Abstract
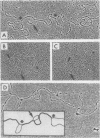
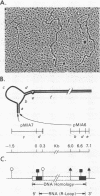
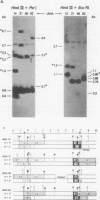
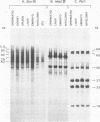
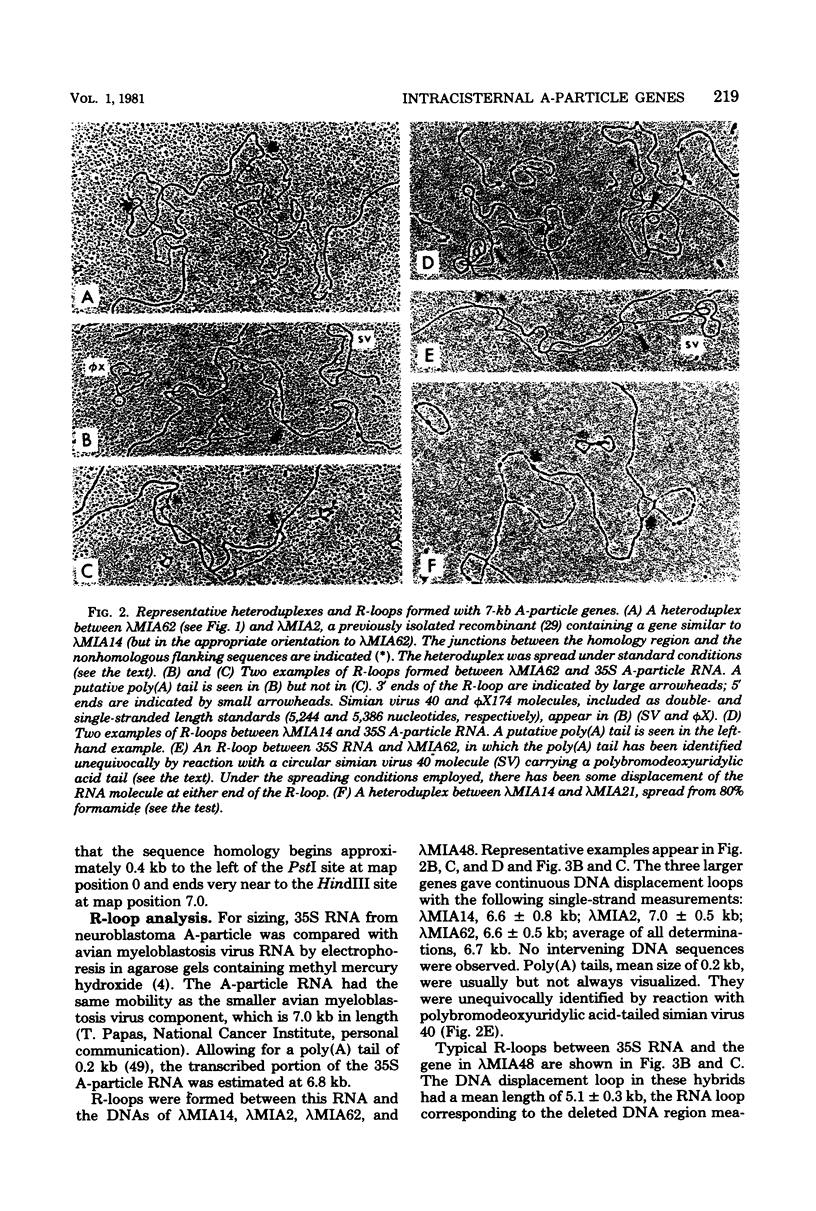
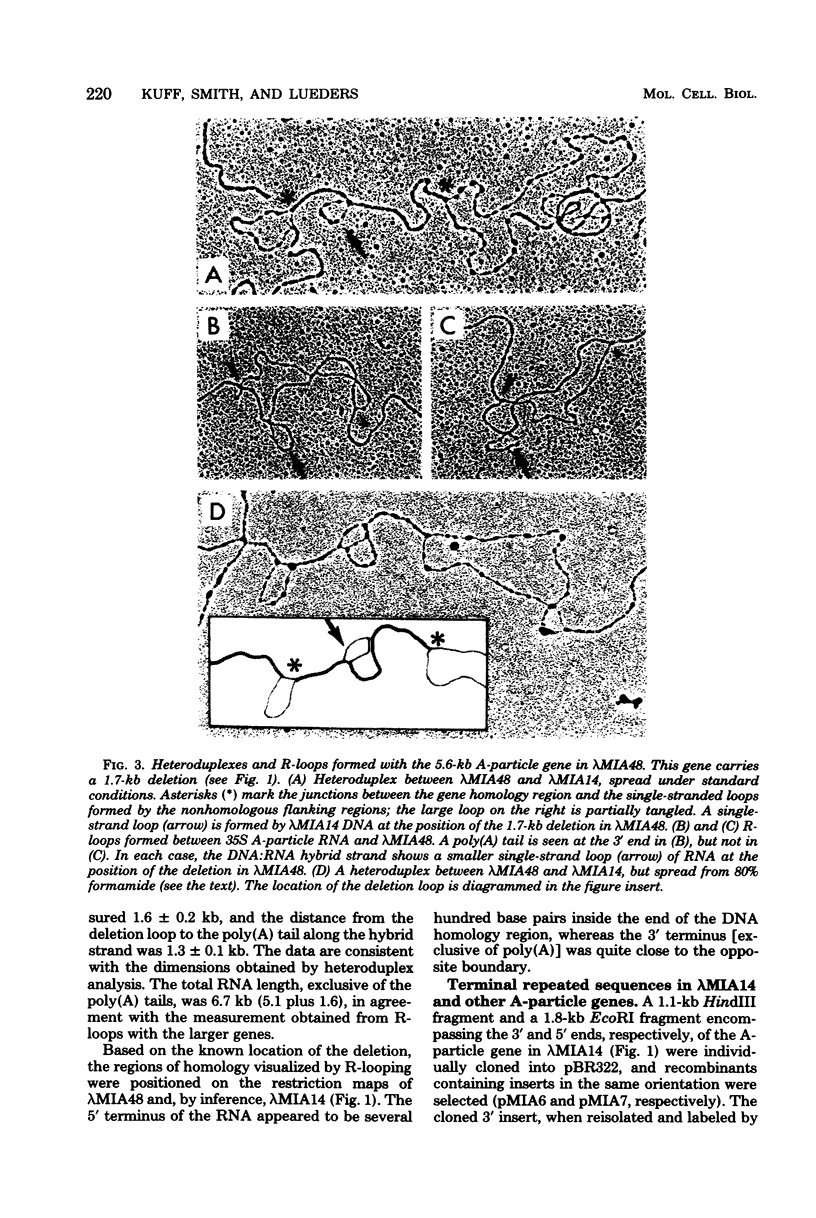
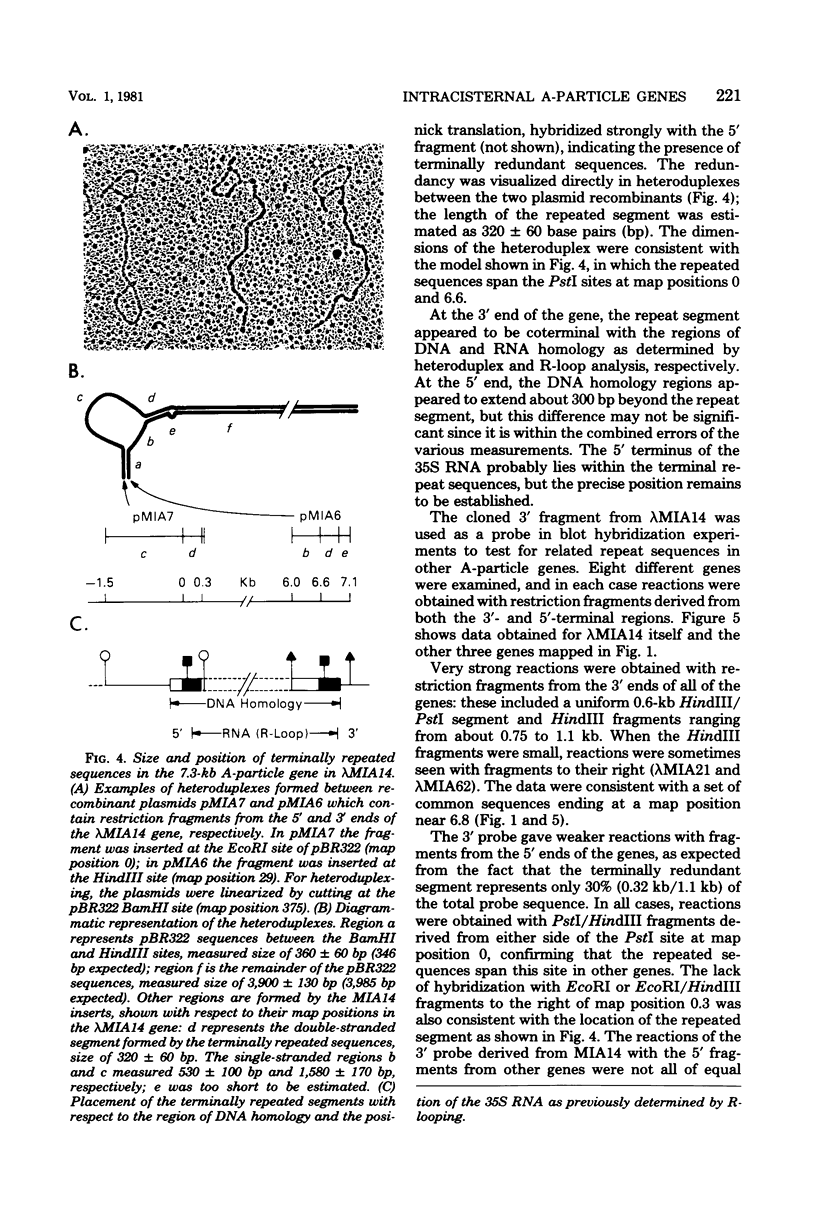
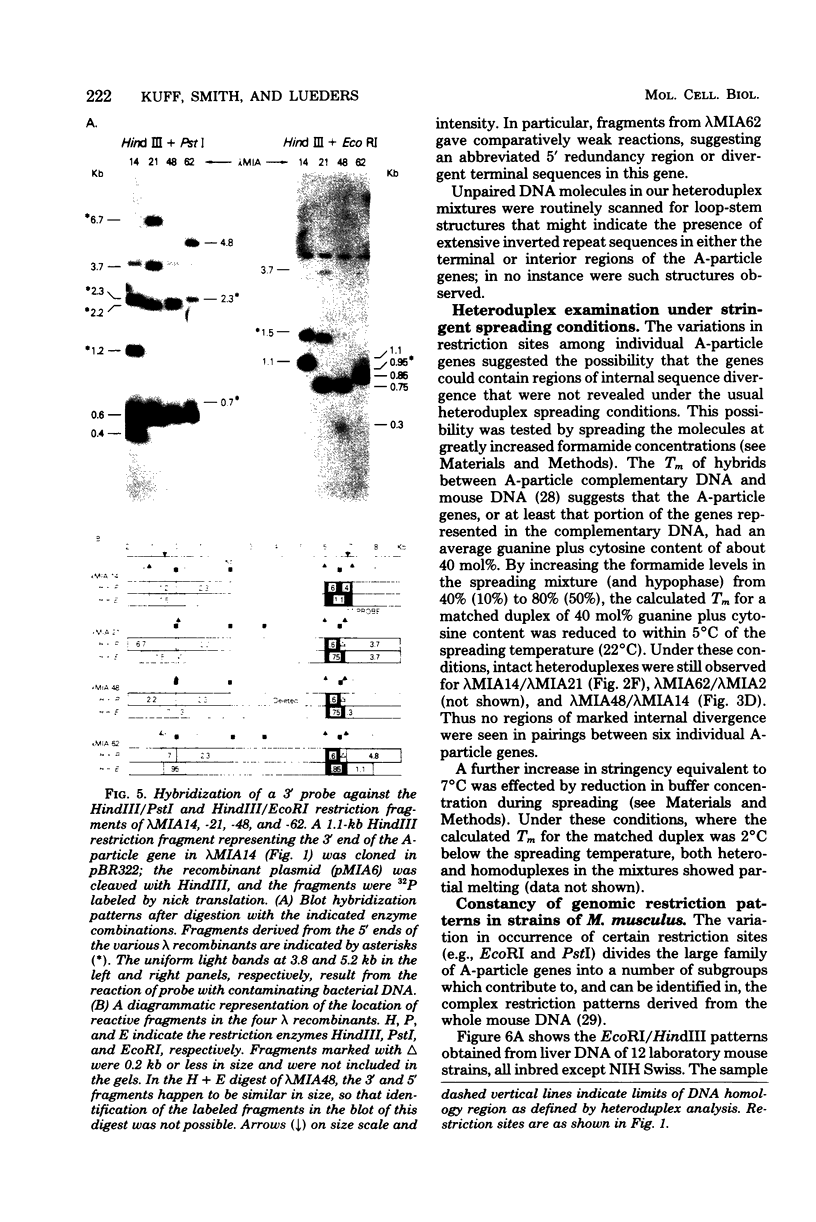
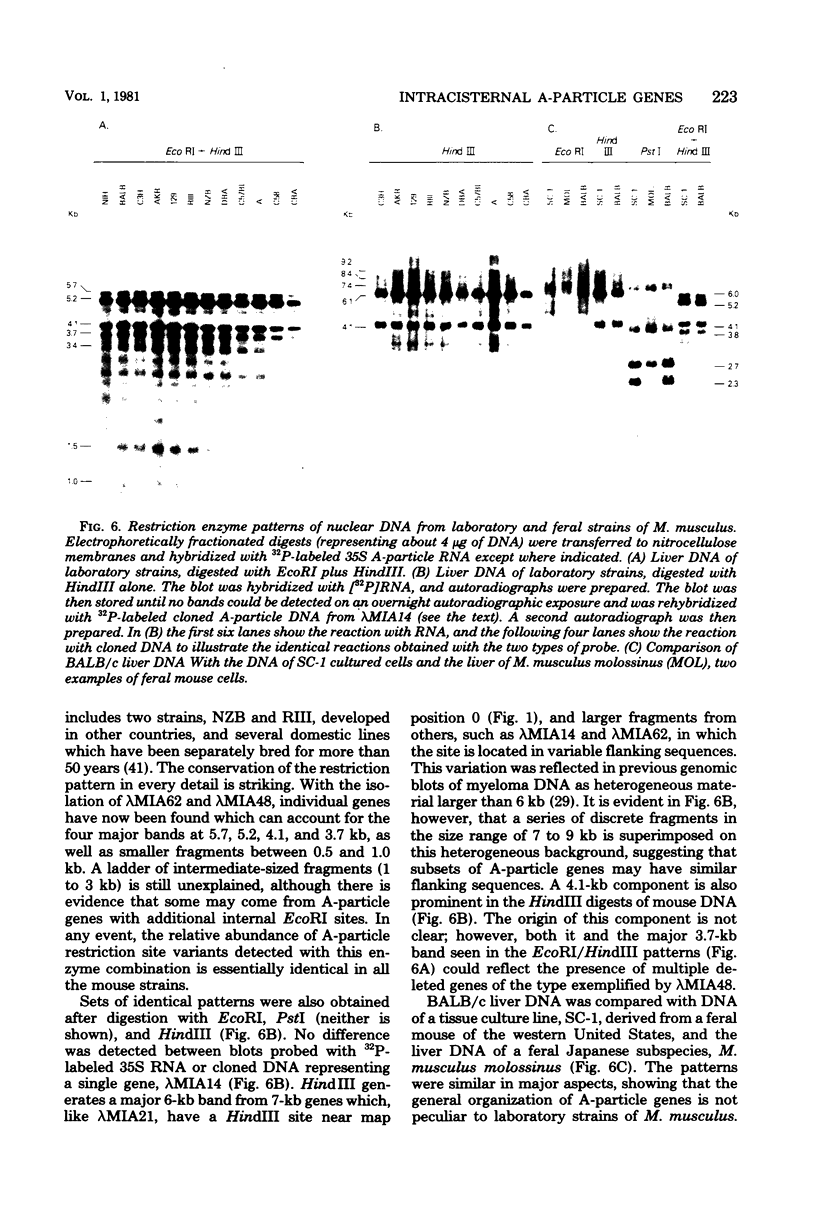
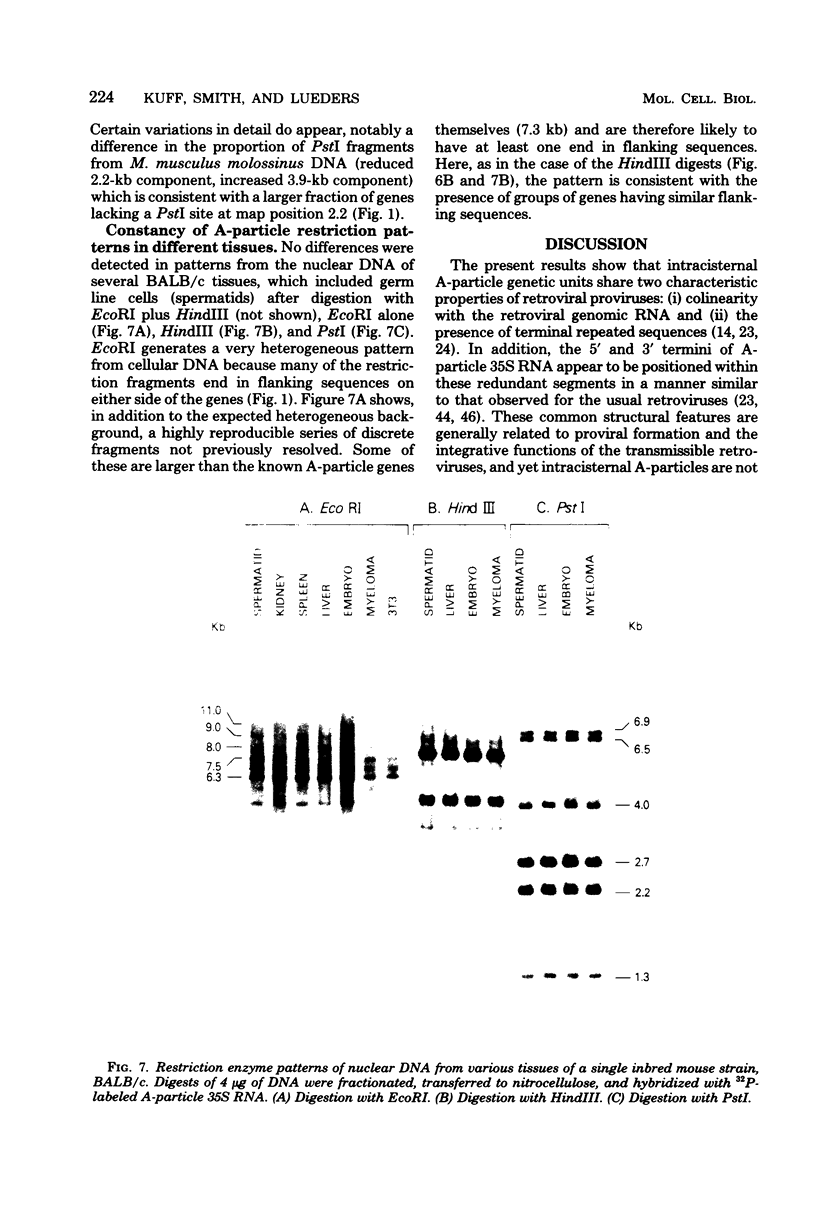
The structural organization of intracisternal A-particle genes has been studied, using isolates from a mouse gene library in lambda phage Charon 4A. The predominant gene form among the isolates was 7.3 kilobases (kb) in length. R-loops between the 7-kb (35S) A-particle genomic ribonucleic acid and several of these genes were colinear, with no visible evidence of intervening deoxyribonucleic acid sequences. One recombinant was found with an A-particle gene that contained a 1.7-kb deletion. Using the deletion as a reference, the deoxyribonucleic acid and ribonucleic acid homology regions were localized with respect to one another and to the restriction map: the 5' terminus of the ribonucleic acid was several hundred base pairs within the 5' end of the deoxyribonucleic acid homology region. Restriction endonuclease fragments encompassing the 5' and 3' regions of one 7.3-kb gene were separately subcloned into pBR322. Heteroduplexes