Abstract
Ferulic acid, a component of the plants Angelica sinensis (Oliv.) Diels and Ligusticum chuanxiong Hort, exerts a neuroprotective effect by regulating various signaling pathways. This study showed that ferulic acid treatment prevents the injury-induced increase of collapsin response mediator protein 2 (CRMP-2) in focal cerebral ischemia. Glycogen synthase kinase-3β (GSK-3β) regulates CRMP-2 function through phosphorylation of CRMP-2. Moreover, the pro-apoptotic activity of GSK-3β is inactivated by phosphorylation by Akt. This study investigated whether ferulic acid modulates the expression of CRMP-2 and its upstream targets, Akt and GSK-3β, in focal cerebral ischemia. Male rats were treated immediately with ferulic acid (100 mg/kg, i.v.) or vehicle after middle cerebral artery occlusion (MCAO), and then cerebral cortices were collected 24 hr after MCAO. MCAO resulted in decreased levels of phospho-Akt and phospho-GSK-3β, while ferulic acid treatment prevented the decrease in the levels of these proteins. Moreover, phospho-CRMP-2 and CRMP-2 levels increased during MCAO, whereas ferulic acid attenuated these injury-induced increases. These results demonstrate that ferulic acid regulates the Akt/GSK-3β/CRMP-2 signaling pathway in focal cerebral ischemic injury, thereby protecting against brain injury.
Keywords: Ferulic acid, neuroprotection, Akt, GSK-3β, CRMP-2
Ferulic acid (4-hydroxy-3-methoxycinnamic acid) is a well-known phenolic compound present in Angelica sinensis (Oliv.) Diels and Ligusticum chuanxiong Hort, which are plants used to treat stroke in traditional Chinese medicine. Ferulic acid has potent antioxidant and anti-aging properties [1-3]. Ferulic acid exerts beneficial and biological effects against various diseases such as cancer, diabetes, and neurodegenerative diseases [3-5]. Ferulic acid reduces focal cerebral ischemic injury by modulating the expression of apoptosis-related proteins and regulating inflammatory reactions [6-9]. Moreover, ferulic acid protects cortical neuronal cells against glutamate toxicity and modulates the phosphatidylinositol 3-kinase (PI3K) and extracellular signal-regulated kinase (ERK) pathways [10].
The PI3K/Akt pathway is an important signaling pathway involved in cell growth, cell survival, apoptosis, and metabolism [11,12]. Activation of the PI3K/Akt pathway suppresses neuronal cell death from cerebral ischemia and enhances cells survival [13-15]. Akt phosphorylates several pro-apoptotic proteins, such as Bad and glycogen synthase kinase-3β (GSK-3β), resulting in inactivation of these apoptotic proteins [16]. GSK-3β increases caspase-3 activity and induces apoptotic cell death after transient global ischemia; however, its pro-apoptotic activity is inhibited by phosphorylation by Akt [16,17]. Moreover, GSK-3β phosphorylates its downstream target, collapsin response mediator protein 2 (CRMP-2). CRMP-2 is a microtubule-binding protein that enhances microtubule polymerization and stabilization. CRMP-2 abundantly exists in the growing axons of hippocampal neurons and mediates neuronal differentiation and growth. The ability of CRMP-2 to bind to microtubules is suppressed by phosphorylation [18,19,20]. GSK-3β phosphorylates CRMP-2, thereby inhibiting microtubule polymerization and stabilization, which in turn inhibits axonal elongation [21,22]. We previously reported that ferulic acid has a neuroprotective effect against focal cerebral ischemia [8,9]. In this study, we demonstrate that ferulic acid attenuates the focal cerebral ischemia-induced increase in CRMP-2 using a proteomics approach. We hypothesize that ferulic acid exerts its effects by modulating the Akt/GSK3β/CRMP-2 pathway in ischemic brain injury. Thus, we also investigated the phosphorylation of Akt, GSK3β, and CRMP-2 in response to ferulic acid treatment in a focal cerebral ischemia.
