Background: Polysialylated glycoproteins play an import role during numerous biological processes.
Results: Polysialylated ST8SiaII and NCAM are components of mammalian semen and are partially associated with spermatozoa.
Conclusion: Polysialic acid represents a further glyco-motif in mammalian ejaculates, which is known to influence the immune system.
Significance: Administration of polysialic acid during insemination might be useful to increase the number of spermatozoa escaping the female immune system.
Keywords: Cell Adhesion, Fertilization, Polysaccharide, Post-translational Modification, Spermatozoa, Mammalian Sperm, NCAM, Polysialic Acid, Polysialyltransferase, ST8SiaII
Abstract
Fertilization in animals is a complex sequence of several biochemical events beginning with the insemination into the female reproductive tract and, finally, leading to embryogenesis. Studies by Kitajima and co-workers (Miyata, S., Sato, C., and Kitajima, K. (2007) Trends Glycosci. Glyc, 19, 85–98) demonstrated the presence of polysialic acid (polySia) on sea urchin sperm. Based on these results, we became interested in the potential involvement of sialic acid polymers in mammalian fertilization. Therefore, we isolated human sperm and performed analyses, including Western blotting and mild 1,2-diamino-4,5-methylenedioxybenzene-HPLC, that revealed the presence α2,8-linked polySia chains. Further analysis by a glyco-proteomics approach led to the identification of two polySia carriers. Interestingly, besides the neural cell adhesion molecule, the polysialyltransferase ST8SiaII has also been found to be a target for polysialylation. Further analysis of testis and epididymis tissue sections demonstrated that only epithelial cells of the caput were polySia-positive. During the epididymal transit, polySia carriers were partially integrated into the sperm membrane of the postacrosomal region. Because polySia is known to counteract histone as well as neutrophil extracellular trap-mediated cytotoxicity against host cells, which plays a role after insemination, we propose that polySia in semen represents a cytoprotective element to increase the number of vital sperm.
Introduction
In vertebrates, the highly negatively charged carbohydrate polysialic acid (polySia)3 is known to influence the regulation of cell-cell contact and repulsion, for example (1). In mammals, polySia consists of α2,8-linked N-acetylneuraminic acid (Neu5Ac) residues, and the chain length of these polymers can exceed 60 sialic acid residues (2–6). Whereas most extracellular glycoproteins are modified by monosialyl residues, polysialylation is restricted to a small number of N- and O-glycosylated proteins. The best characterized target for polysialylation is the neural cell adhesion molecule NCAM (7). Here, glycans at the fifth and sixth N-glycosylation site can be post-translationally modified with sialic acid polymers (8–11). The modification of NCAM with polySia creates a bulky and highly negatively charged moiety, which leads to an inhibition of the homophilic trans as well as cis interaction between NCAM molecules modulating the adhesive properties of eukaryotic cells (12–17).
More recently, four additional polysialylated glycoproteins have been identified as follows: (i) a not clearly specified α-subunit of a voltage-gated sodium channel in adult rat brain (18); (ii) a soluble form of CD36 in murine and human milk (19); (iii) neuropilin-2 on human mature dendritic cells (20), and (iv) the synaptic cell adhesion molecule 1 in postnatal murine brain (21).
In mammals, the generation of polySia depends on the presence of the α2,8-polysialyltransferases ST8SiaII and ST8SiaIV. Deletion of both enzymes in mice leads to a mortal phenotype because polySia is involved in the development of several essential organs like the brain, heart, kidney, pancreas, and the respiratory tract (22–25). Interestingly, both polysialyltransferases are able to polysialylate their N-glycans in cell culture experiments (26–28). However, such an autopolysialylation has been, so far, not reported in vivo.
PolySia is not only expressed in vertebrates but also in other clades (29) like sea urchins (30). In contrast to vertebrates, however, also α2,9- and α2,5-O-glycolyl-linked polySia exist in addition to the α2,8-linked form. Kitajima and co-workers (31) show that polySia mediates important functions of sea urchin sperm. For instance, α2,9-linked polySia is suggested to influence the motility of sea urchin sperm, and α2,5-O-glycolyl-linked polySia is discussed to potentiate the acrosome reaction (30). The function of α2,8-linked polySia has not been studied to date.
Because beneficial organs are often independently developed in different species during evolution (e.g. eye of vertebrates and sepia), we investigated in this study mammalian semen for the existence of polysialylated glycoproteins. Our data reveal the presence of polysialylated ST8SiaII besides polysialylated NCAM in mammalian semen. Thus, polySia carriers may influence processes localized in the female reproductive tract.
EXPERIMENTAL PROCEDURES
Materials
NCAM-specific monoclonal antibody (mAb) 123C3 (32, 33) and polySia-specific mAb 735 (33) as well as inactive and active endoneuraminidase (endoN) were purified as described previously (34, 35). mAbs against human ST8SiaII and ST8SiaIV were purchased from Sigma.