between the two subclones revealed an approximately 300-base pair segment of terminally redundant sequences. The cloned 3' fragment hybridized with restriction fragments from the 5' end of several other A-particle genes, demonstrating the presence of common (though not necessarily identical) terminally repeated sequences. A-particle genes varied in the occurrence of specific restriction sites at characteristic internal loci. However, heteroduplexes between several variant 7.3-kb genes showed continuous homology regions even when spread under stringent hybridization conditions. The relative abundance of restriction site variants was highly conserved in 12 laboratory strains of Mus musculus, in embryonic and adult tissues of a single inbred strain, and in the SC-1 cell line of feral mouse origin, but appeared to differ in a feral Japanese substrain, Mus musculus molossinus. Some evidence suggests that subsets of A-particle genes may have similar flanking sequences. The results are discussed in terms of the evolution of this multigene family.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacheler L. T., Fan H. Multiple integration sites for Moloney murine leukemia virus in productively infected mouse fibroblasts. J Virol. 1979 Jun;30(3):657–667. doi: 10.1128/jvi.30.3.657-667.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender W., Davidson N. Mapping of poly(A) sequences in the electron microscope reveals unusual structure of type C oncornavirus RNA molecules. Cell. 1976 Apr;7(4):595–607. doi: 10.1016/0092-8674(76)90210-5. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Biczysko W., Pienkowski M., Solter D., Koprowski H. Virus particles in early mouse embryos. J Natl Cancer Inst. 1973 Sep;51(3):1041–1050. doi: 10.1093/jnci/51.3.1041. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Szollosi D. Intracisternal A particles in ova and preimplantation stages of the mouse. Nat New Biol. 1973 May 16;243(124):91–93. [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Sherr C. J., Schidlovsky G., Todaro G. J. A new class of genetically transmitted retravirus isolated from Mus cervicolor. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3579–3583. doi: 10.1073/pnas.73.10.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Sherr C. J., Todaro G. J. A new class of murine retroviruses: immunological and biochemical comparison of novel isolates from Mus cervicolor and Mus caroli. Virology. 1977 Jul 15;80(2):401–406. doi: 10.1016/s0042-6822(77)80015-9. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Chase D. G., Pikó L. Expression of A- and C-type particles in early mouse embryos. J Natl Cancer Inst. 1973 Dec;51(6):1971–1975. doi: 10.1093/jnci/51.6.1971. