Table 2 and Online Supplementary Table S1). Thirty-seven (20%) patients had a hospitalization event within 60 days before metronomic therapy initiation. Median number of cycles of metronomic therapy was 1 (range 1–5) with 47 (25%) receiving 2 or more.
Table 2.
Toxicities within 90 days prior to initiation of metronomic therapy.
Response and survival
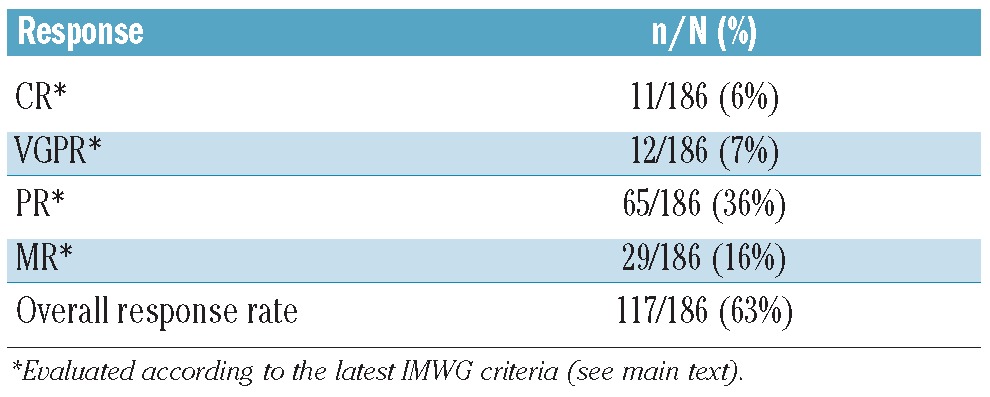
According to IMWG criteria,14 11 (6%) of the 186 metronomically-treated population achieved complete response (CR), 12 (7%) achieved very good partial response (vgPR), 65 (36%) achieved partial response (PR), and 29 (16%) achieved minimal response (MR), for an overall response rate (ORR) of 117 of 186 (63%) (Table 3). Stable disease (SD) was achieved by 33 (18%) of the patients, with only 36 of 186 (19%) having disease progression. The median time to best response was 27 days (range 6–203). CR occurred most frequently in patients with ISS stage 1 disease.