Separation of Vital Sperm
For enrichment of vital human sperm, a swim-up procedure was applied. For this purpose, 1 ml of native ejaculate was stacked under 5 ml of TALP medium (2 mm CaCl2, 3.1 mm KCl, 0.4 mm MgCl2, 100 mm NaCl, 25 mm NaHCO3, 0.3 mm NaH2PO4, 1 mm sodium pyruvate, 10 mm HEPES, 21.6 mm sodium lactate, 20% fetal bovine serum (v/v)). After incubation at 37 °C and 5% CO2 for 60 min, 3 ml of the supernatant of each well were isolated and centrifuged for 10 min at 700 × g. Enriched mobile sperm were used for further analyses.
Protein Extraction and Isolation of Polysialylated Proteins
Whole ejaculate was diluted in water and homogenized. Precipitation of proteins was performed by adding 4 parts of acetone at −20 °C per part of sample. After incubation at −20 °C overnight, the protein pellet was isolated by centrifugation and lyophilized to dryness.
Alternatively, polysialylated proteins were isolated from enriched vital sperm by affinity precipitation. To this end, samples were homogenized in lysis buffer (50 mm Tris/HCl (pH 8.0), 5 mm EDTA, 150 mm NaCl, 1% Triton X-100 (w/v), 0.5% sodium deoxycholate (w/v), 1 mm PMSF, 1 mm aprotinin, and 1 mm leupeptin) on ice. Homogenized samples were incubated for 2 h at 4 °C on a shaker and centrifuged for 1 h at 4 °C. For affinity precipitation, inactivated endoN was coupled to tosyl-activated magnetic Dynabeads® M-280 (Invitrogen) according to the manufacturer's instructions and incubated with the supernatant overnight at 4 °C on a shaker. Subsequently, beads were washed twice each with 1 ml of washing buffer 1 (20 mm Tris/HCl (pH 8.0), 150 mm NaCl, 0.5% Triton X-100 (w/v)) and washing buffer 2 (20 mm Tris/HCl (pH 8.0), 150 mm NaCl). Polysialylated proteins were disconnected from magnetic beads with elution buffer (100 mm triethylamine, 150 mm NaCl) and dried down in a vacuum concentrator.
HPLC Analysis of Sialic Acid Polymers
For detection of internal α2,8-linked sialic acid residues, the C7/C9 method described by Sato et al. (36) was applied. After oxidation, reduction, and fluorescence labeling, the resulting DMB derivatives were analyzed on a Superspher® 100 C-18 column (250 × 40 mm, Merck) at 40 °C using a Merck-Hitachi HPLC system (37). Mobile phases methanol/acetonitrile/water/trifluoroacetic acid (TFA) (4:4:92:0.1) (M1) and methanol/acetonitrile/water/TFA (45:45:10:0.1) (M2) were used for separation of DMB-labeled sialic acids. A linear gradient was applied from 0 to 20% M2 in 35 min at a flow rate of 0.3 ml/min.
The degree of polymerization of polySia chains was analyzed by DMB-HPLC analysis (2, 39). To this end, purified polySia carriers were dissolved in 80 μl of DMB reaction buffer and incubated for 24 h at 4 °C. The reaction was stopped by adding 20 μl of 1 mm NaOH, and released polySia chains were separated on a DNAPac PA-100 column (Dionex, Idstein, Germany) by HPLC (37). MilliQ water (eluent (E) 1) and 1 m NaNO3 (E2) were used as eluents at a flow rate of 1 ml/min. Elution was performed by the following gradient: t0 min = 0% E2; t5 min = 1% E2; t15 min = 10% E2; and t60 min = 50% E2.
SDS-PAGE and Western Blotting
Total protein lysates as well as purified polysialylated proteins were separated by 10% SDS-PAGE under reducing conditions and, subsequently, transferred onto a PVDF membrane. Binding of anti-polySia, anti-NCAM, as well as anti-ST8SiaII and ST8SiaIV mAbs was detected by use of horseradish peroxidase-conjugated secondary antibodies (Dako, Hamburg, Germany) and chemiluminescence SuperSignal kit (Thermo Fisher, Kehl, Germany). Prior to SDS-PAGE, a part of the samples was treated with endoN or peptide-N-glycosidase F (PNGaseF). For the release of N-glycans, immunoprecipitates were resuspended in 40 mm DTT, 0.5% SDS and boiled for 10 min. After adjusting the sample buffer to the final concentration of 50 mm sodium phosphate (pH 7.5), 40 mm DTT, 0.5% SDS (w/v), and 1% Nonidet P-40 (v/v), precipitates were incubated with PNGaseF (50 units/ml) (Roche Applied Science) for 16 h at 37 °C. The endoN digest (1 μg/ml) was performed in lysis buffer for 16 h at 37 °C.