Materials and Methods
Animals and ferulic acid treatment
Male Sprague-Dawley rats (210-230 g, n=40) were housed under temperature (25℃) and lighting (12/12 light/dark cycle). All experimental protocols for animal use were approved by Institutional Animal Care and Use Committee at Gyeongsang National University (GNU-120806-R0034). Animals were randomly divided four groups as follows: vehicle+sham group, ferulic acid+sham group, vehicle+middle cerebral artery occlusion (MCAO) group, and ferulic acid+MCAO group (n=10 per group). Ferulic acid (Sigma, St. Louis, MO, USA) was dissolved in normal saline as the vehicle. Ferulic acid (100 mg/kg) or vehicle was injected intravenously immediately after MCAO [6].
Middle cerebral artery occlusion
The focal cerebral ischemia model was generated by operating middle cerebral artery occlusion (MCAO), as a previously described with some modification [23]. Animals were anesthetized with sodium pentobarbital (30 mg/kg, intraperitoneal injection) before MCAO. Briefly, the right common carotid artery was exposed and all branches of external carotid artery were isolated. The common carotid artery were clamped with vascular clips, and the external carotid artery was cut. A 4/0 nylon filament with rounded tip by heating was inserted into the right external carotid artery and was carefully advanced into the internal carotid artery. The filament was advanced until the origin of the right MCA. The body temperature was maintained at approximately 37℃ using a heating pad. Sham-operated animals were received the same surgical procedure without a nylon filament insertion. The right cortices were isolated at 24 hr after MCAO.
Two-dimensional gel electrophoresis, image analysis, and protein identification
A proteomic approach was carried out as a previously described method [9]. The right cerebral cortices were dissolved in lysis buffer (8 M urea, 4% CHAPS, ampholytes and 40 mM Tris-HCl) and centrifuged at 16,000 g for 20 min at 4℃. The supernatant was isolated and Bradford assay (Bio-Rad, Hercules, CA, USA) was used to determine protein concentration. Total proteins (50 µg) were mixed with rehydration solution and isoelectric focusing (IEF) was performed for 13 hr using the immobilized pH gradients gel strips (IPG, pH 4-7, pH 6-9, 17 cm, Bio-Rad). The protein samples were focused on Protean IEF Cell (Bio-Rad) at 20℃ as follows: 250 V (15 min), 10000 V (3 hr), and then 10,000 V to 50,000 V. After IEF, electrophoresis was performed with gradient gel (7.5-17.5%) at 10 mA/gel for 10 hr (Protein-XI, Bio-Rad). The gels were stained with silver stain solution (0.2% silver nitrate, 0.75 mL/L formaldehyde) and were scanned using Agfar ARCUS 1200™ (Agfar-Gevaert, Mortsel, BEL). The PDQuest 2-D analysis software (Bio-Rad) was matched, analyzed, and visualized protein spots. Protein spots were subjected to trypsin digestion and peptides were extracted using extraction buffer (5% trifluoroacetic acid in 50% acetonitrile) and the extracted peptides were dried using a vacuum centrifuge for 20 min. The protein samples were analyzed by a Voyager-DE™ STR biospectrometry workstation (Applied Biosystem, Forster City, CA, USA). The database searches were carried out using MS-Fit and ProFound program. SWISS-PROT and NCBI were used as the protein sequence databases.
Western blot analysis
The right cerebral cortices were quickly extracted and frozen in liquid nitrogen. The samples were homogenized in lysis buffer [1% Triton X-100, 1 mM EDTA in 1xPBS (pH 7.4)] containing 10 mM leupeptin and 200 µM phenylmethylsulfonyl fluoride, were centrifuged at 15,000 g for 20 min at 4℃. The supernatants were collected and total protein concentration was measured. Total protein (30 µg) from each sample was run on a 10% SDS-polyacrylamide gel electrophoresis. The gels were transferred on the poly-vinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% skim milk solution and washed in Tris-buffered saline containing 0.1% Tween-20 (TBST), and then incubated with the following antibodies : anti-Akt, anti-phopho-Akt (Ser 473), anti-GSK-3β, anti-phopho-GSK-3β (Ser 9), anti-CRMP-2, anti-phospho-CRMP-2 (Thr 514) (diluted 1:1000, Cell Signaling Technology, Beverly, MA, USA), and anti-3β-actin (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) as primary antibody. After washing with TBST, the membrane was incubated with horseradish perxoxidase-conjugated goat anti-rabbit IgG (1:5000, Pierce, Rockford, IL, USA) and the signals were detected with an enhanced chemiluminescence Western blot detection kit (GE Healthcare, Little Chalfont, Buckinghamshire, UK) according to the manufacturer's manuals. The intensity analysis of Western blot analysis was carried out using SigmaGel 1.0 (Jandel Scientific, San Rafael, CA, USA) and SigmaPlot 4.0 (SPSS Inc., Point Richmond, CA, USA).