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. A very large repeating unit of mouse DNA containing the 18S, 28S and 5.8S rRNA genes. Cell. 1977 Aug;11(4):795–805. doi: 10.1016/0092-8674(77)90292-6. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J., Fink G. R. Insertion of the eukaryotic transposable element Ty1 creates a 5-base pair duplication. Nature. 1980 Jul 24;286(5771):352–356. doi: 10.1038/286352a0. [DOI] [PubMed] [Google Scholar]
- Gafner J., Philippsen P. The yeast transposon Ty1 generates duplications of target DNA on insertion. Nature. 1980 Jul 24;286(5771):414–418. doi: 10.1038/286414a0. [DOI] [PubMed] [Google Scholar]
- Groner B., Hynes N. E. Number and location of mouse mammary tumor virus proviral DNA in mouse DNA of normal tissue and of mammary tumors. J Virol. 1980 Mar;33(3):1013–1025. doi: 10.1128/jvi.33.3.1013-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Howk R. S., Troxler D. H., Lowy D., Duesberg P. H., Scolnick E. M. Identification of a 30S RNA with properties of a defective type C virus in murine cells. J Virol. 1978 Jan;25(1):115–123. doi: 10.1128/jvi.25.1.115-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Keshet E., O'Rear J. J., Temin H. M. DNA of noninfectious and infectious integrated spleen necrosis virus (SNV) is colinear with unintegrated SNV DNA and not grossly abnormal. Cell. 1979 Jan;16(1):51–61. doi: 10.1016/0092-8674(79)90187-9. [DOI] [PubMed] [Google Scholar]