Glycoproteomics Approach
Eluted polysialylated proteins were separated by electrophoresis on 10% ready-to-use SDS gels (Bio-Rad), followed by in-gel digestion with trypsin (Promega, Mannheim, Germany) as described in detail previously (21). Extracted peptides were separated on a C18 column (PepMap, 3 μm, 75 μm × 100 mm, Dionex) using an Ultimate nanoLC system (Dionex) with a linear gradient from 10% acetonitrile, 0.1% formic acid to 60% acetonitrile, 0.1% formic acid. Peptides were directly spotted by a Probot (Dionex) onto a matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) steel target (Bruker Daltonics, Bremen, Germany) and mixed with an equal volume of 2,5-dihydrobenzoic acid (DHB) matrix (7.5 mg DHB/ml, 1% phosphoric acid, 50% acetonitrile). Peptide mass fingerprints of tryptic digests were generated by MALDI-TOF-MS using an Ultraflex I TOF/TOF mass spectrometer (Bruker Daltonics). MS and MS/MS spectra were acquired in positive reflector mode using FlexControl 2.4 software and analyzed by the FlexAnalysis software 3.0 (both Bruker Daltonics). External calibration of mass spectra was carried out using peptide calibration standard for MS (Bruker-Daltonics), and annotations of fragment ions in the MS/MS mode were performed according to Ref. 40.
Immunohistochemistry/Immunofluorescence
Paraffin-embedded testis and epididymis tissue (formalin-fixed) sections were cut into 5-μm serial sections. After rehydration in xylene and following descending ethanol series, sections were incubated with blocking solution for 5 min, followed by incubation with the primary antibody mAb 735 (10 μg/ml PBS containing 2% (w/v) bovine serum albumin (BSA)) overnight at 4 °C. As negative control for the polySia staining and before incubation with mAb, 735 tissue sections were pretreated with endoN (3 μg/ml in PBS containing 0.1% BSA) overnight at 37 °C. For staining the Envision+ system, the HRP kit (Dako) was used. The stained sections were counterstained with hemalaun (Roth, Karlsruhe, Germany).
For immunofluorescence, sperm were washed with PBS, 0.1% BSA (pH 7.4) and centrifuged at 700 × g by discarding the supernatant. Purified sperm were fixed in PBS (pH 7.4) containing 2% formaldehyde (v/v) for 30 min at 22 °C. After fixation, sperm were washed with PBS, 0.1% BSA. For negative control of the polySia staining as well as the staining against NCAM and ST8SiaII, sperm were pretreated with endoN (3 μg/ml in PBS, 0.1% BSA) overnight at 37 °C. Primary antibodies were incubated overnight at 4 °C. For the visualization of the acrosome, biotinylated peanut agglutinin (20 μg/ml in PBS/0.1% BSA) (Vector Laboratories Burlingame, CA) was used. Fluorescein isothiocyanate (FITC)-conjugated or rhodamine-conjugated (Dianova, Hamburg, Germany) secondary antibodies against mouse IgG and biotin were used for visualization of the primary antibodies. All images were taken with a Leica DMR microscope and processed by MetaMorph® Microscopy Automation and Image Analysis Software ©2012 Molecular Devices, LLC.
mRNA Analyses of ST8SiaII and ST8SiaIV and NCAM
Total cellular RNA was prepared from rat epididymis at the time of explantation by RNeasy mini kit (Qiagen, Hilden, Germany). Subsequently, first strand cDNA was generated by iScript cDNA synthesis kit (Bio-Rad).
Intron-spanning primers were used to amplify ST8SiaII, ST8SiaIV, and NCAM targets using SYBR Green real time PCR master mixes (Invitrogen) according to the manufacturer's instructions. Primers used in quantitative PCR expression analyses are as follows: NCAM forward 5′-CAAAAATGACGAAGCCGAAT-3′ and NCAM reverse 5′-GTGGACGTTCTCCAGGTGAT-3′; ST8SiaII forward 5′-GTGGAGTGGGTCAATGCTCT-3′ and ST8SiaII reverse 5′-GGTACTTGACGGGGTTCTGA-3′; and ST8SiaIV forward 5′-GGACTGAGGAGCACCAAGAG-3′ and ST8SiaIV reverse 5′-AGCCTTGTACGGAATGTTGG-3′.
cDNA samples were denatured at 95 °C for 3:30 min, followed by 35 cycles of each for 20 s at 95 °C, 20 s at 60 °C, and 20 s at 72 °C. For melt curve analysis, PCR samples were slowly heated from 72 to 95 °C and finally stored at 20 °C. Amplified products were separated by gel electrophoresis using 2% agarose gel.