Statistical analysis
All data are expressed as mean±SEM. The results in each group were compared by one-way analysis of variance (ANOVA) followed by to post-hoc Scheffe's test. The difference for comparison was considered significant at *P<0.05.
Results
Levels of CRMP-2 in vehicle- and ferulic acid-treated animals
We previously reported the neuroprotective effects of ferulic acid against focal ischemic brain injury [8]. Ferulic acid treatment decreased infarct volume and brain damage in response to MCAO [8]. In this study, using a proteomics approach, we identified an increase in expression of CRMP-2 in the cerebral cortices of vehicle+MCAO animals (Figure 1). Ferulic acid treatment attenuated the MCAO-induced increase in CRMP-2 protein expression. CRMP-2 levels were 1.53±0.03 and 1.10±0.02 in the vehicle+MCAO and ferulic acid+MCAO animals, respectively.
Figure 1.
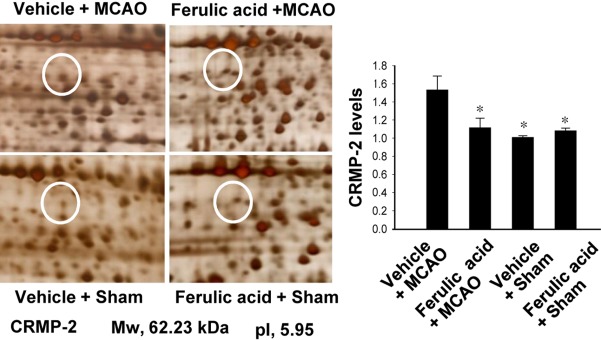
Collapsin response mediator protein 2 (CRMP-2) protein spots identified by MALDI-TOF in the cerebral cortex from vehicle+middle cerebral artery occlusion (MCAO), ferulic acid+MCAO, vehicle+sham, ferulic acid+sham animals. Circles indicate the CRMP-2 protein spots. The intensity of spots was measured using PDQuest software. The ratio of intensity is described as spots intensity of these animals to spots intensity of sham+vehicle animals. Data (n=5) are shown as mean±SEM. *P<0.05. Mw and IP indicate molecular weight and isoelectrical point, respectively.