- Keshet E., Shaul Y., Kaminchik J., Aviv H. Heterogeneity of "virus-like" genes encoding retrovirus-associated 30S RNA and their organization within the mouse genome. Cell. 1980 Jun;20(2):431–439. doi: 10.1016/0092-8674(80)90629-7. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Callahan R., Howk R. S. Immunological relationship between the structural proteins of intracisternal A-particles of Mus musculus and the M432 retrovirus of Mus cervicolor. J Virol. 1980 Mar;33(3):1211–1214. doi: 10.1128/jvi.33.3.1211-1214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K., Scolnick E. M. Nucleotide sequence relationship between intracisternal type A particles of Mus musculus and an endogenous retrovirus (M432) of Mus cervicolor. J Virol. 1978 Oct;28(1):66–74. doi: 10.1128/jvi.28.1.66-74.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Intracisternal A-particle genes: identification in the genome of Mus musculus and comparison of multiple isolates from a mouse gene library. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3571–3575. doi: 10.1073/pnas.77.6.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences associated with intracisternal A particles are reiterated in the mouse genome. Cell. 1977 Dec;12(4):963–972. doi: 10.1016/0092-8674(77)90161-1. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Segal S., Kuff E. L. RNA sequences specifically associated with mouse intracisternal A particles. Cell. 1977 May;11(1):83–94. doi: 10.1016/0092-8674(77)90319-1. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire K. R., Asofsky R. M., Potter M., Kuff E. L. Macroglobulin-producing plasma-cell tumor in mice: identification of a new light chain. Science. 1965 Oct 15;150(3694):360–363. [PubMed] [Google Scholar]
- Meistrich M. L. Separation of spermatogenic cells and nuclei from rodent testes. Methods Cell Biol. 1977;15:15–54. doi: 10.1016/s0091-679x(08)60207-1. [DOI] [PubMed] [Google Scholar]
- Ono M., Cole M. D., White A. T., Huang R. C. Sequence organization of cloned intracisternal A particle genes. Cell. 1980 Sep;21(2):465–473. doi: 10.1016/0092-8674(80)90483-3. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Segal S., Lueders K. K., Kuff E. L. RNA associated with murine intracisternal type A particles codes for the main particle protein. J Virol. 1978 Jul;27(1):118–126. doi: 10.1128/jvi.27.1.118-126.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., Straus N. A. Relatedness of mouse satellite deoxyribonucleic acid to deoxyribonucleic acid of various Mus species. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3546–3550. doi: 10.1073/pnas.70.12.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staats J. Standardized nomenclature for inbred strains of mice: seventh listing for the International Committee on Standardized Genetic Nomenclature for Mice. Cancer Res. 1980 Jul;40(7):2083–2128. [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Verma I. M., Lerner R. A. Nucleotide sequence of Moloney leukemia virus: 3' end reveals details of replications, analogy to bacterial transposons, and an unexpected gene. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3302–3306. doi: 10.1073/pnas.77.6.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., Goddard J. G., Berns A., Verma I. M. Structure of Moloney murine leukemia viral DNA: nucleotide sequence of the 5' long terminal repeat and adjacent cellular sequences. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3307–3311. doi: 10.1073/pnas.77.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. G., Blattner F. R. Construction and characterization of the hybrid bacteriophage lambda Charon vectors for DNA cloning. J Virol. 1979 Feb;29(2):555–575. doi: 10.1128/jvi.29.2.555-575.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Reitz M. S., Jr, Trainor C. D., Gallo R. C. Murine intracisternal type A particles: a biochemical characterization. J Virol. 1975 Oct;16(4):887–896. doi: 10.1128/jvi.16.4.887-896.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]