RESULTS
Detection of polySia in Human Semen
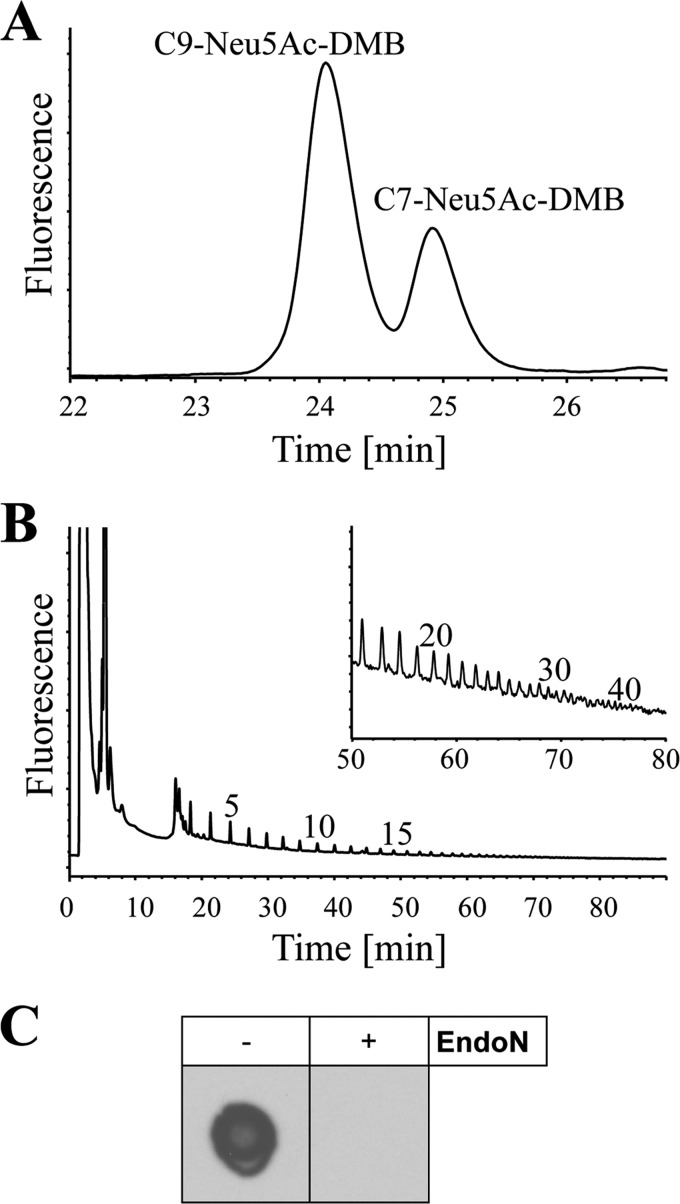
For the detection of internal sialic acid residues in human semen, the proteins thereof were subjected to C7/C9 analysis after acetone precipitation. To this end, all terminal sialic acid molecules were transformed into C7 residues by periodate oxidation. Because of the α2,8-linkage, internal sialic residues were protected against oxidation because no vicinal hydroxyl groups existed, which are necessary for oxidation. In a last step, all sialic acid residues were released under acidic conditions and labeled with DMB. Resulting C7-Neu5Ac-DMB and C9-Neu5Ac-DMB were separated by reverse phase-HPLC (Fig. 1A). The detection of C9-Neu5Ac-DMB after periodate oxidation indicated the presence of internal α2,8-linked sialic acid residues linked to proteins of human semen. However, the analysis of di-, tri-, and oligomers of Neu5Ac might also lead to comparable chromatograms. Hence, detection of internal sialic acid residues is not unambiguous proof for the presence of polySia.
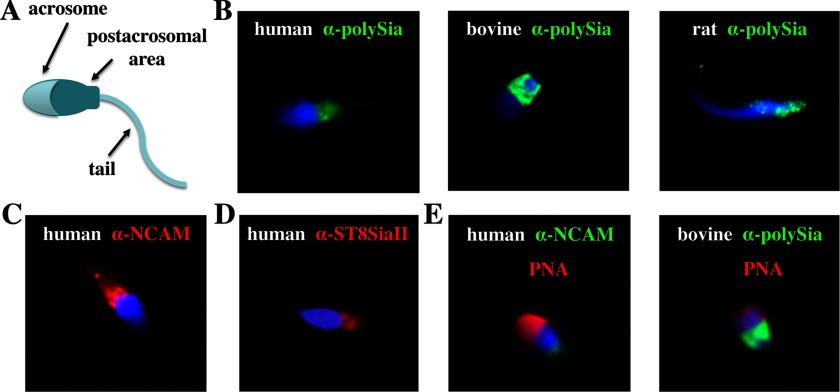
FIGURE 1.
