Abstract
Graft failure is a major complication after unrelated cord blood transplantation. Presence of HLA-antibodies before cord blood transplantation may impact graft failure. To analyze the effect of anti-HLA antibodies on unrelated cord blood transplantation outcomes, we analyzed 294 unrelated cord blood transplant recipients after reduced intensity conditioning regimen. The majority of the patients (82%) were transplanted for malignancies, 60% with double-unrelated cord blood transplant, 63% were HLA mismatched. Retrospectively, pre-unrelated cord blood transplant serum was tested for HLA-Ab using Luminex™ platform. Results were interpreted as mean fluorescence intensity (MFI) against donor-specific mismatch. Among 62 recipients (23%) who had anti-HLA antibodies before unrelated cord blood transplant, 14 patients had donor specific anti-HLA antibodies (DSA) (7 were donor-specific anti-HLA antibodies for single unrelated cord blood transplant and 7 for double unrelated cord blood transplant). Donor specific anti-HLA antibodies threshold ranged from 1620–17629 of mean fluorescence intensity (MFI). Cumulative incidence of Day-60 neutrophil engraftment was 76%: 44% for recipients with donor specific anti-HLA antibodies and 81% in those without donor specific anti-HLA antibodies (P=0.006). The cumulative incidence of 1-year transplant related mortality was 46% in patients with donor specific anti-HLA antibodies and 32% in those without antibodies (P=0.06). The presence of donor specific anti-HLA antibodies was associated with a trend for decreased survival rate (42% vs. 29%; P=0.07). Donor specific anti-HLA antibody in recipients of unrelated cord blood transplant is associated with graft failure and decreased survival. Patient’s screening for donor specific anti-HLA antibodies before unrelated cord blood transplantation is recommended before choosing an HLA mismatched cord blood unit. Whenever possible it is important to avoid selecting a unit for which the patient has donor specific anti-HLA antibodies.
Introduction
Unrelated cord blood transplantation (UCBT) is an alternative option for patients without a suitable HLA matched donor.1 Tolerance of some degree of HLA mismatch, with relatively low rates of both acute and chronic graft-versus-host disease (GvHD) makes UCBT an interesting source of hematopoietic stem cells for both pediatric and adult transplants.
Different studies comparing UCBT to matched unrelated donor transplant2–4 showed no significant difference in disease free survival in children and adults; however, probability of neutrophil and platelet engraftment is lower and delayed after UCBT. Many approaches are currently available that aim to improve engraftment after UCBT, such as the use of double UCBT (dUCBT) and reduced intensity conditioning regimen (RIC).5 However, the hematopoietic engraftment remains lower after double UCBT when compared to other stem cell sources.6
Other strategies7,8 are under investigation to prevent graft failure (GF) by the intrabone infusion of UCB and the co-infusion of expanded single UCB unit.9 Nevertheless, delayed engraftment and GF continue to be a major concern after UCBT.
Importantly, the detection of patient-, donor-, disease-and transplant-related factors that are associated with engraftment may help clinicians to choose the best UCB unit and to improve the techniques of transplantation. Since most UCBT are performed with HLA mismatched CB units,10 the presence of anti-HLA (donor specific antibodies, DSA) in the patients against the UCB unit can be an issue for engraftment. Anti-HLA antibodies before transplant may occur due to the alloimunization to HLA through blood transfusions, pregnancy and also in some unexposed individuals.11–14 In unrelated donor recipients, Spellman et al. reported pre-transplant anti-HLA antibodies associated to GF and higher mortality. Interestingly, in this case control study, higher frequency of DSA anti HLA DP was reported.13
In the UCBT setting, few studies with controversial results are available on the impact of DSA and outcomes. Two series reporting respectively 38615 and 73 patients15 receiving single or double UCBT showed an increased risk of GF and lower survival for patients with positive DSA. However, another report showed no association between the presence of DSA and transplant outcomes in 126 dUCBT recipients.16
In order to address the impact of the anti-HLA antibodies on outcomes after UCBT, in an independent dataset and using standardized methodologies for detection of DSA, we performed a retrospective registry-based analysis including only patients given an RIC UCBT in 16 French transplant centers, from 2000 to 2011.
Design and Methods
Patients
Using the Eurocord database, we selected 415 UCBT recipients (n=360 first transplant) after RIC regimen. Immunology laboratories of French transplant centers were requested to perform standardized tests for the detection of DSA retrospectively when samples were available and if not yet tested. Therefore, 294 patients were assessed for anti-HLA antibodies screening before UCBT and were included in this analysis. All patients received a single (sUCBT) or double unmanipulated UCB unit as their first graft after an RIC. RIC was defined as a conditioning regimen not containing either total body irradiation (TBI) with a dose equal or superior to 6 Gy, or a dose of oral busulfan equal or superior to 8 mg/kg when administered orally, or to 6.4 mg/kg intravenously.
