Abstract
Uterine smooth muscle tumor is very rare in laboratory rats and, there has been no report in the wild rodents. Among a total of 400 wild rats captured in Gyeonggi, Gangwon, and Chungbuk provinces of Korea in 2007, 2010, and 2011, we found a uterine spindle cell tumor, diagnosed as smooth muscle cell origin based on differential features of histology and immunohistochemistry. Its incidence was very low, like in the laboratory rats, as under 0.5% for female. Considering generally applied histological and cellular criteria, this case was difficult in differential diagnosis between benign and malignant. Ki-67 labeling index was therefore further investigated, and it ranged from 26.4 to 37.6% in the 10 different areas, representing an average of 32.9±0.05%. The Ki-67 labeling index of neoplastic cells near the necrotic area was recorded as 83.5%. According to such high Ki-67 labeling index, it was more likely a malignant leiomyosarcoma, assenting to the previous proposal that Ki-67 labeling index is a significant criterion to differentiate between malignant and benign in the smooth muscle tumors.
Keywords: Ki-67, leiomyosarcoma, Rattus norvegicus, rat
Leiomyoma and leiomyosarcoma are respectively benign and malignant tumor originating from smooth muscle cells [1]. They can occur anywhere since smooth muscle is widely dispersed throughout the body, such as the gastrointestinal and genital tracts, respiratory and vascular systems, hair follicle-associated arrector pilae muscle, and the uveal tract of the eye [1]. Smooth muscle tumor is relatively common in dogs, compared to other domestic animals including the cat and the horse. In the dog, leiomyoma frequently occurs in the stomach (25%), vagina and cervix (19%), urinary bladder (9%) and uterus (8%), whereas the small intestine (29%), cecum (15%), spleen (13%), urinary bladder (10%) and stomach (7%) are the frequently affected sites of leiomyosarcoma [1]. In dogs, leiomyosarcoma incidence was 0.5% in the Cornel files from 1977 to 1997 [1].
Smooth muscle tumors are less common in laboratory animals, with the incidence being under only 0.5% in an untreated group in a 2-year carcinogenicity studies in the NTP technical report [2]. Bullock and Curtis reported only 4 (0.01%) cases of leiomyoma and 2 cases (<0.01%) of leiomyosarcoma in 31,868 rats, and Crain (1958) and Bullock and Curtis (1930) reported only 2 cases of leiomyoma and no incidence of leiomyosarcoma in 369 Wistar rats, respectively [3,4].
Leiomyosarcoma is differentiated from the benign smooth muscle tumor, leiomyoma, based on several clinical and pathological criteria such as metastasis, invasion, cellular atypia, relatively high mitotic index, areas of necrosis, and so on [1]. However, despite these criteria, it is difficult in many cases to clearly differentiate leiomyosarcoma from the well-differentiated form of leiomyoma without significant evidence such as metastasis and invasion.
In the present study, we report a case of spontaneious unterine leiomyosarcoma in a wild rat, Rattus norvegicus, which was one of the rats captured around cow or pig farms in some provinces of Korea. Grossly and microscopically, this case was difficult to clearly differentiate between leiomyoma and leiomyosarcoma, but it was finally diagnosed as leiomyosarcoma on the basis of the high labeling index of the cell proliferation marker, Ki-67.
Among the total 400 wild rats captured in Gyeonggi, Gangwon, and Chungbuk provinces of Korea in 2007, 2010, and 2011, a uterine tumor mass was found, representing that its incidence was under 0.5% for female. Grossly, there were masses in both side of the uterus (Figure 1). The mass in the right was whitish in color and rubbery in hardness and 2.5×1.5 cm in size, while the left one was a soft mass containing yellow-whitish cheese-like contents. The cross section of the right mass was homogenously whitish in color without lobulation.
Figure 1.
