Abstract
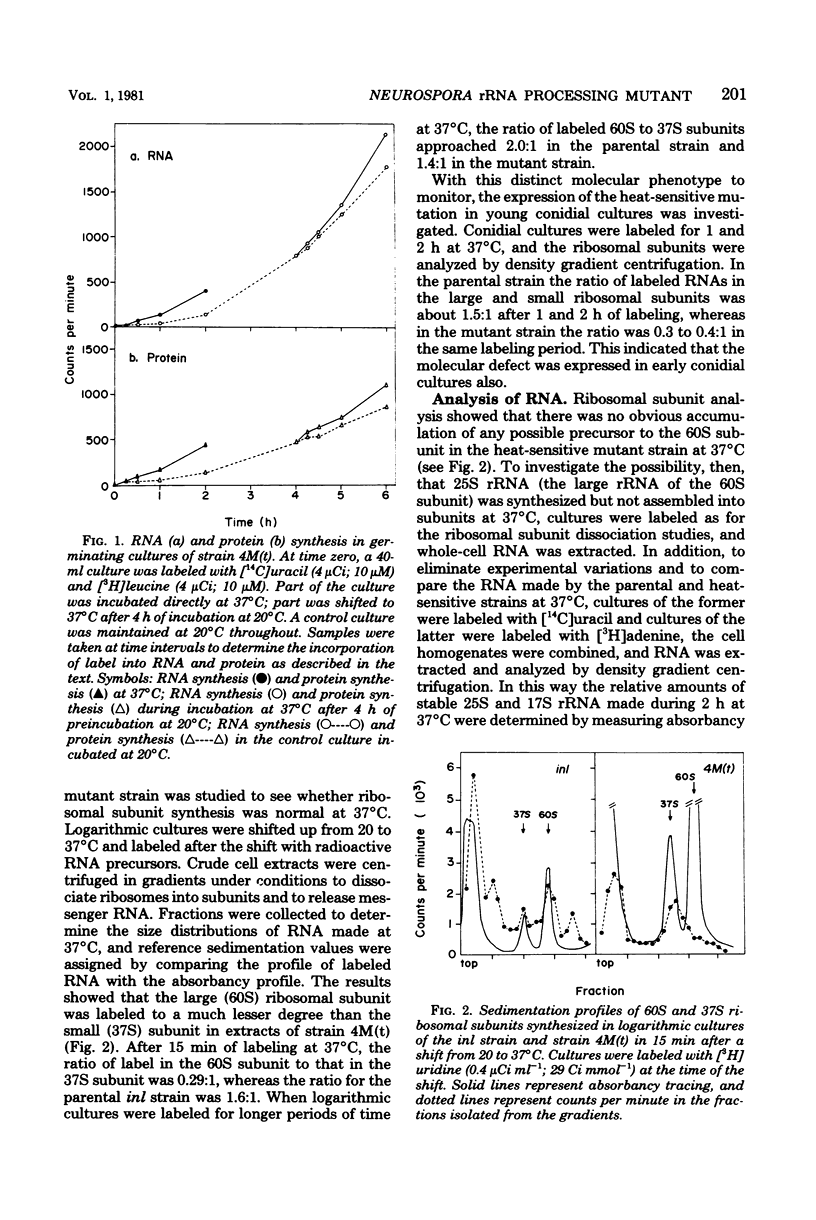
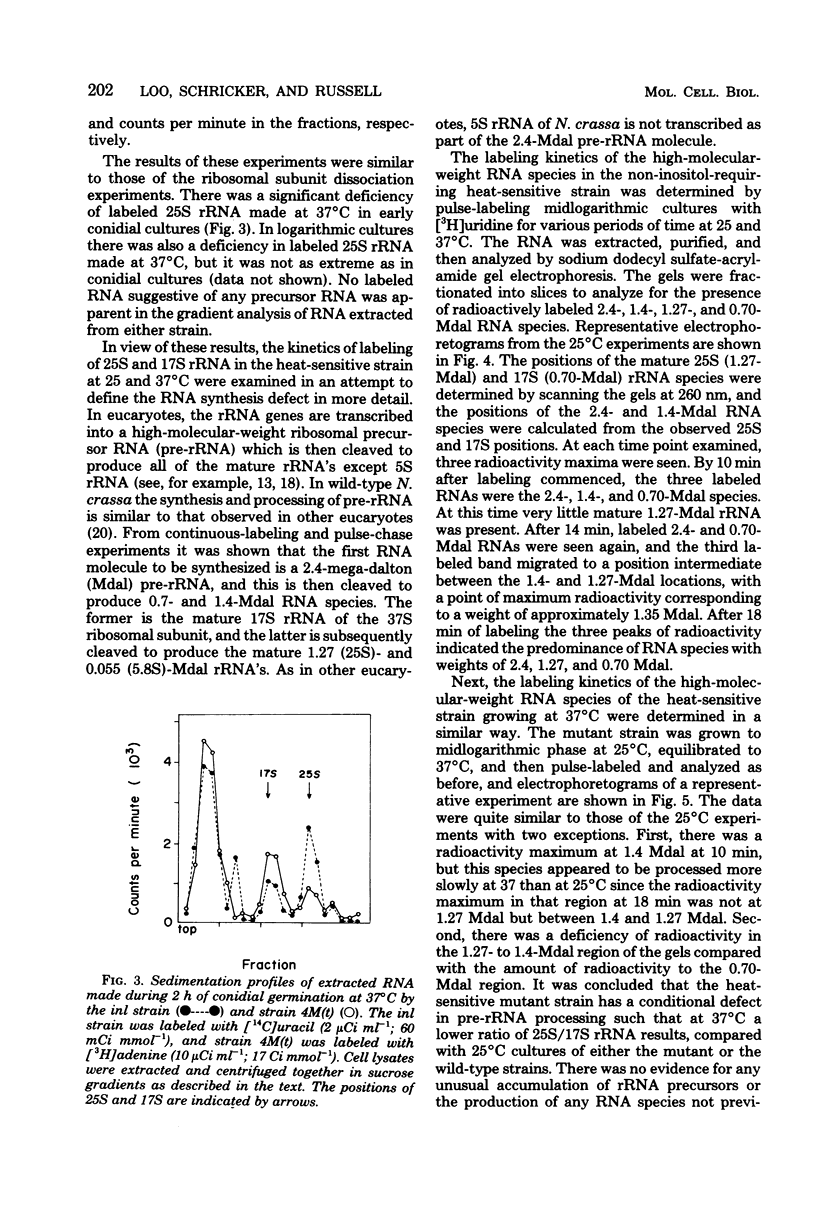
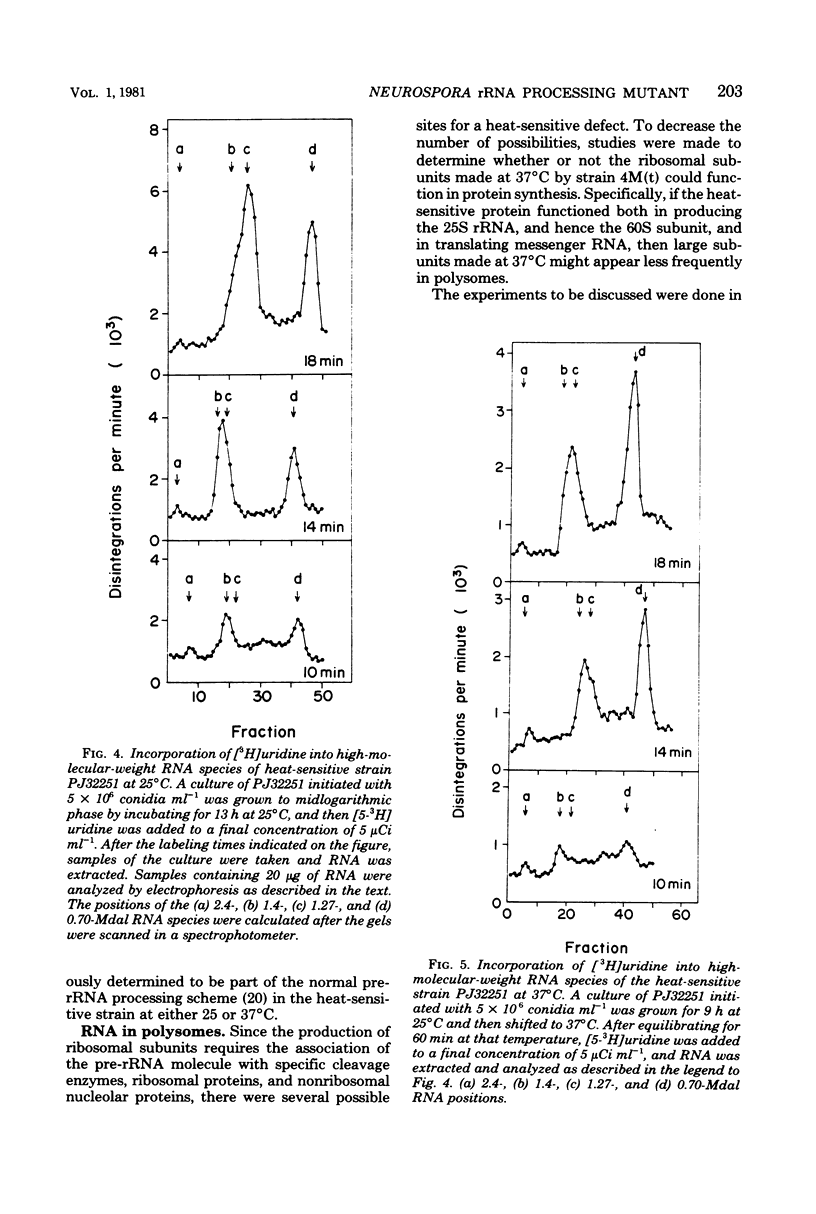
A heat-sensitive mutant strain of Neurospora crassa, 4M(t), was studied in an attempt to define its molecular lesion. The mutant strain is inhibited in conidial germination and mycelial extension at the nonpermissive temperature (37 degrees C). Macromolecular synthesis studies showed that both ribonucleic acid (RNA) and protein syntheses are inhibited when 4-h cultures are shifted from 20 to 37 degrees C. Density gradient analysis of ribosomal subunits made at 37 degrees C indicated that strain 4M(t) is deficient in the accumulation of 60S ribosomal subunits in that the ratio of 60S/37S subunits was 0.29:1 compared with 1.6:1 for the parental strain. This phenotype was shown to be the result of a slow rate of processing of, and a deficiency in the amount of, the immediate precursor to 25S ribosomal RNA (the large RNA of the 60S subunit) in the sequence of events constituting the production of mature ribosomal RNAs from the primary transcript of the ribosomal deoxyribonucleic acid, the precursor ribosomal RNA molecule. Analysis of polysomes suggested that the heat-sensitive gene product might function in both the assembly and the function of the 60S ribosomal subunit, since there was a smaller proportion of newly made 60S subunits synthesized at 37 degrees C in the polysome region of the gradients than in the monosome-plus-subunit region. The ribosomal RNA processing defect is apparently responsible for the observed defects in germination and macromolecular synthesis at 37 degrees C, but the precise molecular lesion is not known. On the basis of these results, the heat-sensitive mutant allele in the 4M(t) strain is considered to define the rip1 (ribosome production) gene locus.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew C., Hopper A. K., Hall B. D. A yeast mutant defective in the processing of 27S r-RNA precursor. Mol Gen Genet. 1976 Feb 27;144(1):29–37. doi: 10.1007/BF00277300. [DOI] [PubMed] [Google Scholar]