Data on patients’ and graft characteristics, as well as on outcomes, were collected using the Eurocord standard forms (100-day and yearly follow-up forms). The Institutional Review Boards of the Eurocord-Netcord scientific committee approved this study. According to EBMT rules, patients provided informed consent allowing for data submission to EBMT and Eurocord database and use of data for research in accordance with the Declaration of Helsinki.
Antibody testing
Pre-transplant patients’ sera samples cryopreserved in each participating center were tested retrospectively, unless previously analyzed before the transplantation. The patient serum was collected and tested or stored (for later testing) within a maximum of 30 days before UCBT, according to transplant center protocol for patients and donor selection.
HLA antibodies screening was performed either by an Elisa method (Lambda Antigen Tray™ Mixed Class I & II -One Lambda Inc., Canoga Park, CA, USA or Dynaship-Dynal) or by a Luminex assay (LABScreen Mixed class I and II, One Lambda Inc., Canoga Park, CA, USA or Lifecodes Lifescreen Deluxe-Gen-Probe Inc., San Diego, CA, USA). In our series, 85% of the antibody identification was performed with the Luminex from One Lambda Inc. All laboratories participating in this study were accredited by the EFI (European Federation for Immunogenetics). Laboratories performed the Luminex typing according to the manufacturer’s recommendations and the laboratory guidelines.
All samples with a positive screening were further evaluated using the Luminex-based single antigen flow beads to determine HLA specificities (LABScreen Single Antigen class I and II, One Lambda Inc., Canoga Park, CA, USA or Lifecodes LSA class I or class II -Gen-Probe Inc., San Diego, CA, USA). Samples were considered positive for specific HLA antigens based on a background adjusted for mean fluorescence intensity (MFI) greater than 1000. All the DSA+ cases but one were identified using the OneLambda Luminex single antigen technique.
Definitions and end points
A patient was considered anti-HLA antibody negative when the initial screening test was negative. Each patient with a positive screening for anti-HLA antibodies was further investigated for donor specific anti-HLA antibodies (DSA) with a Luminex Single Antigen assay. Patients with anti-HLA antibodies directed against an antigen present in the UCB infused, or in at least one of the UCB infused in the case of double cord transplantation were defined as DSA positive cases.
Neutrophil engraftment was defined as achieving absolute neutrophil count (ANC) of 0.5×109/L or over for three consecutive days, and platelet recovery was defined as achieving platelet count of 20×109/L or over unsupported by platelet transfusions for seven days. Primary graft failure was defined as never having achieved ANC of 0.5×109/L or over lasting for at least three consecutive days or ANC of 0.5x109/L or over without donor engraftment (autologous recovery). Secondary graft failure was defined as having achieved ANC of 0.5×109/L or over with subsequent decline or loss of donor engraftment. Full donor chimerism was defined as 95% or over leukocytes of donor origin in peripheral blood or marrow samples, measured by different techniques according to each transplant center’s protocol. Autologous reconstitution was defined as 95% or over leukocytes of recipient origin. Mixed chimerism was defined by the presence of more than 5% but less than 95% leukocytes of donor origin. The diagnosis and grading of acute and chronic GvHD was assigned by the transplant center using standard criteria.17 Transplant-related mortality (TRM) was defined as the time from transplantation to death not related to disease recurrence or progression. Overall survival (OS) was calculated from the date of UCBT until death or last observation alive.
Statistical analysis
Median values and ranges were used for continuous variables and percentages for categorical variables (Table 1). For each continuous variable, the study population was initially split into quartiles and in two groups by the median. The probabilities of OS and LFS were calculated using the Kaplan-Meier method and the log rank test for univariate comparisons.18 The probabilities of neutrophil engraftment, grade II–IV acute and chronic GvHD, relapse and NRM were calculated with the cumulative incidence (CI) estimator. Multivariate analyses were performed using Fine and Gray’s proportional hazards regression model.19 Variables that reached a P value of 0.15 in the univariate analysis were included in the initial models, and variables were eliminated one at a time in a stepwise fashion in order to only keep variables that reached a P value of 0.05 or less in the final model. P values were two-sided. Statistical analyses were performed with SPSS (SPSS Inc., Chicago, IL, USA) and Splus (MathSoft Inc., Seattle, WA, USA).