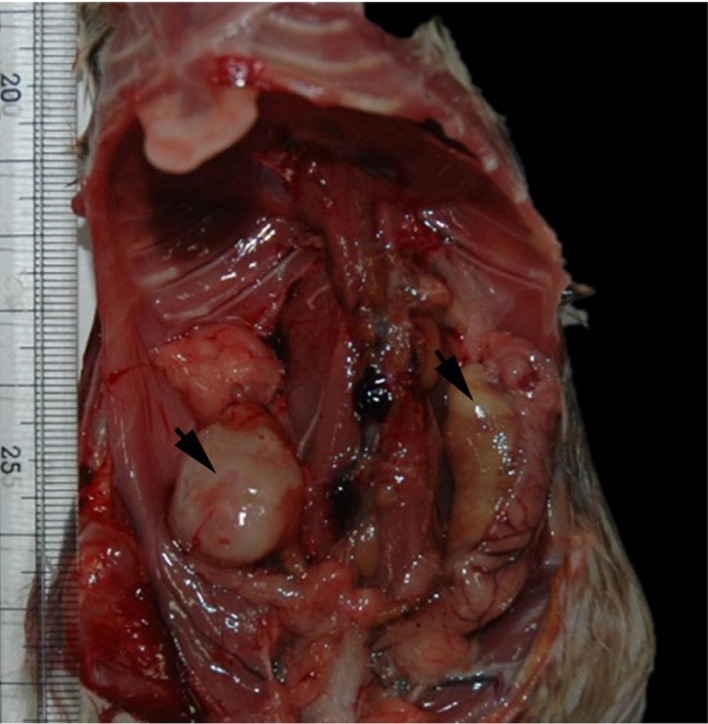
Gross finding of the masses (arrows) in both side of the uterus.
The uterus with the masses was fixed in 10% neutral buffered formalin and then processed into paraffin-embedded tissue blocks. The 4 µm tissue sections were prepared for hematoxylin and eosin staining and microscopic examination. Based on the gross and histological examinations, the mass in the left was defined as an abscess, while the right one was defined as a uterine neoplasm.
To identify the cellular origin of the neoplasm, we performed immunohistochemistry for vimentin, cytokeratin, desmin, smooth muscle actin, and S-100. Briefly, the deparaffinized tissue sections were hydrated and heated in the 0.01 M citrate buffer (pH 6.0) for 20 minutes for antigen retrieval. Thereafter, the tissue sections were immersed in 3% hydrogen peroxide in methanol for 20 minutes at room temperature to block endogenous peroxide activity. After washing, the diluted primary antibodies for mouse monoclonal anti-vimentin (DAKO North America Inc., Carpinteria, CA, USA; 1:100), mouse monoclonal anti-cytokeratin (DAKO North America Inc., Carpinteria, CA, USA; 1:50), mouse monoclonal anti-desmin (CELL MARQUE, Rocklin, CA, USA; 1:100), mouse monoclonal anti-smooth muscle actin (CELL MARQUE, Rocklin, CA, USA; 1:300), and rabbit polyclonal anti-S100 (DAKO North America Inc., Carpinteria, CA, USA; 1:100) were applied to the tissue sections. Thereafter, the tissue sections were incubated with the respective secondary antibodies supplied by the ABC (Avidin-Biotin Complex) kit (Vector Laboratories Inc., Burlingam, CA, USA) for 60 minutes at room temperature. The sections were then visualized by 3,3'-diaminobenzidine and counterstained with Mayer's hematoxylin. To evaluate the cell proliferating activity of the neoplastic cells, we also performed immunohistochemical staining for rabbit monoclonal anti-human Ki-67 (Acris Antibodies GmbH, Herford, Germany; 1:250) according to the same protocol as that of other antigens described above. To determine the Ki-67 labeling index, the positive cells were counted in 10 randomly selected high-power fields (HPF, ×400), and the percentage of positive cells to total counted cell number (positive cells/more than 2,000 neoplastic cells ×100%) was calculated.