Table 3.
Patients’ responses.
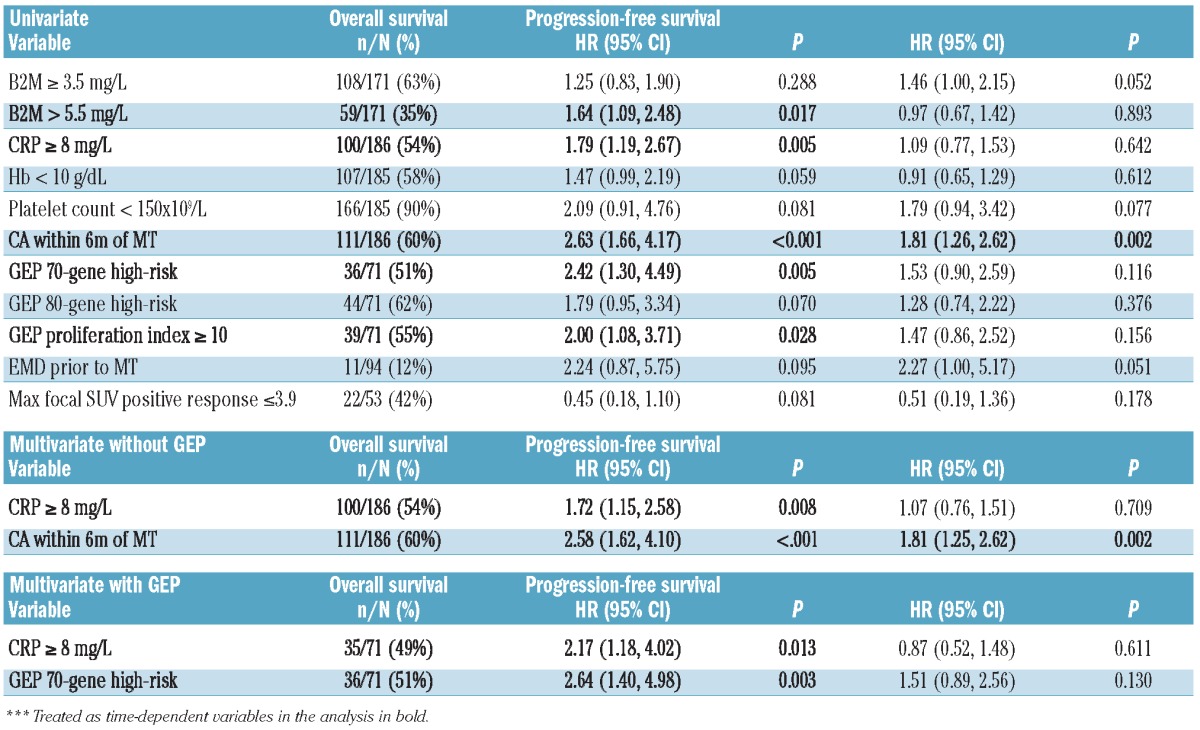
Median durations of OS and PFS were 11.2 and 3.6 months, respectively (Figure 1). OS and PFS were not linked to the level of response achieved (Figure 2). Neither OS nor PFS were affected by age (≥ 65 years), serum albumin (< 3.5 g/dL), LDH (≥ 190 U/L) or creatinine (≥ 2.0 mg/dL) (data not shown). Importantly, OS and PFS were both adversely affected by the presence of CA (Figure 3). OS was inferior with C-reactive protein (CRP) 8.0 mg/L or over (P=0.005), B2M more than 5.5 mg/L (P=0.017) (Table 4). CRP 8.0 mg/L or over and CA retained significance in multivariate analysis (HR=1.72, P=0.008 and HR=2.58, P<0.001, respectively).
Figure 1.
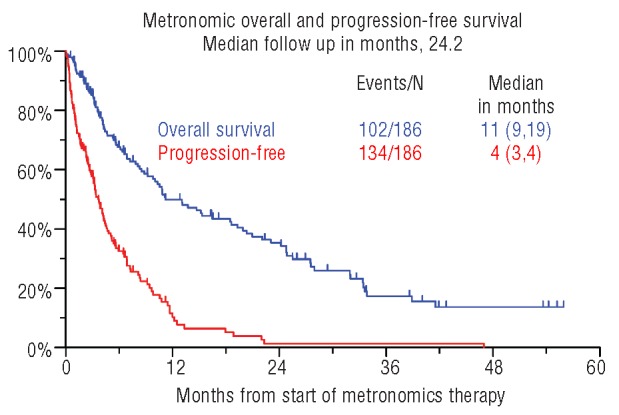
Kaplan-Meier plots of overall survival (OS) and progression-free survival (PFS) from initiation of metronomic therapy.
Figure 2.