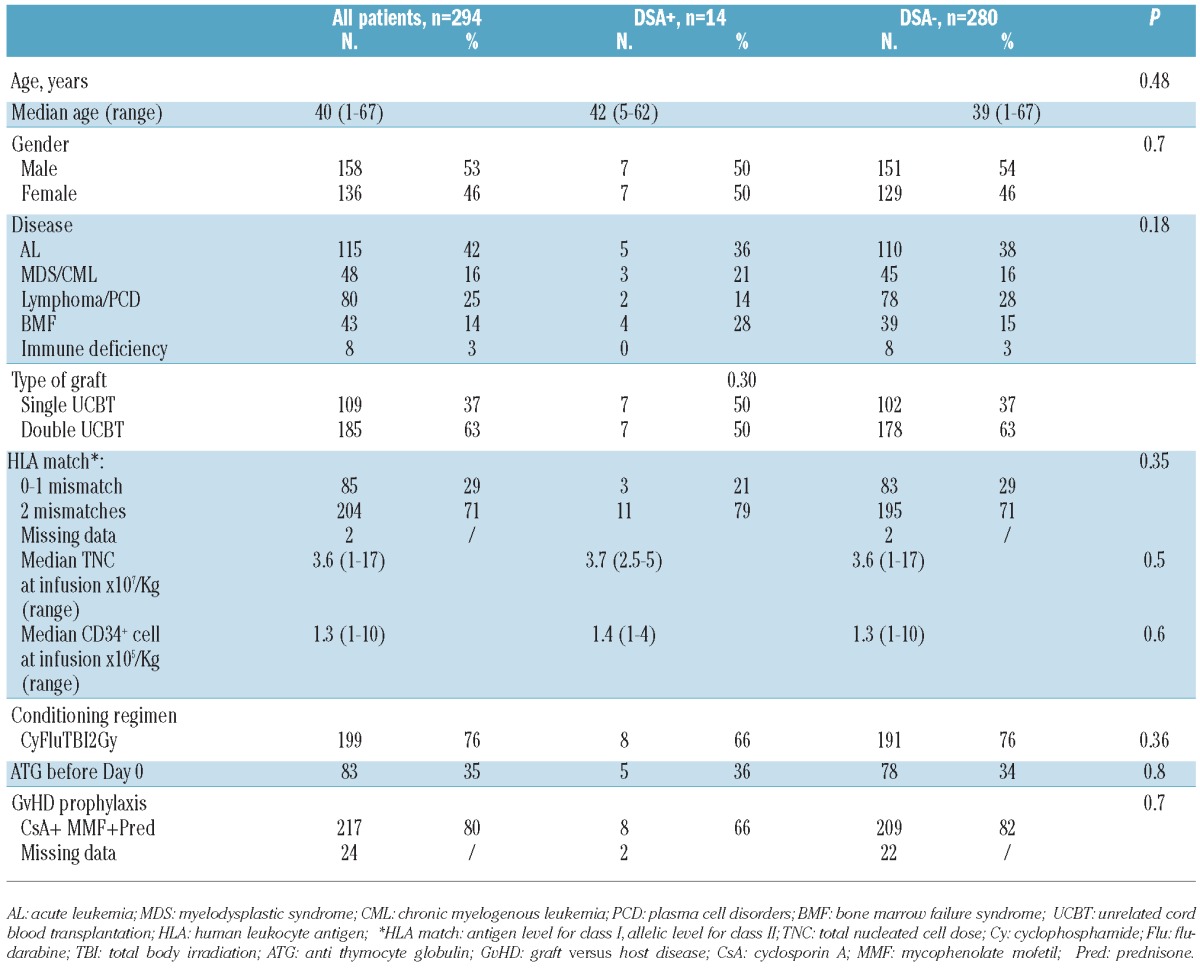
Table 1.
Patients’ and transplant characteristics.
Results
Patients’ characteristics
Baseline patients’ and transplant characteristics are shown in Table 1. In summary, 39% were transplanted for acute leukemia, 37% were transplanted with a single UCBT and 63% a double UCBT. Most of the patients received UCB units with 4/6 HLA disparity (antigen level for HLA-A and B, allelic level for DRB1).
All patients received an RIC regimen. Median infused total nucleated cell dose (TNC) was 3.6×107/kg (range 1–17×107/kg) and median CD34+ cell dose was 1.6×105/kg (range 1–10×105/kg).
Anti-HLA antibodies
Of the 294 cases tested 62 (21%) were anti-HLA antibody positive.