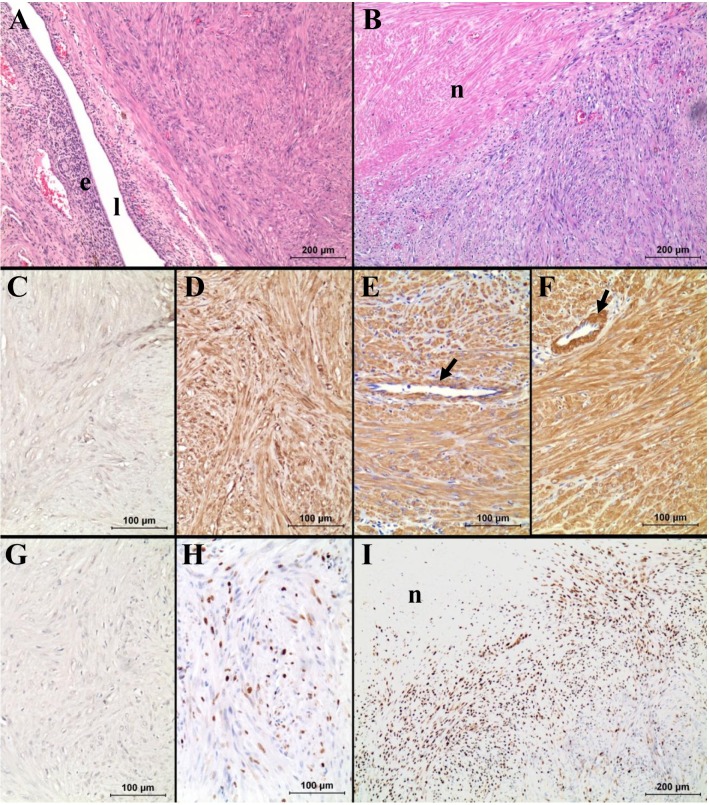
Histologically, the neoplasm was surrounded by comprehensive perimetrial connective tissue. However, it was not clear whether the neoplastic cells are inwardly invading into the endometrium or just compressing the endometrium by growing expansion without invasion. The tumor was composed of uniform spindle shapes of neoplastic cells, irregularly interlacing in different directions, with abundant eosinophilic cytoplasm (Figure 2A). The boundaries between the cells were not distinct. The vesicular nuclei were slightly variable in size and shape with granular chromatin, but they were generally elongated cigar-shaped with blunt ends (Figure 2A). A large necrotic area was present in the central area of the neoplasm (Figure 2B). Mitotic figures were rare (0~2 under ×400 magnification). There was a localized inflammatory area composed of brown-pigmented macrophages, lymphocytes, and plasma cells with multifocal purulent areas. Based on the histological characteristics of neoplastic cells, it was most likely considered as leiomyoma or the well-differentiated type of leiomyosarcoma. Because we could not find any other tumor mass in the animal, it was finally diagnosed as a primary smooth muscle tumor originating from the unterine myometrium. Similar to a previous study [5], uterine smooth muscle tumor was confirmed in our study by positive immunostains for vimentin, desmin and smooth muscle actin and a negative stain for cytokeratin and S-100 (Figure 2).
Figure 2.
Histopathology (A, B) and immunohistochemistry for cytokeratin (C), vimentin (D), desmin (E), smooth muscle actin (F), S-100 (G) and Ki-67 (H, I). Note the strong immunoreactivity of the neoplastic cells for vimentin, desmin and smooth muscle actin, same as the smooth muscle fibers of blood vessels (arrows in E and F). Also note the very high Ki-67 index of the neoplastic cells near the necrotic area ("n" in B and I). In A, "e" and "l" represent endometrium and lumen, respectively.
Histologically, leiomyomas are typically composed of smooth muscle cells with bland, uniform, cigar-shaped nuclei, arranged in interlacing bundles, showing little or no mitotic activity [1,2,6]. On the other hand, typical leiomyosarcoma is a highly cellular tumor composed, at least in some places, of intersecting fascicles of large spindled cells with markedly pleomorphic nuclei; also, multinucleated giant cells are a frequent finding [1,2,6]. Numerous mitotic figures (usually over 10 per 10 HPF) and frequent atypical mitotic figures are also seen [6]. Coagulative tumor cell necrosis, an irregular border and vascular invasion are typically observed [1,2].
However, it was a critical question whether this particular case was benign or malignant. There have been difficulties in many cases in differentiating between leiomyoma and well-differentiated leiomyosarcoma. It has been proposed that invasion, high mitotic index, and tumor necrosis are the factors that allow differentiation [1,2,6]. However, these criteria are not absolutely definitive, such as in this present case. No metastasis was found, and the neoplasm was well-circumscribed by connective tissue. The neoplastic cells were well-differentiated, uniformly similar to the morphology of normal smooth muscle cells without prominent atypia. In addition, mitotic figures were very rare, with the index being no higher than 5 mitoses/10 HPF, which is an average mitotic index of canine leiomyoma [1,7]. These characteristics correspond to the benign smooth muscle tumor, leiomyoma. However, a necrotic area was noted in the central of the tumor (Figure 2B), which is more common in malignancy. In addition, the boundaries between the neoplasm and endometrium were neither distinct nor encapsulated.