Detection of polySia in human semen. A, terminal and internal sialic acid residues of the protein fraction of human semen were visualized by the C7/C9 method. After subsequent metaperiodate oxidation, reduction, hydrolysis, and DMB labeling, C7-Neu5Ac-DMB and C9-Neu5Ac-DMB were separated by reverse phase-HPLC. Internal sialic acid residues resulted in the detection of C9-Neu5Ac-DMB, whereas terminal ones are detected as C7-Neu5Ac-DMB. B, for characterization of the polySia chain length, protein fraction of the whole ejaculate was subjected to the mild DMB method. DMB-labeled sialic acid polymers were separated by anion exchange chromatography according to the chain length. Respective numbers of sialic acid residues are given for single peaks on top of the profiles. C, proteins were transferred to a PVDF membrane, and polySia was visualized with the mAb 735. For negative control polySia was degraded with endoN.
For this reason, the chain length was analyzed in more detail. Therefore, polySia chains were cleaved off the glycans under mild acidic conditions and tagged directly with DMB at the reducing end. Fluorescently labeled chains were separated by anion exchange chromatography according to the degree of polymerization (2, 3, 42). As shown in Fig. 1B, the obtained chromatographic profiles show that polySia chains can include more than 40 sialic acid residues.
In addition to the chemical detection of polySia, we performed a dot blot analysis with a mAb against α2,8-linked polySia (Fig. 1C). The protein fraction of human ejaculate revealed a strong polySia signal, whereas the immune staining was abolished after the degradation of polySia by endoN. Taken together, the chemical and immunological analyses of human semen disclosed the existence of α2,8-linked polySia chains, which can reach a length of more than 40 sialic acid residues.
ST8SiaII and NCAM Are Polysialylated
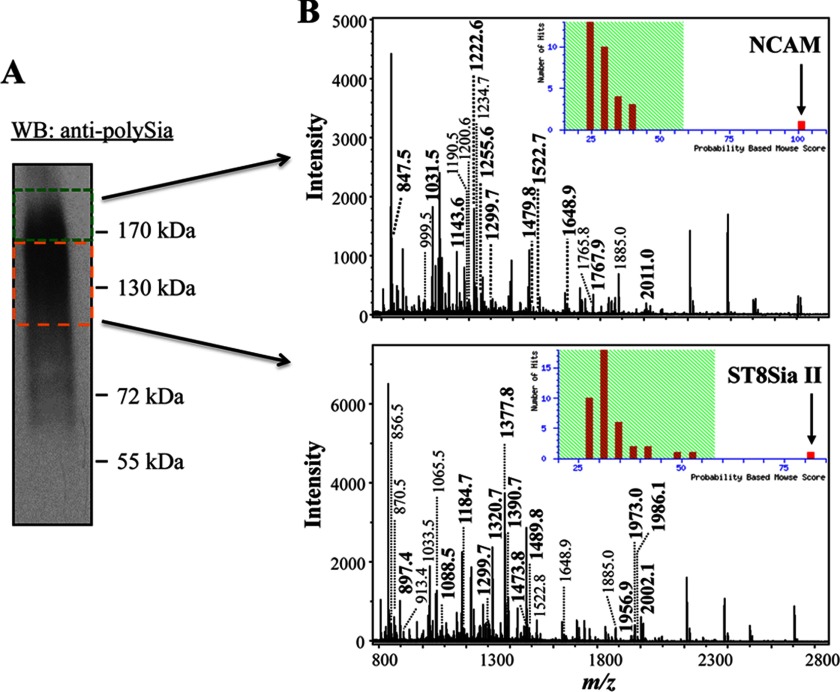
For identification of the polysialylated proteins, rat epididymis was used storing maturated spermatozoa. At first, the polySia carriers were purified from epididymal lysates using magnetic beads, which were coated with enzymatically inactive endoN. EndoN contains an extended binding site for polySia, and targeted mutation of active site residues gives rise to an inactive form that works as an efficient polySia-specific lectin (35, 43). Purified polySia carriers were subjected to Western blotting against polySia. As shown in Fig. 2A, polySia immunostaining displayed a typical diffuse band for polysialylated proteins in a region between 100 and 200 kDa. To generate peptide mass fingerprints of the polysialylated glycoproteins, SDS-PAGE was performed, and two gel slices in the area of the immune signal against polySia were used for tryptic in gel digest. Resulting peptides were extracted, separated by reverse phase-nanoLC and directly spotted onto a MALDI-TOF-MS carrier for MS(/MS) analysis. Resulting mass spectra were utilized for database search leading to the identification of ST8SiaII and NCAM (Fig. 2B). The findings were confirmed by additional fragmentation analyses of selected peptides (supplemental Fig. 1).
FIGURE 2.