Of the positive cases, 14 were positive against the UCB (DSA+). Among the 14 DSA+, 8 had antibodies against HLA class I antigens, 3 had antibodies against HLA class II and 3 against class I and II. The groups of patients with and without DSA were similar in respect to recipients’ age at UCBT, proportion of gender disparity between donor and recipients, type of diagnosis and in graft composition (Table 1). All the 14 patients with DSA received a minimum TNC dose of 2.5×107/Kg at infusion.
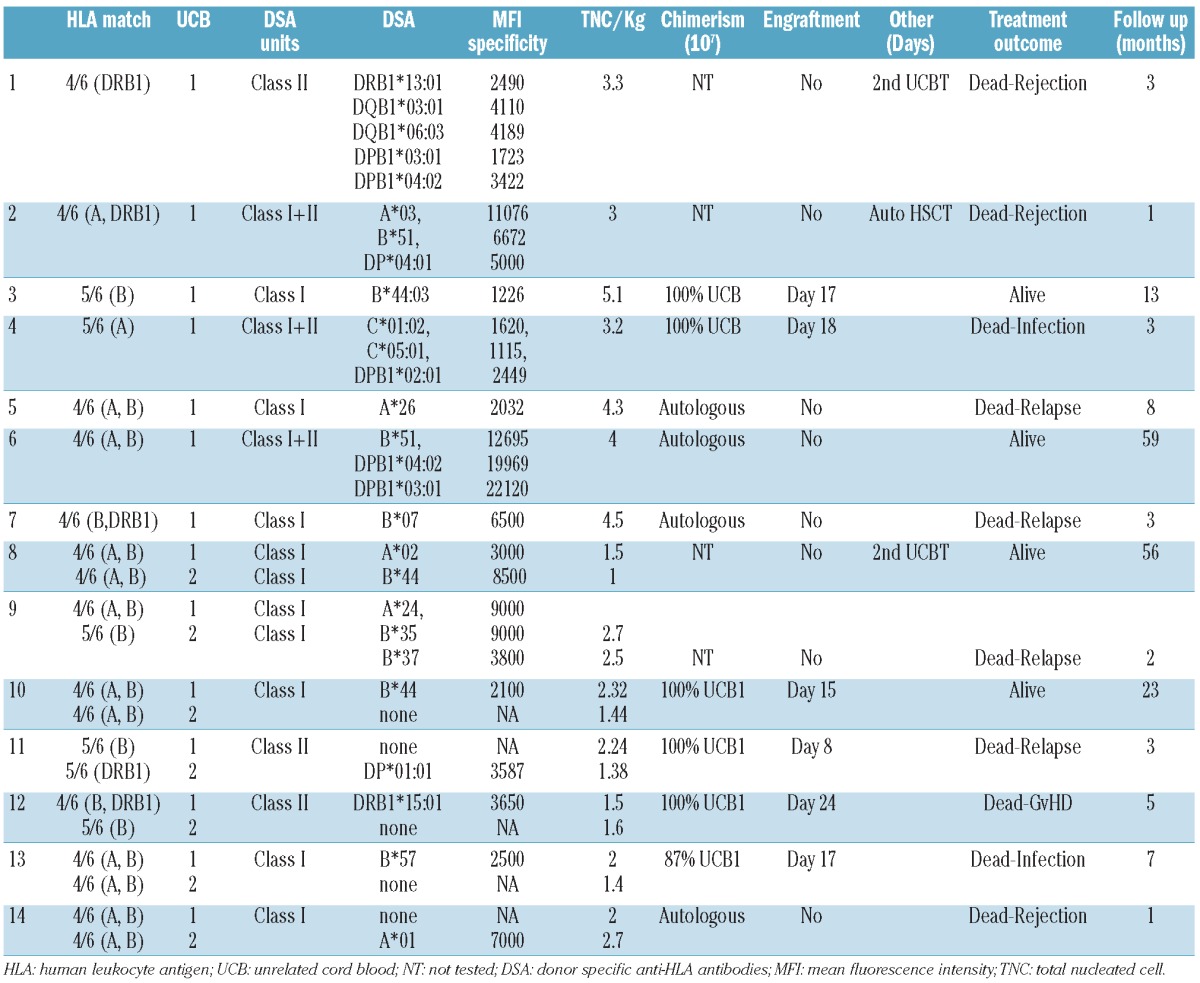
Specificity of the patients transplanted with DSA and their outcomes are shown in Table 2.
Table 2.
Patients with DSA positivity against UCB units and their outcome.
DSA and engraftment
CI of Day-60 neutrophil engraftment was 78±1%, and the median time to engraftment was 20 (range 13–60) days. Seventy-three patients experienced graft failure. No significant difference was found in the CI of engraftment for patients with HLA-Ab not specific to antigens present in the UCB units (n=48) when compared with the antibody negative group.