Because of the controversy between benign and malignancy in diagnosis, labeling index Ki-67, a cell proliferation marker, has been considered because the labeling index of Ki-67 has been shown to be useful for differential diagnosis in uterine smooth muscle tumors as well as p53 and progesterone receptor [8-11]. According to the previous study, a labeling index of >15% indicated malignancy, although some leiomyosarcoma showed under 10% labeling index [8]. So far, no leiomyoma indicating >30% of labeling index was reported [11]. In the present study, the labeling index of Ki-67 ranged from 26.4% to 37.6%, representing an average of 32.9±0.05% (Figure 2F). The Ki-67 labeling index of neoplastic cells near the necrotic area was very high, as recorded 83.5% (Figure 2I). Thus, this case was more likely to be leiomyosarcoma rather than benign leiomyoma.
Based on the results of histological and immunohistochemical analyses on the mesenchymal cell tumor occurred in the uterus of a wild rat, this case was diagnosed as a smooth muscle tumor on the boundary between benign and malignant in the decision criteria. According to the high Ki-67 labeling index, it was more likely a malignant leiomyosarcoma, assenting to the previous proposal that Ki-67 labeling index is a significant criterion to differentiate between malignant and benign in the smooth muscle tumors on the boundary. The incidence of uterine smooth muscle tumor was very low in the wild rat, similar to that in the laboratory rat.
Acknowledgments
This research was supported by Institute of Veterinary Science, Kangwon National University, Korea.
References
- 1.Cooper BJ, Valentine BA. Tumors of muscles. In: Meuten DJ, editor. Tumor in Domestic Animals. 4th ed. Ames: Iowa State Press; 2002. pp. 319–363. [Google Scholar]
- 2.Joel RL, Micheal PJ. Oviduct, Uterus, and Vagina. In: Boorman GA, Eustis SL, Elwell MR, editors. Pathology of the Fischer Rat - Reference and Atlas. San Diego: Academic Press; 1990. pp. 443–459. [Google Scholar]
- 3.Bullock FD, Curtis MR. Spontaneous Tumors of the Rat. Cancer Res. 1930;14:1–115. [Google Scholar]
- 4.Crain RC. Spontaneous tumors in the Rochester strain of the Wistar rat. Am J Pathol. 1958;34(2):311–335. [PMC free article] [PubMed] [Google Scholar]
- 5.Yi JY, Kim YH, Yoon BI. Primary subcutaneous leiomyosarcoma of the hamster hind leg. J Vet Med Sci. 2008;70(5):517–520. doi: 10.1292/jvms.70.517. [DOI] [PubMed] [Google Scholar]
- 6.Anisimov VN, Nikonov AA. Tumours of the vagina, uterus and oviduct. In: Turusov VS, Mohr U, editors. Pathology of tumours in laboratory animals. volume 1. Tumours of the rat. 2nd ed. Oxford: Oxford University Press; 1990. pp. 445–471. [PubMed] [Google Scholar]
- 7.Johnson GC, Miller MA, Ramos-Vara JA. Comparison of argyrophilic nucleolar organizer regions (AgNORs) and mitotic index in distinguishing benign from malignant canine smooth muscle tumors and in separating inflammatory hyperplasia from neoplastic lesions of the urinary bladder mucosa. J Vet Diagn Invest. 1995;7(1):127–136. doi: 10.1177/104063879500700120. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Yang B. Immunohistochemical analysis of p16, p53, and Ki-67 expression in uterine smooth muscle tumors. Int J Gynecol Pathol. 2008;27(3):326–332. doi: 10.1097/PGP.0b013e31815ea7f5. [DOI] [PubMed] [Google Scholar]
- 9.Mayerhofer K, Lozanov P, Bodner K, Bodner-Adler B, Kimberger O, Czerwenka K. Ki-67 expression in patients with uterine leiomyomas, uterine smooth muscle tumors of uncertain malignant potential (STUMP) and uterine leiomyosarcomas (LMS) Acta Obstet Gynecol Scand. 2004;83(11):1085–1088. doi: 10.1111/j.0001-6349.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 10.Mittal K, Demopoulos RI. MIB-1 (Ki-67), p53, estrogen receptor, and progesterone receptor expression in uterine smooth muscle tumors. Hum Pathol. 2001;32(9):984–987. doi: 10.1053/hupa.2001.27113. [DOI] [PubMed] [Google Scholar]
- 11.O'Neill CJ, McBride HA, Connolly LE, McCluggage WG. Uterine leiomyosarcomas are characterized by high p16, p53 and MIB1 expression in comparison with usual leiomyomas, leiomyoma variants and smooth muscle tumours of uncertain malignant potential. Histopathology. 2007;50(7):851–858. doi: 10.1111/j.1365-2559.2007.02699.x. [DOI] [PubMed] [Google Scholar]