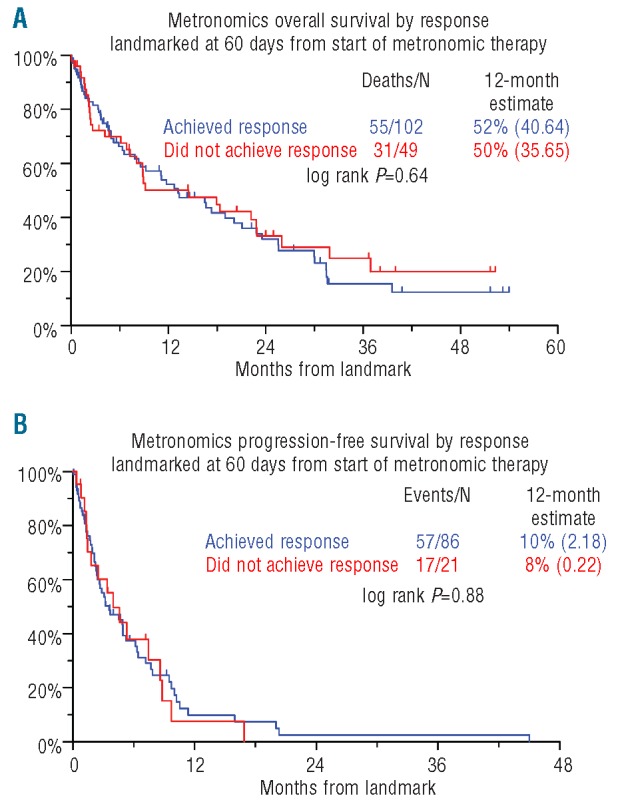
Kaplan-Meier plots of overall survival (OS) (A) and progression-free survival (PFS) (B) according to response by IMWG criteria (MR or better) from 60 days from initiation of metronomic therapy.
Figure 3.
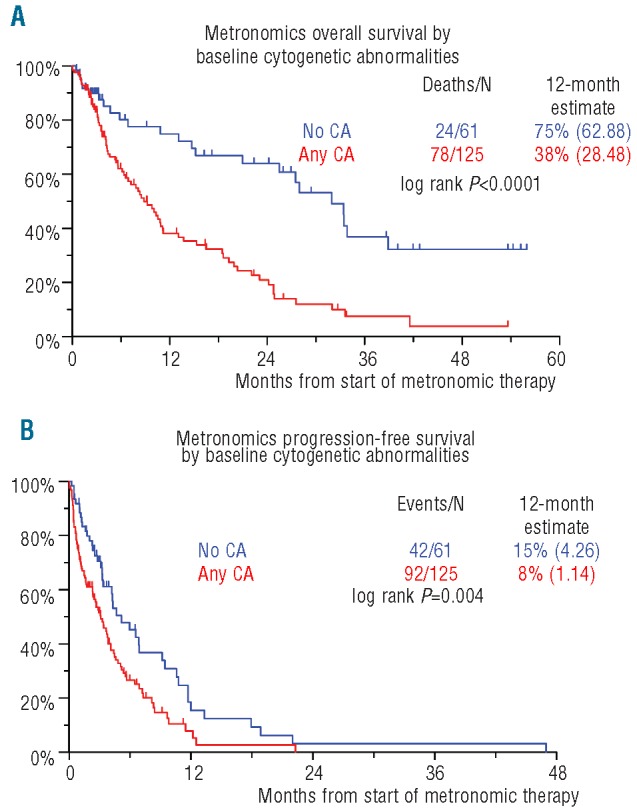
Kaplan-Meier plots of overall survival (OS) (A) and progression-free survival (PFS) (B) from initiation of metronomic therapy by baseline cytogenetic abnormalities (CA).
Table 4.
Cox proportional hazards regression modeling for OS and PFS.
For the 71 patients with available GEP within six months prior to the initiation of metronomic therapy, OS was inferior in high-risk MM in both the GEP70 and GEP80 models (HR=2.42, P=0.005 for GEP70 and HR=1.79, P=0.07 for GEP80; univariate analysis) (Figure 4A). Proliferation Index as measured by GEP was an important correlate to OS in univariate analysis (HR=2.00, P=0.028). GEP70 risk designation also correlated with OS using the multivariate regression model (HR=2.64, P=0.003), along with CRP 8.0 mg/dl or over (HR=2.17, P=0.013). Superior results in terms of OS were evident for the group of patients who were both low risk in the GEP70 model and had a normal base-line karyotype (P=0.0002) (Figure 5A). In the context of GEP data, no variable could achieve statistical significance for PFS regardless of whether CA were taken into account or not (Figures 4B and 5B).
Figure 4.