Levels of phospho-Akt and phospho-GSK-3β in vehicle- and ferulic acid-treated animals
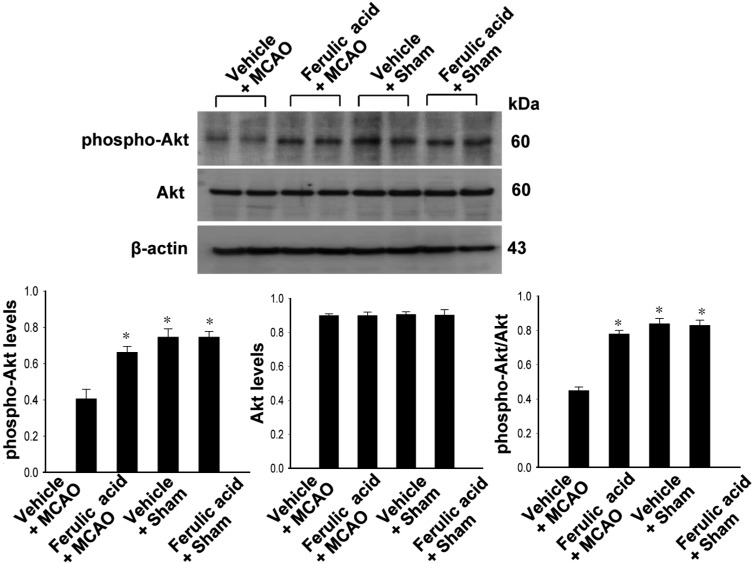
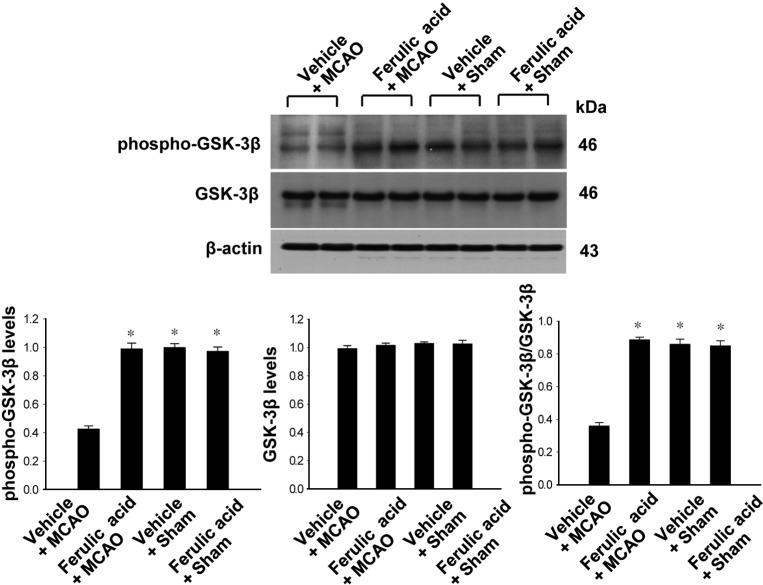
Figures 2 and 3 showed the levels of phosphorylated Akt and GSK-3β in the cerebral cortex of the MCAO group treated with ferulic acid or vehicle. MCAO injury resulted in decreased phospho-Akt and phospho-GSK-3β expression, while ferulic acid treatment attenuated the injury-induced decreases in the levels of these proteins. Phospho-Akt levels were 0.41±0.04 and 0.66±0.03 in the vehicle+MCAO and ferulic acid+MCAO animals, respectively (Figure 2). Moreover, phospho-GSK-3β levels were 0.43±0.02 and 0.99±0.04 in the vehicle+MCAO and ferulic acid+MCAO animals, respectively (Figure 3). However, these proteins were expressed at similar levels in sham-operated animals treated with vehicle or ferulic acid. Total Akt and GSK-3β levels were similar in the vehicle- and ferulic acid-treated animals during MCAO (Figure 2, 3).
Figure 2.
Western blot analysis of phospho-Akt and Akt in the cerebral cortex from vehicle+middle cerebral artery occlusion (MCAO), ferulic acid+MCAO, vehicle+sham, ferulic acid+sham animals. Each lane represents an individual experimental animal. Densitometric analysis is represented as these proteins intensity to β-actin intensity. Molecular weight (kDa) is depicted at right. Data (n=5) are represented as mean±SEM. *P<0.05.
Figure 3.
Western blot analysis of phospho-GSK-3β and GSK-3β in the cerebral cortex from vehicle + middle cerebral artery occlusion (MCAO), ferulic acid+MCAO, vehicle+sham, ferulic acid+sham animals. Each lane represents an individual experimental animal. Densitometric analysis is represented as these proteins intensity to β-actin intensity. Molecular weight (kDa) is depicted at right. Data (n=5) are represented as mean±SEM. *P<0.05.