CI engraftment was 79% in case of absence of antibody (n=232), and was 81% for no-DSA group (n=48) (P=0.28).
The CI of engraftment was 77% for DSA negative and 44% for DSA positive patients (P=0.003) (Figure 1). Three out of the 8 patients with DSA directed to class I antigen engrafted, and 2 out of the 3 with DSA against class II antigen, and one out of the 3 against both class I and class II antigens engrafted as well.
Figure 1.
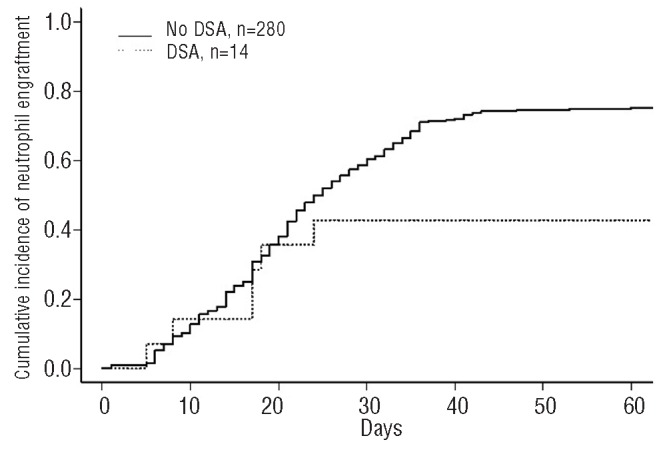
The cumulative incidence of engraftment at 60 days by DSA. The solid line represents no DSA and the dashed line represents the DSA.
Univariate analysis showed the presence of DSA associated with engraftment (P=0.003). The other factors associated with lower myeloid engraftment were patients transplanted before 2008 (67% vs. 80%; P=0.003) and non-malignant disease (64% vs. 76%; P=0.005). Prognostic factors tested in univariate analysis were donor/recipient gender mismatch, recipient age, prior autologous transplant, type of graft (single or double UCBT), TNC doses at collection and infusion, number of HLA disparities, use of ATG before Day 0 and presence of anti-HLA Ab non-donor-specific. In multivariate analysis, the only factor independently associated with neutrophil recovery was the presence of DSA before transplant (HR 1.69, 95%CI: 1.2–12.6; P=0.002).
Sixty-five of the patients without pre-transplant DSA failed to engraft. Fourteen patients received a second allogeneic stem cell transplantation, 24 had autologous reconstitution and 27 did not receive any subsequent treatment (26 of whom died in a median time of 53 days).
The CI of Day 180-platelet engraftment was 62%. Only 3 out of 14 patients with DSA had platelet recovery (21%). In the patients without DSA (n=280), the CI of platelet engraftment was 73%.
Impact of level of mean fluorescence intensity for DSA positive patients on engraftment
Of the 14 patients with DSA, the median level of MFI was 3900. The intensity of DSA measured by MFI was associated with graft failure.
All the 6 patients with DSA who engrafted had MFI lower than median. The median MFI among the DSA patients who experienced graft failure was 7750 (range 2032–19969), and it was 2474 (range 1226–3650) in the DSA patients who achieved engraftment (P=0.004) (Table 2).
Of the 14 patients with DSA, 7 were transplanted with an sUCB, and 2 of these engrafted. The level of the MFI in these cases with engraftment was under 2500. On the other hand, with the exception of one case, all patients who presented graft failure had a high level of DSA (MFI 4110–22120). Of the 7 patients with DSA and transplanted with dUCB, 3 engrafted with a unit that had an intermediate level of DSA (MFIs 2100, 3650 and 2500). In the remaining 4 cases, one engrafted with the unit without DSAs (MFIs against the non-engrafting unit were 3685), and 3 did not engraft. In these last 3 cases, there was at least one unit with a high level of DSA (MFI 8500, 9000, and 7000).
Graft-versus-host disease and relapse
CI of grade II–IV acute GvHD and 3-year chronic GvHD was 31±4% and 22±3%. The CI of aGvHD was 21% in patients with DSA; however, the number of DSA patients at risk was very small, as 7 out of 14 patients with DSA died within 100 days of rejection or early relapse. No disease- or transplant-related factors were associated with occurrence of aGvHD (such as patient and donor gender mismatch, patient age, prior autologous transplant, type of graft, TNC dose at collection and infusion, number of HLA disparities, use of ATG before Day 0 and presence of non-donor-specific anti-HLA Ab).
For the 244 patients transplanted for malignant disease the CI of relapse at three years was 34±4%. Four out of 11 patients with malignant disease and DSA (36%) relapsed after UCBT.