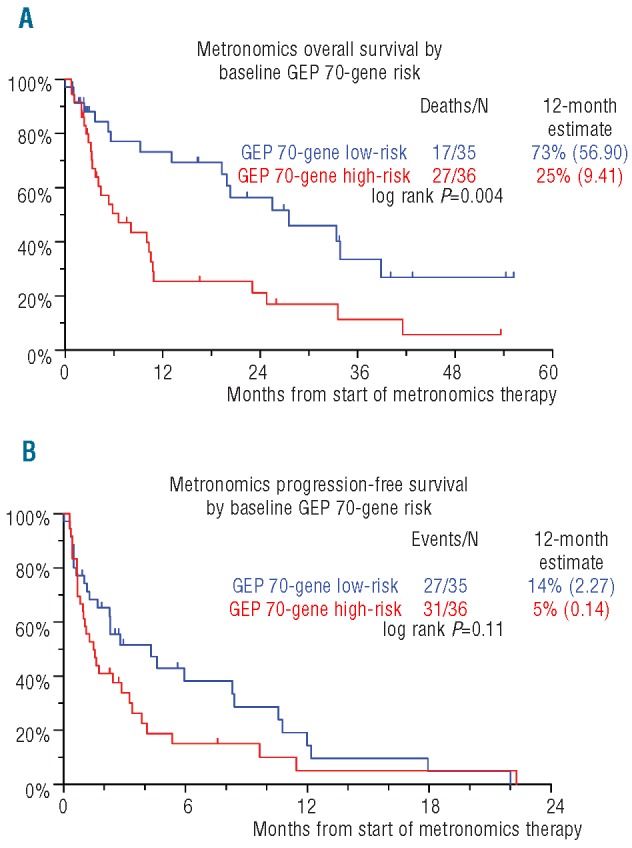
Kaplan-Meier plots of overall survival (OS) (A) and progression-free survival (PFS) (B) from initiation of metronomic therapy by baseline 70-gene Gene Expression Profile (GEP) risk status.
Figure 5.
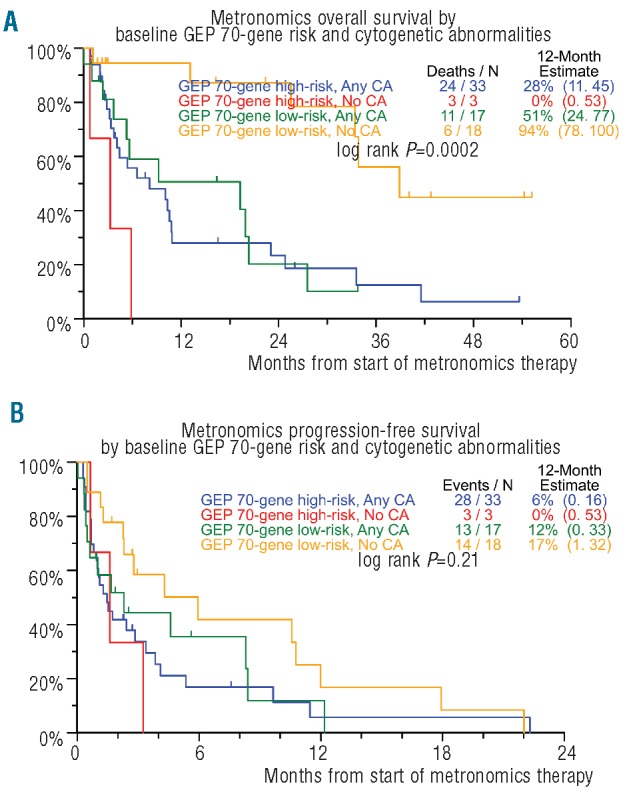
Kaplan-Meier plots of overall survival (OS) (A) and progression-free survival (PFS) (B) from initiation of metronomic therapy by combined baseline cytogenetic abnormalities (CA) and Gene Expression Profile (GEP) risk status.