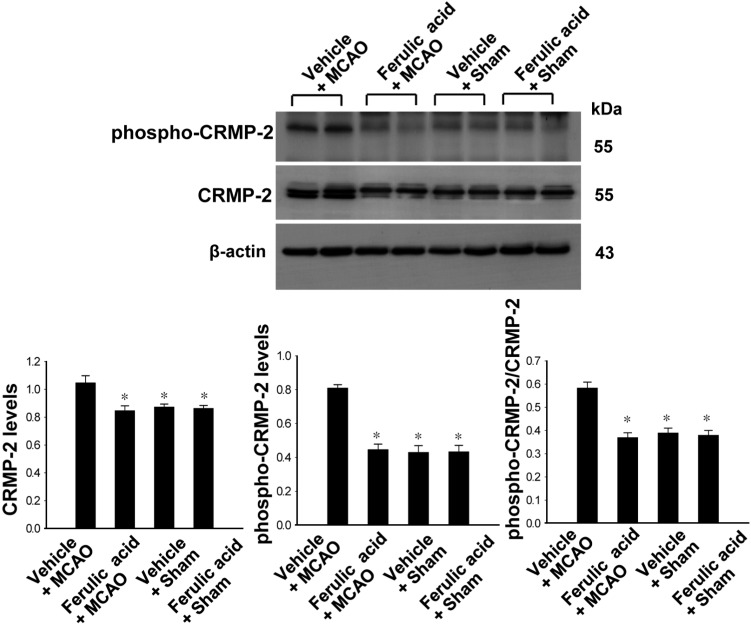
Levels of phospho-CRMP-2 and CRMP-2 in vehicle- and ferulic acid-treated animals
We confirmed CRMP-2 expression in ischemic brain injury using Western blot analysis. CRMP-2 is a downstream target of GSK-3β. CRMP-2 expression was increased in vehicle+MCAO animals, whereas the increase in CRMP-2 levels was attenuated in ferulic acid+MCAO animals (Figure 4). CRMP-2 levels were 1.05±0.05 and 0.84±0.03 in the vehicle+MCAO and ferulic acid+MCAO animals, respectively. Moreover, phospho-CRMP-2 levels were increased in the vehicle+MCAO-operated groups, while ferulic acid treatment prevented the MCAO-induced increase in the phospho-CRMP-2 level. Phospho-CRMP-2 levels were 0.81±0.02 and 0.44±0.03 in the vehicle+MCAO and ferulic acid+MCAO animals, respectively. In sham-operated animals, CRMP-2 and phospho-CRMP-2 proteins levels in the vehicle-treated groups were similar to those in the ferulic acid-treated groups.
Figure 4.
Western blot analysis of phospho-CRMP-2 and CRMP-2 in the cerebral cortex from vehicle+middle cerebral artery occlusion (MCAO), ferulic acid+MCAO, vehicle+sham, ferulic acid+sham animals. Each lane represents an individual experimental animal. Densitometric analysis is represented as these proteins intensity to β-actin intensity. Molecular weight (kDa) is depicted at right. Data (n=5) are represented as mean±SEM. *P<0.05.
Discussion
Ferulic acid plays a neuroprotective role against transient focal cerebral ischemia through its anti-oxidant and anti-apoptotic effects [6,7]. We previously confirmed that ferulic acid reduces brain damages and prevents neuronal cell death by modulating various signaling pathways [8,9]. In the present study, we demonstrated that ferulic acid regulates Akt and its downstream target, GSK-3β.
GSK-3β is a serine/threonine protein kinase and a critical downstream target of the Akt signaling pathway. The phosphorylation of GSK-3β by Akt inactivates its pro-apoptotic activity, leads to inhibition of apoptosis. We found that focal cerebral ischemia induced a decrease in phospho-GSK-3β levels and that ferulic acid attenuated this decrease in phospho-GSK-3β. Ferulic acid also prevented the brain injury-induced decrease in expression of phospho-Akt, a protein upstream of GSK-3β. We recently reported that ferulic acid exerts a neuroprotective effect by activating the Akt signaling pathway [8]. In this study, we focused on GSK-3β expression in cerebral ischemic injury. Ferulic acid prevented brain injury-induced decreases in phospho-Akt and phospho-GSK-3β levels. However, total Akt and GSK-3β levels were constitutively maintained in cerebral ischemia regardless of ferulic acid treatment. In transient global ischemia, GSK-3β leads to cell injury by increasing caspase-3 activity [17]. We previously showed that ferulic acid attenuated the MCAO-induced activation of caspase-3 [8]. To be more precise, caspase-3 expression increases during focal cerebral ischemia and ferulic acid treatment attenuates this injury-induced increase in caspase-3. A decrease in caspase-3 activity leads to inhibition of apoptotic cell death. Phosphorylation of GSK-3β is a critical event for the inhibition of apoptotic cell death. We clearly showed in this study that ferulic acid attenuated the injury-induced decrease in phospho-GSK-3β expression. The maintenance of phospho-GSK-3β levels by ferulic acid contribute to the inactivation of caspase-3.