- Brand R. C., Planta R. J. The molecular weights of yeast ribosomal precursor RNAs. Mol Biol Rep. 1975 Dec;2(4):321–325. doi: 10.1007/BF00357019. [DOI] [PubMed] [Google Scholar]
- Brown D. D. The genes for ribosomal RNA and their transcription during amphibian development. Curr Top Dev Biol. 1967;2:47–73. doi: 10.1016/s0070-2153(08)60283-5. [DOI] [PubMed] [Google Scholar]
- Burdon R. H. Ribonucleic acid maturation in animal cells. Prog Nucleic Acid Res Mol Biol. 1971;11:33–79. doi: 10.1016/s0079-6603(08)60325-6. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Kohne D. E., Dutta S. K. Ribosomal RNA genes of Neurospora: isolation and characterization. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3256–3259. doi: 10.1073/pnas.69.11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B., Wellauer P. K. A reinvestigation of 5' leads to 3' polarity in 40S ribosomal RNA precursor of Xenopus laevis. Cell. 1976 Jul;8(3):443–448. doi: 10.1016/0092-8674(76)90157-4. [DOI] [PubMed] [Google Scholar]
- Klootwijk J., de Jonge P., Planta R. J. The primary transcript of the ribosomal repeating unit in yeast. Nucleic Acids Res. 1979 Jan;6(1):27–39. doi: 10.1093/nar/6.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumlauf R., Marzluf G. A. Characterization of the sequence complexity and organization of the Neurospora crassa genome. Biochemistry. 1979 Aug 21;18(17):3705–3713. doi: 10.1021/bi00584a011. [DOI] [PubMed] [Google Scholar]
- LESTER H. E., GROSS S. R. Efficient method for selection of auxotrophic mutants of Neurospora. Science. 1959 Feb 27;129(3348):572–572. doi: 10.1126/science.129.3348.572. [DOI] [PubMed] [Google Scholar]