Of the 186 patients, 53 had PET examinations before and after metronomic therapy within two months from initiation and did not receive other intervening therapy. Positive response in maximum standardized uptake FDG values (SUVmax) to values 3.9 or less tended to improve OS (HR=0.45, P=0.081) and was unrelated to PFS. EMD diagnosed by PET-CT was present in 11 cases and showed a clear tendency towards statistical significance to adversely affect both OS and PFS (HR=2.24, P=0.095 and HR=2.27, P=0.051 for OS and PFS, respectively) (Table 4). Out of these 11 patients, 8 responded to metronomic treatment with a greater than 50% reduction in tumor size and suppression of SUV to base-line levels.
Toxicity
Patients were monitored for toxicity for two months after the initiation of metronomic therapy (Table 5 and Online Supplementary Table S2). Most patients were treated in an outpatient unit (177 of 186, 95%). Secondary admissions due to any cause (regimen-related toxicity or MM) occurred in 59 patients (32%), and secondary admissions thought to be due to regimen-related toxicity were estimated to have occurred in 37 patients (20%). Treatment-related mortality (TRM) was only 1% (2 of 186). Grade 4 leukopenia, anemia, and thrombocytopenia due to metronomic therapy occurred in 108 (58%), 12 (6%), and 147 (79%) patients, respectively. Hematologic toxicity was difficult to evaluate as the majority of the patients had pre-existing cytopenia (Table 2). Interestingly, evolving treatment-related clinically important cytopenia was largely restricted to those with pre-existing moderate to severe cytopenia. The detailed impact of metronomic therapy in hematologic toxicity is shown in Online Supplementary Table S3 and Online Supplementary Figures S1-S3. Grade 4 and grade 5 hemorrhagic events occurred in one patient each; the grade 5 hemorrhagic event resulted in the patient undergoing surgery. Despite neutropenia in the majority of patients, grade 3–4 neutropenic fever occurred in only 2%.
Table 5.
Toxicities within 60 days of initiation of metronomic therapy.
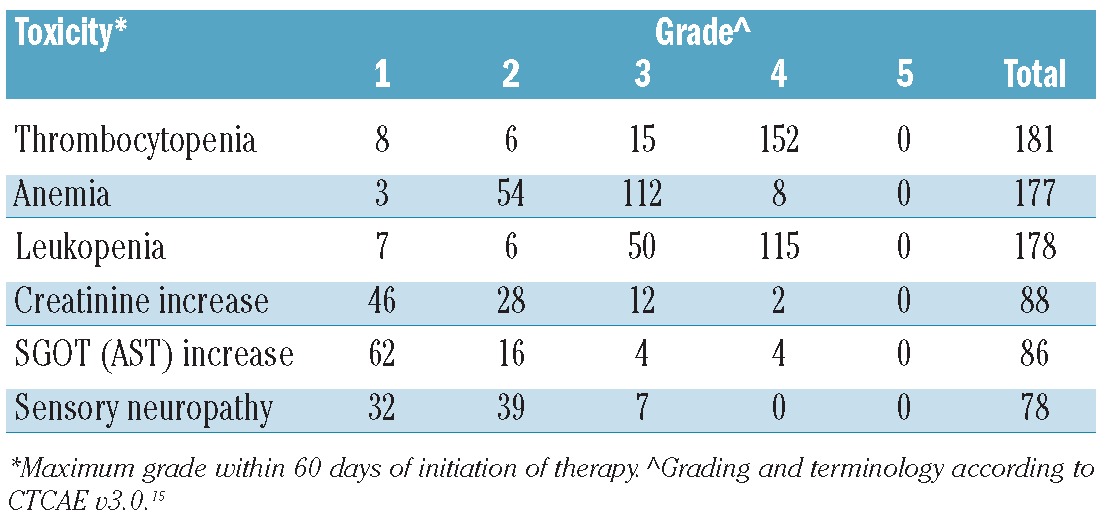
The most common non-hematologic grade 3–4 toxicities were metabolic events: 86 patients (46%) incurred hypophosphatemia, 42 (23%) hypokalemia, 41 (22%) hyponatremia, 41 (22%) hyperglycemia, and 29 (16%) hypocalcemia. Fatigue, malaise, or lethargy was encountered in 31 (17%). Colitis and stomatitis/pharyngitis were recorded in only 31 of 186 (17%) and were not associated with serious complications. The most frequent cardiovascular complication was hypotension (23 of 186, 12%) with only 3 (2%) having toxicity grades equal or greater than 3. The incidence of thromboembolic events, regardless of severity, was 7 of 186 (4%). Renal toxicities and metabolic abnormalities were common but brief in duration and easily manageable. A temporary increase in creatinine was the most common renal toxicity. Liver toxicity as shown by a mild increase in liver enzymes (> grade 2) was observed in 86 (42%) patients. Treatment-associated liver dysfunction over grade 3 occurred in 4 (2%) patients with no grade 5 