Identification of ST8SiaII and NCAM as polySia carriers. A, polysialylated proteins were purified from rat epididymis lysates using magnetic beads, which were coated with inactive endoN and separated by SDS-PAGE for Western blotting (WB) and tryptic in gel digest. Two gel slices (see labeling) were cut for tryptic in gel digest. B, after reduction, carbamidomethylation and treatment with trypsin resulting peptides were extracted, separated by nano-LC, and directly spotted onto a MALDI-TOF-MS target for MS analysis. Database search (mascot) revealed NCAM and ST8SiaII as polySia carriers with significant scores (inset). Identified peptides of NCAM and ST8SiaII are printed in boldface. Signals representing tryptic peptides of trypsin, keratin, actin, myosin, and spectrin were detected in consequence of sample preparation and were not labeled.
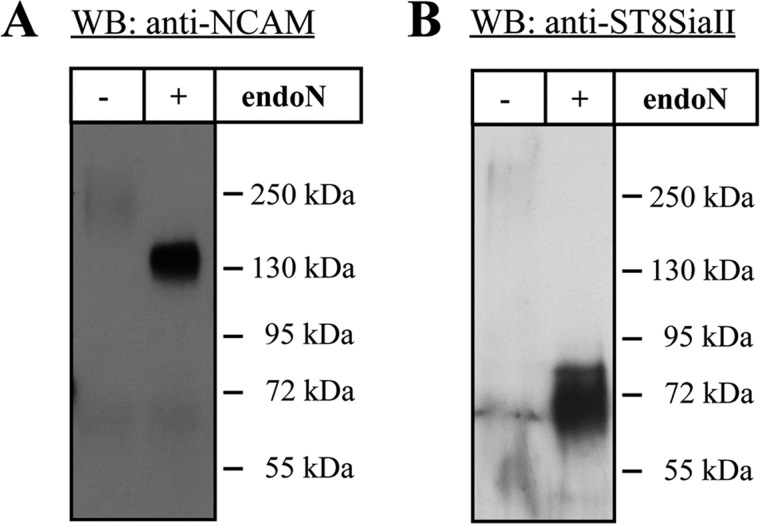
After MS(/MS)-based identification of ST8SiaII and NCAM in rat epididymal tissue, their presence in human semen was approved by Western blotting. Purification of the polySia carriers with magnetic endoN beads and immunostaining against ST8SiaII as well as NCAM were employed. Whereas the polysialylated forms of both glycoproteins were hardly (NCAM) or not (ST8SiaII) detectable, the underlying proteins NCAM-140 and ST8SiaII could be visualized after removal of polySia by endoN treatment (Fig. 3). The poor visibility of the polysialylated forms of NCAM and ST8SiaII can be explained by the bulky and highly negative properties of polySia. The antibody-protein binding might be similarly inhibited as the NCAM-NCAM interaction.
FIGURE 3.
Detection of polysialylated NCAM and ST8SiaII using human sperm. For Western blotting (WB) against NCAM (A) and ST8SiaII (B), polysialylated proteins were purified from mobile human sperm lysates using magnetic beads, which were coated with inactive endoN. Western blot analyses were performed with or without previous endoN treatment. Apparent molecular masses of standard proteins are indicated in kDa.
PolySia Chains Are Attached to N-Glycans of the Fifth Potential N-Glycosylation Site of ST8SiaII
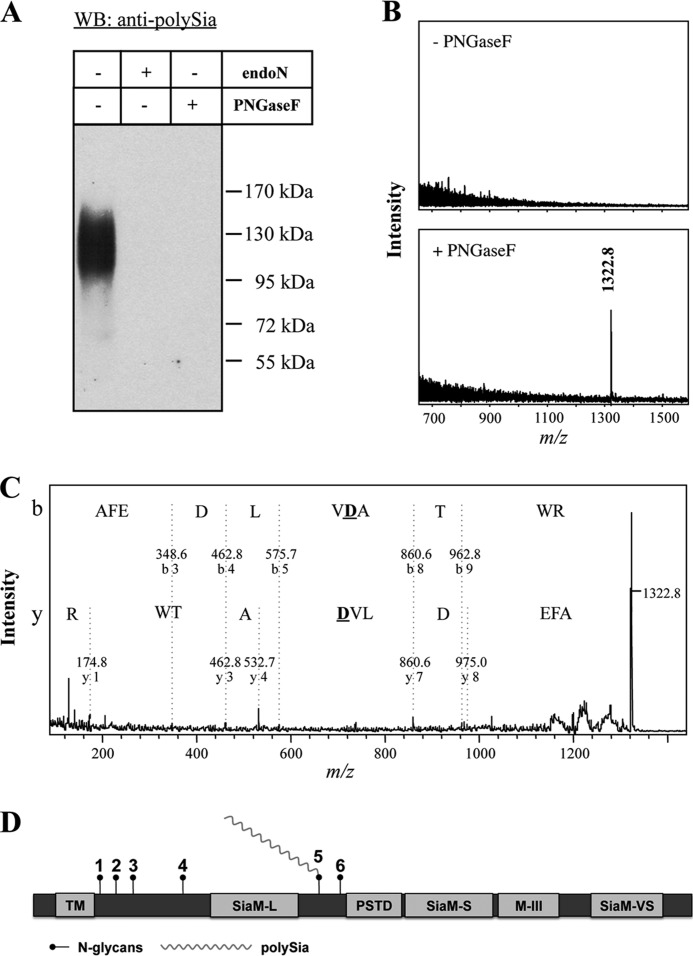
In the case of NCAM, it is well known that N-glycans at the fifth and sixth N-glycosylation site can be polysialylated (8–11). To dissect whether N- or O-glycans of ST8SiaII were modified by polySia, purified polysialylated glycoproteins of human semen were treated with PNGaseF. Resulting de-N-glycosylated proteins were exposed to Western blotting using a mAb against polySia. After the release of N-glycans, the immunostaining was abolished indicating that polySia chains together with the N-glycans were released with PNGaseF (Fig. 4A).