- Liau M. C., Hurlbert R. B. The topographical order of 18 S and 20 S ribosomal ribonucleic acids within the 45 S precursor molecule. J Mol Biol. 1975 Oct 25;98(2):321–332. doi: 10.1016/s0022-2836(75)80121-5. [DOI] [PubMed] [Google Scholar]
- Loo M. Neurospora crassa temperature-sensitive mutant apparently defective in protein synthesis. J Bacteriol. 1975 Jan;121(1):286–295. doi: 10.1128/jb.121.1.286-295.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden B. E., Salim M. The methylated nucleotide sequences in HELA cell ribosomal RNA and its precursors. J Mol Biol. 1974 Sep 5;88(1):133–152. doi: 10.1016/0022-2836(74)90299-x. [DOI] [PubMed] [Google Scholar]
- Mirkes P. E., McCalley B. Synthesis of polyadenylic acid-containing ribonucleic acid during the germination of Neurospora crassa conidia. J Bacteriol. 1976 Jan;125(1):174–180. doi: 10.1128/jb.125.1.174-180.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkes P. E. Polysomes, ribonucleic acid, and protein synthesis during germination of Neurospora crassa conidia. J Bacteriol. 1974 Jan;117(1):196–202. doi: 10.1128/jb.117.1.196-202.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev N., Georgiev O. I., Venkov P. V., Hadjiolov A. A. The 37 S precursor to ribosomal RNA is the primary transcript of ribosomal RNA genes in Saccharomyces cerevisiae. J Mol Biol. 1979 Jan 25;127(3):297–308. doi: 10.1016/0022-2836(79)90331-0. [DOI] [PubMed] [Google Scholar]
- Perry R. P. Processing of RNA. Annu Rev Biochem. 1976;45:605–629. doi: 10.1146/annurev.bi.45.070176.003133. [DOI] [PubMed] [Google Scholar]
- Rungger D., Crippa M. The primary ribosomal DNA transcript in eukaryotes. Prog Biophys Mol Biol. 1977;31(3):247–269. doi: 10.1016/0079-6107(78)90010-x. [DOI] [PubMed] [Google Scholar]