Figure 2.
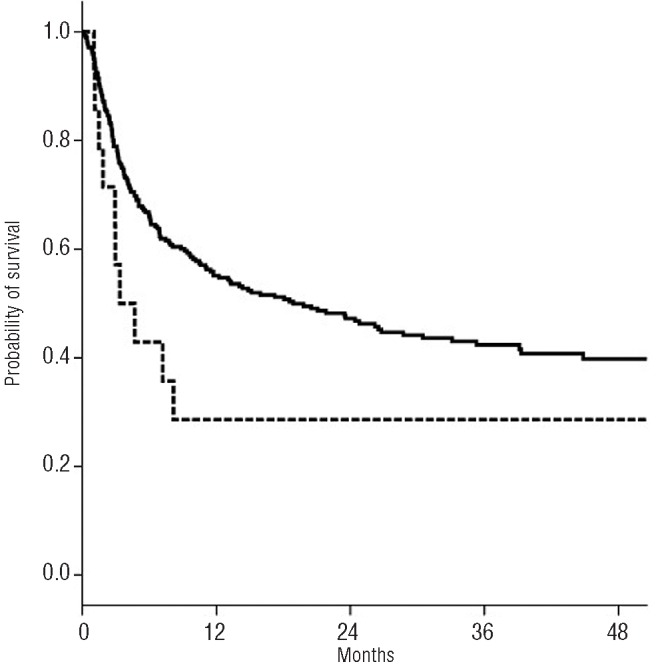
Probability of 3-year overall survival by DSA. The solid line represents no DSA and the dashed line represents the DSA.
Transplant-related mortality and overall survival
CI of TRM at one year was 36±4%. Overall, 170 patients died, and the causes of death included relapse (n=55), GvHD (n=38), infection (n=33), rejection (n=11), multi-organ failure (n=16), toxicity (n=9), and other causes not specified (n=8). No significant difference was found in the CI of TRM for patients with HLA-Ab not specific to antigens present in the UCB units, no-DSA (n=48), when compared with the DSA group (n=14) and with the antibodies negative group (n=232). CI of TRM was 32% in case of absence of antibody and 36% for the no-DSA group (P=0.48). The CI of TRM was 46%±8 in patients with DSA and 32%±4 in those without antibodies (P=0.06). Graft failure as time-dependent variable was associated with higher risk of TRM (P=0.002) and lower OS (P=0.002).
The probability of overall survival at three years was 42+3% with a median follow up of 36 months. The presence of DSA was associated with lower survival (42% vs. 29%; P=0.07). Also, patients transplanted with well matched UCB (6/6 or 5/6) had a 3-year probability of survival of 50% compared to 36% (P=0.02) for those receiving UCB with 2 or more HLA mismatches. Survival was better for patients receiving UCB graft with TNC over 3.6×107/Kg (47% vs. 36%; P=0.04).
Discussion
In this retrospective study, we have found that the presence of donor specific HLA antibodies is an important factor for engraftment and, consequently, mortality after reduced intensity single or double UCBT. The impact of DSA on outcomes after transplantation has been extensively described in solid organ transplantation20 in which HLA matching is not as important as in hematopoietic stem cell transplantation (HSCT). The effect of DSA has also been to some extent described in the HSCT literature, but with the advent of alternative HSCT donor this issue has been revisited. In the context of unrelated adult HSCT, Spellman et al.13 reported the results of a case control study showing higher GF rate when pre-transplant DSA were detected in the recipients of 10/10 HLA matched unrelated donors. Therefore, presence of DSA against HLA-DP was associated with more than 60% of the risk of GF. Recently, in the haploidentical transplantation setting21 and in HLA mismatched UCBT,15,16,22,23 the presence of DSA has also been described as a factor increasing graft failure. In our study, including 294 patients with a median follow up of three years, we showed that the presence of DSA before UCBT is associated with delayed engraftment and GF. This finding translated in a trend of increased TRM and decreased survival.