incidents recorded. Sensory neuropathy was evident in 82 (44%) patients undergoing treatment with 46 of 186 (25%) grade 2 or worse. The incidence of this toxicity prior to the initiation of metronomic therapy was 72 of 186 (39%) and 32 of 186 (17%), respectively, as mentioned above. Any subsequent increase in the severity of neurological toxicity was mostly transient, returning to baseline in the vast majority of cases. A complete list of all grade 3 or greater toxicities is available in the Online Supplementary Table S2.
Discussion
Research on tumor angiogenesis began with the pioneering work of Folkman in the early 1970s.18 The relationship between bone marrow angiogenesis and MM is well established.19,20 In 1991, Kerbel suggested that anti-cancer agents may be able to target tumor vasculature after observing that many of the endothelial of tumor blood vessels are immature and constantly proliferating.21 Ten years later, Klement et al.22 and Browder et al.23 showed that mice bearing subcutaneous tumors responded to frequent repeated dosages of low-dose chemotherapy despite resistance to conventional dosing. Hanahan et al.,24 coined the term “metronomic” to refer to this type of treatment, which now means the administration of frequent low doses of chemotherapeutic agents, with no prolonged breaks in therapy. It is hypothesized that chemotherapy administered metronomically inhibits tumor growth primarily by blocking tumor neo-angiogenesis, while at the same time significantly reducing undesirable toxic effects. This hypothesis was the conceptual basis for the design of metronomic therapy for MM. The therapy includes metronomically-dosed doxorubicin and cisplatin along with thalidomide, a drug with well-established anti-angiogenic properties. Bortezomib, which is known to affect the bone marrow microenvironment,25 was included, along with rapamycin, another drug with known anti-angiogenic effect.26 While this regimen represents a more ‘intense’ approach over a shorter time period than that usually practiced in other metronomic regimens, the dosages used and the mode of administration are very different from conventional chemotherapy and are aimed at an anti-angiogenic effect.
In this population of patients with relapsed/refractory MM receiving metronomic therapy, the most important prognostic factors for OS were presence of cytogenetic abnormalities and CRP levels above 8.0 mg/L. These results support the importance of the cytogenetically defined risk in MM in every stage of the disease.27 High CRP values in MM have been recognized as a valid prognostic factor since 1992.28 In our cohort of metronomically-treated MM patients, response according to IMWG criteria did not correlate with OS or PFS. In contrast, we saw a beneficial OS trend in correlation with PET data suggesting that tumor burden as measured by conventional MM markers may not be accurate in relapsed/refractory MM, especially at the end stage of the disease. Thus, in end-stage MM, absence of progression rather than response to therapy according to IMWG criteria seems to be the clinically more relevant end point.