- Russell P. J., Hammett J. R., Selker E. U. Neurospora crassa cytoplasmic ribosomes; ribosomal ribonucleic acid synthesis in the wild type. J Bacteriol. 1976 Aug;127(2):785–793. doi: 10.1128/jb.127.2.785-793.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlitt S. C., Russell P. J. Neurospora crassa cytoplasmic ribosomes: isolation and characterization of a cold-sensitive mutant defective in ribosome biosynthesis. J Bacteriol. 1974 Nov;120(2):666–671. doi: 10.1128/jb.120.2.666-671.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siev M., Weinberg R., Penman S. The selective interruption of nucleolar RNA synthesis in HeLa cells by cordycepin. J Cell Biol. 1969 May;41(2):510–520. doi: 10.1083/jcb.41.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniolo D., Basilico C. Processing of ribosomal RNA in a temperature sensitive mutant of BHK cells. Biochim Biophys Acta. 1976 Apr 2;425(4):409–418. doi: 10.1016/0005-2787(76)90005-8. [DOI] [PubMed] [Google Scholar]
- Toniolo D., Meiss H. K., Basilico C. A temperature-sensitive mutation affecting 28S ribosomal RNA production in mammalian cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1273–1277. doi: 10.1073/pnas.70.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapman J., Planta R. J. Detailed analysis of the ribosomal RNA synthesis in yeast. Biochim Biophys Acta. 1975 Dec 4;414(2):115–125. doi: 10.1016/0005-2787(75)90214-2. [DOI] [PubMed] [Google Scholar]
- Udem S. A., Warner J. R. Ribosomal RNA synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972 Mar 28;65(2):227–242. doi: 10.1016/0022-2836(72)90279-3. [DOI] [PubMed] [Google Scholar]
- Udem S. A., Warner J. R. The cytoplasmic maturation of a ribosomal precursor ribonucleic acid in yeast. J Biol Chem. 1973 Feb 25;248(4):1412–1416. [PubMed] [Google Scholar]
- van den Bos R. C., Retèl J., Planta R. J. The size and the location of the ribosomal RNA segments in ribosomal precursor RNA of yeast. Biochim Biophys Acta. 1971 Mar 25;232(3):494–508. doi: 10.1016/0005-2787(71)90603-4. [DOI] [PubMed] [Google Scholar]