The effect of antibodies on engraftment was only detected when the specificity corresponded to the donor antigen. We did not find any difference in the engraftment rate for patients who had antibodies against an HLA not present on the UCB. Takanashi et al.22 also reported an increased risk of graft rejection after single UCBT and MAC, with 38% probability of engraftment in patients with DSA (n=20). In the setting of dUCBT, two reports showed controversial results. The Dana Farber group15 reported the impact of DSA (n=18) on engraftment in 73 dUCBT recipients, and the effect on mortality was found only in the group with DSA directed against both cord blood units (n=7). However, Brunstein et al.16 showed no association between the presence of DSA and transplant outcomes in a series of 126 dUCBT recipients after MAC or RIC (n=18 DSA+) in which the threshold for the detection of DSA was 500 MFI, lower than in our study. Importantly, similar to the results showed by Cutler et al., in our series, a higher MFI level was associated with an increase in GF, providing further evidence that the use of UCB corresponding to a DSA with a high MFI level in the recipient should be avoided. The impact of DSA with low or intermediate level of MFI seems less clear and further studies with larger series of patients are required to determine the optimal recipient’s MFI cut-off value for UCB selection. In our study, all but one patient with DSA and MFI lower than the median, engrafted. However, we could not draw a definitive conclusion on the cut-off level of MFI based on the small series sample.
Other HLA-related immunological factors, such as HLA-A/B high resolution typing, HLA-C matching,10 the HLA vector direction24 may play a role in graft failure and other outcomes. Recently, we and others reported a lower TRM and better survival after UCBT when selecting a non-inherited maternal antigen (NIMA)-matched UCB unit.25,26
It is important to highlight that the use of cord blood transplants for patients with non-malignant disorders, such as bone marrow failure syndromes (BMFS)27 or hemoglobinopathies and the use of RIC regimens are known factors associated with low engraftment rate. In our study, 14% of the population was transplanted for BMFS, but in the multivariate analysis adjusted for the type of diagnosis, the effect of DSA was independently associated with graft failure (P=0.002). Another important factor, which may have an impact on transplant results, is the T-cell depletion using ATG before Day 0. In the mismatched donor setting, ATG was often given to facilitate engraftment. More recently, conditioning regimens without ATG are more frequently used, especially in the double UCBT setting. In our series of patients, ATG use was reported in 35% of the population. Interestingly, 4 of the 14 patients with DSA received ATG as part of conditioning regimen and 3 of them engrafted. It is interesting to note that the frequency of DSA in the transplant population varies in different publications. This may be because of the different distribution of gender, age, parity status and specific clinical practices. In the studies mentioned above,15,16,22 the DSA frequencies range from 0.5% in the Japanese group to around 20% in the American reports. In France, the screening for donor anti-HLA antibodies has been performed by several transplant centers since 2009; therefore, the physicians selecting the donor may have been aware of the presence of DSA, and, consequently, may have preferred to select a different unit. This could have accounted for the relatively low incidence of DSA reported in our series (0.5%). Moreover, in our series, 12 of 14 patients with DSA were transplanted before 2009, showing the importance of including these criteria before selecting the UCB unit.
Another probable explanation for the low frequency of alloimmunization in our series is the routine use in France since the 1980s of leukocyte reduced cellular blood components (platelets and red cells).28
Strategies such as plasma or B-cell depletion to minimize the detrimental effect of DSA on transplant outcomes might be considered. However, the benefit of long-lasting immune depletion should be carefully considered in the cord blood transplant setting, and, more importantly, to avoid choosing a unit when the patient has DSA against the CB unit whenever possible. Given our results, with the objective of improving final outcomes of UCBT, we recommend that the recipient screening and the identification of specific anti-HLA Ab should be considered and performed using standardized methodology as soon as the patient has an indication for an UCBT, as part of donor selection strategy. DSA search is an effective tool to be included in the algorithm for the best UCB unit selection.
Acknowledgments
The following authors contributed as treating physician from transplant centers (in alphabetical order of the city): Prof. P Guardiola, CHR Angers, Angers; Prof. JY Cahn, A Michallon Hospital, Grenoble; Prof. M Michallet, E Herriot Hospital, Lyon; Prof. V Mialou, IHOP Hospital, Lyon; Prof. G Michel, La Timone Hospital, Marseille; Prof. JF Rossi, Lapeyronie Hospital, Montpellier; Prof. A Sirvent, Arnaud de Villeneuve Hospital, Montpellier; Prof. P Bordigoni, Brabois Hospital, Nancy; Prof. M Mohty, Hotel Dieu Hospital, Nantes; Prof. L Mannone, l’Archet Hospital, Nice; Prof. G Socié, Saint-Louis Hospital, Paris; Prof. F Guilhot, J Bernard Hospital, Poitiers; Prof. H Tilly, Centre Henry Becquerel Hospital, Rouen; Prof. D Guyotat, Hopital Nord, Institut de Cancérologie de la Loire, Saint-Etienne.