In an outpatient setting, the vast majority (95%) of the patients tolerated their metronomic therapy regimen quite well. The dominant toxicity encountered related to aggravation of pre-existing myelosuppression, possibly due to abrogation by the P-glycoprotein efflux pump at the hematopoietic stem cell level.29,30 We speculate that continuous exposure of MM and hematopoietic stem cells to doxorubicin suppressed the effect of the MDR-1 gene and thus exerted the observed anti-myeloma effect.31 Of the non-hematologic side effects, metabolic abnormalities and toxicities involving the kidney, liver, and gastrointestinal systems dominated but were transient and easily manageable. These outcomes are in accordance with the general toxicity profile of metronomic therapy in various types of solid tumors9,11,12 or lymphomas.32 Also, of our 186 patients receiving metronomic therapy, 46 (25%) subsequently received melphalan-based autologous transplant supported by stem cells collected earlier in the disease course, further highlighting the favorable and transient toxicity profile of the metronomic regimen in relapsed/refractory MM.
In conclusion, metronomic therapy is an effective salvage treatment for heavily pre-treated relapsed/refractory MM patients. It results in a relatively high ORR for this patient population, although survival was not influenced by the level of response according to IMWG criteria. The favorable toxicity profile allows the clinician to administer subsequent treatment to patients who are initially ineligible for autologous transplant and/or newer investigational agents. Its overall efficacy makes it a possible therapeutic candidate for earlier stages of MM. Thus, metronomic therapy represents a valuable addition to the clinician’s armamentarium for the heavily pre-treated patients with relapsed/refractory MM.
Acknowledgments
The Office of Grants and Scientific Publications of the University of Arkansas for Medical Sciences provided editorial assistance.
Footnotes
The onine version of this article has a Supplementary Appendix.
Funding
This work was supported by National Cancer Institute (Program Project Grant CA 55819), Bethesda, MD, USA.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Nair B, van Rhee F, Shaughnessy JD, Jr, Anaissie E, Szymonifka J, Hoering A, et al. Superior results of Total Therapy 3 (2003–33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006–66 with VRD maintenance. Blood. 2010;115(21):4168–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Y, Nair B, Shaughnessy JD, Jr, Cartron MA, Haessler J, Anaissie E, et al. Cytogenetic abnormalities in multiple myeloma: poor prognosis linked to concomitant detection in random and focal lesion bone marrow samples and associated with high-risk gene expression profile. Br J Haematol. 2009;145(5):637–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanamura I, Stewart JP, Huang Y, Zhan F, Santra M, Sawyer JR, et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: incidence increases from MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantation. Blood. 2006;108(5):1724–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawyer JR. The prognostic significance of cytogenetics and molecular profiling in multiple myeloma. Cancer Genetics. 2011;204 (1):3–12 [DOI] [PubMed] [Google Scholar]
- 5.Shaughnessy JD, Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109(6):2276–84 [DOI] [PubMed] [Google Scholar]
- 6.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21): 1565–71 [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24(3): 431–6 [DOI] [PubMed] [Google Scholar]
- 8.Barlogie B, Anaissie E, van Rhee F, Shaughnessy JD, Jr, Szymonifka J, Hoering A, et al. Reiterative survival analyses of total therapy 2 for multiple myeloma elucidate follow-up time dependency of prognostic variables and treatment arms. J Clin Oncol. 2010;28(18):3023–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong NS, Buckman RA, Clemons M, Verma S, Dent S, Trudeau ME, et al. Phase I/II trial of metronomic chemotherapy with daily dalteparin and cyclophosphamide, twice-weekly methotrexate, and daily prednisone as therapy for metastatic breast cancer using vascular endothelial growth factor and soluble vascular endothelial growth factor receptor levels as markers of response. J Clin Oncol. 2010;28(5):723–30 [DOI] [PubMed] [Google Scholar]