Phosphorylation of CRMP-2 by GSK-3β inactivates CRMP-2 and allows it to react with neurofibrillary tangles [24]. CRMP-2 can contribute to neuronal polarity and axonal elongation [22,25]. Moreover, CRMP-2 also participates in the pathophysiology of neurological disorders. CRMP-2 is overexpressed in cerebral cortex-induced ischemic brain injury, and upregulation of CRMP-2 levels indicates neuronal cell dysfunction [26]. Levels of phosphorylated CRMP-2 are very high in Alzheimer's disease [24]. We found that ischemic brain injury increased in CRMP-2 levels while ferulic acid treatment maintained CRMP-2 expression at a similar level to that observed in sham-operated animals. Moreover, phosphorylation of CRMP-2 increased in response to cerebral ischemic injury, while ferulic acid treatment attenuated this injury-induced increase in the level of phospho-CRMP-2. The phosphorylation of CRMP-2 decreases its ability to bind to tubulin and inhibits neurite elongation [20]. Thus, the increase in levels of phospho-CRMP-2 during MCAO injury leads to the destruction of cells. The Akt/GSK-3β/CRMP-2 signaling pathway is a critical step for neuroprotective mechanism. We demonstrated that ferulic acid attenuated the MCAO injury-induced decrease in the level of phospho-GSK-3β and the MCAO injury-induced increase in the level of phospho-CRMP-2. Thus, ferulic acid exerts its neuroprotective effects against focal cerebral ischemic injury by modulating the Akt/GSK-3β/CRMP-2 signaling pathway.
Acknowledgments
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MEST)(No.2010-0007881).
References
- 1.Sultana R, Ravagna A, Mohmmad-Abdul H, Calabrese V, Butterfield DA. Ferulic acid ethyl ester protects neurons against amyloid beta-peptide(1-42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. J Neurochem. 2005;92(4):749–758. doi: 10.1111/j.1471-4159.2004.02899.x. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR, Menon VP. Influence of ferulic acid on gamma-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes. Toxicology. 2006;228(2-3):249–258. doi: 10.1016/j.tox.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan M, Sudheer AR, Menon VP. Ferulic Acid: therapeutic potential through its antioxidant property. Ferulic Acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr. 2007;40(2):92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawabata K, Yamamoto T, Hara A, Shimizu M, Yamada Y, Matsunaga K, Tanaka T, Mori H. Modifying effects of ferulic acid on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Lett. 2000;157(1):15–21. doi: 10.1016/s0304-3835(00)00461-4. [DOI] [PubMed] [Google Scholar]
- 5.Ohnishi M, Matuo T, Tsuno T, Hosoda A, Nomura E, Taniguchi H, Sasaki H, Morishita H. Antioxidant activity and hypoglycemic effect of ferulic acid in STZ-induced diabetic mice and KK-Ay mice. Biofactors. 2004;21(1-4):315–319. doi: 10.1002/biof.552210161. [DOI] [PubMed] [Google Scholar]
- 6.Cheng CY, Ho TY, Lee EJ, Su SY, Tang NY, Hsieh CL. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am J Chin Med. 2008;36(6):1105–1119. doi: 10.1142/S0192415X08006570. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CY, Su SY, Tang NY, Ho TY, Chiang SY, Hsieh CL. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008;1209:136–150. doi: 10.1016/j.brainres.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 8.Koh PO. Ferulic acid prevents the cerebral ischemic injury-induced decrease of Akt and Bad phosphorylation. Neurosci Lett. 2012;507(2):156–160. doi: 10.1016/j.neulet.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Koh PO. Ferulic acid prevents the cerebral ischemic injury-induced decreases of astrocytic phosphoprotein PEA-15 and its two phosphorylated forms. Neurosci Lett. 2012;511(2):101–105. doi: 10.1016/j.neulet.