FIGURE 4.
Determination of the polysialylated N-glycosylation site of ST8SiaII. A, purified polySia carriers of rat epididymis were separated by SDS-PAGE. Western blot (WB) analysis was performed using anti-polySia mAb 735 with or without previous endoN or PNGaseF treatment. Apparent molecular masses of standard proteins are indicated in kDa. B, MALDI-TOF mass spectra of precipitated polysialylated glycopeptides before (−) or after (+) PNGaseF treatment and nanoLC separation. Monoisotopic masses of the pseudomolecular ions [M + H]+ are given. C, fragmentation analysis of the de-N-glycosylated peptide by MALDI-TOF-MS/MS. Sequence-specific ions are labeled according to previous studies (44, 45). The conversion of Asn to Asp is illustrated in boldface. D, assignment of a polySia chain to individual N-glycosylation sites of ST8SiaII.
To identify the polysialylated N-glycosylation sites, polysialylated tryptic glycopeptides were purified using magnetic endoN beads. The isolated polysialylated glycopeptides were separated by nanoLC and analyzed by MALDI-TOF-MS with or without previous PNGaseF treatment. In comparison with the untreated sample, de-N-glycosylation with PNGaseF resulted in the detection of one additional mass at m/z 1322.8 (Fig. 4B). This peptide signal matched the calculated deglycosylated peptide mass of the fifth N-glycosylation site after conversion of Asn to Asp by the enzymatic reaction of PNGaseF (Asn219; 213AFEDLVNATWR223). The peptide sequence was additionally proofed by MALDI-TOF-MS/MS (Fig. 4C). The analysis estimated that the signal at m/z 1322.8 represents the deglycosylated N-glycosylation site at Asn219, which is located between the sialyltransferase motif L and the polysialyltransferase domain (Fig. 4D).
PolySia Carriers Are Detectable in the Postacrosomal Region of Mammalian Sperm
Formalin-fixed human, bovine, and rat sperm were used for the visualization of polySia. The immunostaining displayed a polySia-positive area on the surface of the head (Fig. 5, A and B). However, more than 90% of all sperm were polySia-negative in all analyzed species. When sperm were pretreated with endoN, no polySia-positive sperm were detectable. Immunostaining with mAb against NCAM and ST8SiaII using human sperm provided comparable results (Fig. 5, C and D). For the detection of the protein backbone of both polySia carriers, a degradation of polySia with endoN was necessary.
FIGURE 5.
Localization of polySia, NCAM, and ST8SiaII on sperm. A, major areas of a sperm. B, formalin-fixed human, bovine, and rat sperm were stained with an antibody against polySia. Human sperm were used to locate NCAM (C) and ST8SiaII (D). E, acrosomes were visualized with peanut agglutinin (PNA) in addition to NCAM and polySia-positive areas using respective antibodies. Staining of nuclei (blue fluorescence) was achieved by DAPI.
For a more detailed localization of the polySia carriers, the acrosome was visualized using the lectin peanut agglutinin demonstrating that the polysialylated proteins were located in the postacrosomal area of the head as shown for human and bovine sperm (Fig. 5E). In the case of human sperm, polysialylated proteins were primarily present on a part of the postacrosomal area close to the tail. In contrast to human sperm, the complete bovine postacrosomal area was polySia-positive. Comparable results were obtained with rat sperm (data not shown).