Footnotes
Funding
This study was supported by IRGHET (International Research Group on unrelated Hematopoietic Stem Cell Transplantation) and funded in part by the Inserm grant TGIR0805. The authors thank JM Tiercy for helpful discussion.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan KW, et al. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32(4):397–407 [DOI] [PubMed] [Google Scholar]
- 2.Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369(9577): 1947–54 [DOI] [PubMed] [Google Scholar]
- 3.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11(7):653–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351(22): 2276–85 [DOI] [PubMed] [Google Scholar]
- 5.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delaney C, Ratajczak MZ, Laughlin MJ. Strategies to enhance umbilical cord blood stem cell engraftment in adult patients. Expert Rev Hematol. 2010;3(3):273–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha V, Broxmeyer HE. New approaches for improving engraftment after cord blood transplantation. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S126–132 [DOI] [PubMed] [Google Scholar]
- 9.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16 (2):232–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eapen M, Klein JP, Sanz GF, Spellman S, Ruggeri A, Anasetti C, et al. Effect of donor-recipient HLA matching at HLA A, B, C, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. Lancet Oncol. 2011;12(13):1214–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schonewille H, Haak HL, van Zijl AM. Alloimmunization after blood transfusion in patients with hematologic and oncologic diseases. Transfusion. 1999;39(7):763–71 [DOI] [PubMed] [Google Scholar]
- 12.Idica A, Sasaki N, Hardy S, Terasaki P. Unexpected frequencies of HLA antibody specificities present in sera of multitrans-fused patients. Clin Transpl. 2006;139–59 [PubMed] [Google Scholar]
- 13.Spellman S, Bray R, Rosen-Bronson S, Haagenson M, Klein J, Flesch S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115 (13):2704–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morales-Buenrostro LE, Terasaki PI, Marino-Vazquez LA, Lee JH, El-Awar N, Alberu J. “Natural” human leukocyte antigen antibodies found in nonalloimmunized healthy males. Transplantation. 2008;86(8): 1111–5 [DOI] [PubMed] [Google Scholar]
- 15.Cutler C, Kim HT, Sun L, Sese D, Glotzbecker B, Armand P, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011;118(25):6691–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunstein CG, Noreen H, DeFor TE, Maurer D, Miller JS, Wagner JE. Anti-HLA antibodies in double umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2011;17(11):1704–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304 [DOI] [PubMed] [Google Scholar]
- 18.Cox DR. Regression model and life tables. J R Stat Soc B. 1972;34:187–200 [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446): 496–9 [Google Scholar]
- 20.Patel AM, Pancoska C, Mulgaonkar S, Weng FL. Renal transplantation in patients with pre-transplant donor-specific antibodies and negative flow cytometry crossmatches. Am J Transplant. 2007;7(10):2371–7 [DOI] [PubMed] [Google Scholar]
- 21.Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012;47(4):508–15 [DOI] [PubMed] [Google Scholar]
- 22.Takanashi M, Atsuta Y, Fujiwara K, Kodo H, Kai S, Sato H, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116(15):2839–46 [DOI] [PubMed] [Google Scholar]
- 23.Gutman JA, McKinney SK, Pereira S, Warnock SL, Smith AG, Woolfrey AE, et al. Prospective monitoring for alloimmunization in cord blood transplantation: “virtual crossmatch” can be used to demonstrate donor-directed antibodies. Transplantation. 2009;87(3):415–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens CE, Carrier C, Carpenter C, Sung D, Scaradavou A. HLA mismatch direction in cord blood transplantation: impact on outcome and implications for cord blood unit selection. Blood. 2011;118(14):3969–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Rood JJ, Stevens CE, Smits J, Carrier C, Carpenter C, Scaradavou A. Reexposure of cord blood to noninherited maternal HLA antigens improves transplant outcome in hematological malignancies. Proc Natl Acad Sci USA. 2009;106(47):19952–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocha V, Spellman S, Zhang MJ, Ruggeri A, Purtill D, Brady C, et al. Effect of HLA-matching recipients to donor noninherited maternal antigens on outcomes after mismatched umbilical cord blood transplantation for hematologic malignancy. Biol Biol Blood Marrow Transplant. 2012;18(12): 1890–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peffault de Latour R, Purtill D, Ruggeri A, Sanz G, Michel G, Gandemer V, et al. Influence of nucleated cell dose on overall survival of unrelated cord blood transplantation for patients with severe acquired aplastic anemia: a study by eurocord and the aplastic anemia working party of the European group for blood and marrow transplantation. Biol Blood Marrow Transplant. 2011;17(1):78–85 [DOI] [PubMed] [Google Scholar]
- 28.[No authors listed]. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The Trial to Reduce Alloimmunization to Platelets Study Group. N Engl J Med. 1997; 337(26):1861–9 [DOI] [PubMed] [Google Scholar]