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y, Yan EZ, Fan Y, Guo XL, Zhao YJ, Zong ZH, Liu Z. Neuroprotection by sodium ferulate against glutamate-induced apoptosis is mediated by ERK and PI3 kinase pathways. Acta Pharmacol Sin. 2007;28(12):1881–1890. doi: 10.1111/j.1745-7254.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- 11.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–2907. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 12.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29(5):233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Janelidze S, Hu BR, Siesjö P, Siesjö BK. Alterations of Akt1 (PKBalpha) and p70(S6K) in transient focal ischemia. Neurobiol Dis. 2001;8(1):147–154. doi: 10.1006/nbdi.2000.0325. [DOI] [PubMed] [Google Scholar]
- 14.Noshita N, Lewén A, Sugawara T, Chan PH. Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21(12):1442–1450. doi: 10.1097/00004647-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Shibata M, Yamawaki T, Sasaki T, Hattori H, Hamada J, Fukuuchi Y, Okano H, Miura M. Upregulation of Akt phosphorylation at the early stage of middle cerebral artery occlusion in mice. Brain Res. 2002;942(1-2):1–10. doi: 10.1016/s0006-8993(02)02474-5. [DOI] [PubMed] [Google Scholar]
- 16.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 17.Brywe KG, Mallard C, Gustavsson M, Hedtjärn M, Leverin AL, Wang X, Blomgren K, Isgaard J, Hagberg H. IGF-I neuroprotection in the immature brain after hypoxia-ischemia, involvement of Akt and GSK3beta? Eur J Neurosci. 2005;21(6):1489–1502. doi: 10.1111/j.1460-9568.2005.03982.x. [DOI] [PubMed] [Google Scholar]
- 18.Brown M, Jacobs T, Eickholt B, Ferrari G, Teo M, Monfries C, Qi RZ, Leung T, Lim L, Hall C. Alpha2-chimaerin, cyclin-dependent Kinase 5/p35, and its target collapsin response mediator protein-2 are essential components in semaphorin 3A-induced growth-cone collapse. J Neurosci. 2004;24(41):8994–9004. doi: 10.1523/JNEUROSCI.3184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole AR, Knebel A, Morrice NA, Robertson LA, Irving AJ, Connolly CN, Sutherland C. GSK-3 phosphorylation of the Alzheimer epitope within collapsin response mediator proteins regulates axon elongation in primary neurons. J Biol Chem. 2004;279(48):50176–50180. doi: 10.1074/jbc.C400412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120(1):137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Zumbrunn J, Kinoshita K, Hyman AA, Näthke IS. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr Biol. 2001;11(1):44–49. doi: 10.1016/s0960-9822(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 22.Fukata Y, Itoh TJ, Kimura T, Ménager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H, Kaibuchi K. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 2002;4(8):583–591. doi: 10.1038/ncb825. [DOI] [PubMed] [Google Scholar]
- 23.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 24.Gu Y, Hamajima N, Ihara Y. Neurofibrillary tangle-associated collapsin response mediator protein-2 (CRMP-2) is highly phosphorylated on Thr-509, Ser-518, and Ser-522. Biochemistry. 2000;39(15):4267–4275. doi: 10.1021/bi992323h. [DOI] [PubMed] [Google Scholar]
- 25.Inagaki N, Chihara K, Arimura N, Ménager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4(8):781–782. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- 26.Chen A, Liao WP, Lu Q, Wong WS, Wong PT. Upregulation of dihydropyrimidinase-related protein 2, spectrin alpha II chain, heat shock cognate protein 70 pseudogene 1 and tropomodulin 2 after focal cerebral ischemia in rats--a proteomics approach. Neurochem Int. 2007;50(7-8):1078–1086. doi: 10.1016/j.neuint.2006.11.008. [DOI] [PubMed] [Google Scholar]