- 10.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4(6):423–36 [DOI] [PubMed] [Google Scholar]
- 11.Glode LM, Barqawi A, Crighton F, Crawford ED, Kerbel R. Metronomic therapy with cyclophosphamide and dexamethasone for prostate carcinoma. Cancer. 2003;98(8): 1643–8 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Billalabeitia E, Calzas J, Castellano D, Mendiola C, Bezares S, Valentin V, et al. Long-term follow-up of an anthracycline-containing metronomic chemotherapy schedule in advanced breast cancer. Breast J. 2009;15(5):551–3 [DOI] [PubMed] [Google Scholar]
- 13.Holmig K, Stover J, Talamano G, Fassas A, Lee C-K, Tricot G, et al. Bortezomib (VelcadeTM) + AdriamycinTM + Thalidomide + Dexamethasone (VATD) as an Effective Regimen in Patients with Refractory or Relapsed Multiple Myeloma (MM). Blood. 2004;104(11) [Google Scholar]
- 14.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18): 4691–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute Common Toxicity Criteria Version 3.0. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm 2006 [PubMed]
- 16.Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia 2012;26(1):149–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaughnessy JD, Jr, Qu P, Usmani S, Heuck CJ, Zhang Q, Zhou Y, et al. Pharmacogenomics of bortezomib test-dosing identifies hyperexpression of proteasome genes, especially PSMD4, as novel high-risk feature in myeloma treated with Total Therapy 3. Blood. 2011;118(13):3512–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21): 1182–6 [DOI] [PubMed] [Google Scholar]
- 19.Vacca A, Ribatti D, Roncali L, Ranieri G, Serio G, Silvestris F, et al. Bone marrow angiogenesis and progression in multiple myeloma. Br J Haematol. 1994;87(3):503–8 [DOI] [PubMed] [Google Scholar]
- 20.Rajkumar SV, Leong T, Roche PC, Fonseca R, Dispenzieri A, Lacy MQ, et al. Prognostic value of bone marrow angiogenesis in multiple myeloma. Clin Cancer Res. 2000;6(8):3111–6 [PubMed] [Google Scholar]
- 21.Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays. 1991;13(1):31–6 [DOI] [PubMed] [Google Scholar]
- 22.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105(8):R15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60 (7):1878–86 [PubMed] [Google Scholar]
- 24.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105(8): 1045–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roccaro AM, Hideshima T, Raje N, Kumar S, Ishitsuka K, Yasui H, et al. Bortezomib mediates antiangiogenesis in multiple myeloma via direct and indirect effects on endothelial cells. Cancer Res. 2006;66(1): 184–91 [DOI] [PubMed] [Google Scholar]
- 26.Humar R, Kiefer FN, Berns H, Resink TJ, Battegay EJ. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. Faseb J. 2002;16(8):771–80 [DOI] [PubMed] [Google Scholar]
- 27.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109 (5):2106–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bataille R, Boccadoro M, Klein B, Durie B, Pileri A. C-reactive protein and beta-2 microglobulin produce a simple and powerful myeloma staging system. Blood. 1992;80(3):733–7 [PubMed] [Google Scholar]
- 29.Hollister D, Jr, Silver RT, Gordon B, Coleman M. Continuous infusion vincristine and bleomycin with high dose methotrexate for resistant non-Hodgkin’s lymphoma. Cancer. 1982;50(9):1690–4 [DOI] [PubMed] [Google Scholar]
- 30.Calado RT, Falcao RP, Garcia AB, Gabellini SM, Zago MA, Franco RF. Influence of functional MDR1 gene polymorphisms on P-glycoprotein activity in CD34+ hematopoietic stem cells. Haematologica. 2002;87(6): 564–8 [PubMed] [Google Scholar]
- 31.Buda G, Maggini V, Galimberti S, Martino A, Giuliani N, Morabito F, et al. MDR1 polymorphism influences the outcome of multiple myeloma patients. Br J Haematol. 2007;137(5):454–6 [DOI] [PubMed] [Google Scholar]
- 32.Coleman M, Martin P, Ruan J, Furman R, Niesvizky R, Elstrom R, et al. Prednisone, etoposide, procarbazine, and cyclophosphamide (PEP-C) oral combination chemotherapy regimen for recurring/refractory lymphoma: low-dose metronomic, multidrug therapy. Cancer. 2008;112(10): 2228–32 [DOI] [PubMed] [Google Scholar]