Epithelial Cells of the Epididymis Express Polysialylated Proteins
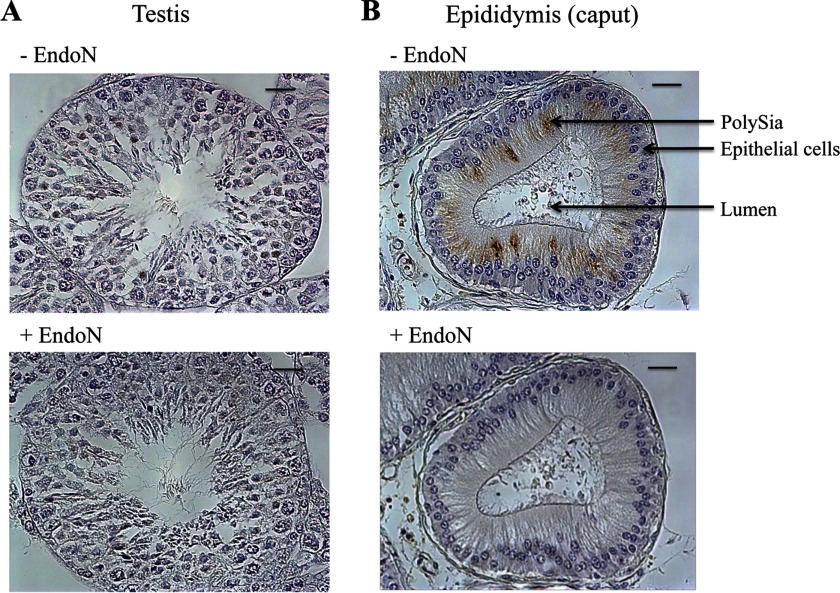
Spermatogenesis takes place in the testis resulting in the generation and release of morphologically differentiated spermatids (44). However, these are neither motile nor able to fertilize. Therefore, maturation of sperm in the epididymis is essential, which comes along, among others, with a change in the cell surface distribution of glycoconjugates. Consequently, it is possible that polySia is present on the surface of all sperm when they leave the testis and that during the maturation in the epididymis the sialic acid polymers are degraded. A second possibility is that polysialylated NCAM and ST8SiaII reach the postacrosomal region during the transfer through the epididymis. Many essential proteins are integrated into the plasma membrane of sperm during the epididymal transit, which are generated and released in so-called epididymosomes by epithelial cells (45). To address this point, paraffin-embedded tissue sections of testis as well as epididymis from mice were examined for polySia. In the tubuli of the testis, no specific immune signal against polySia occurred, including spermatogonia, spermatocytes, spermatids, and Sertoli cells (Fig. 6A). In contrast, a vesicular staining against polySia was visible in epithelial cells of the epididymis (Fig. 6B). The polySia-expressing epithelial cells were located in the caput of the epididymis. Comparable results were obtained when rat and roebuck epididymis were analyzed (data not shown).
FIGURE 6.
Immunohistological localization of polySia in testicular and epididymal tissue. A, paraffin-embedded serial testis (A) and epididymis (B) sections (caput) of mice were stained with a mAb against polySia. For negative control, tissue sections were pretreated with endoN to degrade polySia. Scale bars, 20 μm.
Furthermore, gene transcripts of polysialyltransferases ST8SiaII and ST8SiaIV were analyzed by RT-PCR demonstrating expression of ST8SiaIV in addition to the targets for polysialylation ST8SiaII and NCAM in the epididymis (supplemental Fig. 2). The signal intensity of ST8SiaIV was comparable with the signal intensity of NCAM. However, the expression level of ST8SiaII was lower than the obtained signal for ST8SiaIV and NCAM gene transcripts.
DISCUSSION
In adult mammals, the carbohydrate polymer polySia is mainly expressed in neuronal tissue (1, 22–24). However, more and more reports also associate polysialylated glycoconjugates with other physiological systems (20, 46–49). Because different polySia species contribute to sperm motility and the acrosome reaction of sea urchin sperm (30), we investigated human ejaculates for the presence of polysialylated proteins. Therefore, α2,8-linked polySia could be detected in human semen attached to two different glycoproteins. One of these polySia carriers was NCAM. Surprisingly, the second identified target for polysialylation was the polysialyltransferase ST8SiaII. More than 16 years ago, autopolysialylation of polysialyltransferases was first observed in vitro and later also in cell culture experiments (26, 28, 50, 51). The observed autopolysialylation occurred only in the absence of NCAM and was never observed in vivo so far. Consequently, polysialylation of ST8SiaII and ST8SiaIV was often declared as a biochemical phenomenon, which takes place only under artificial conditions. However, by identifying ST8SiaII as polySia carrier in mammalian semen, we now demonstrate for the first time that polysialylation of polysialyltransferases also occurs in vivo. Besides polysialylated NCAM and ST8SiaII, no further polysialylated glycoproteins could be identified. Nevertheless, a proteomics approach can never completely guarantee that all proteins were identified.
In agreement with in cellulo as well as in vitro experiments concerning the polysialylated glycosylation sites of ST8SiaII, we detected polySia chains on N-glycans of glycosylation site 5 in vivo (52). In addition to this glycosylation site, N-glycans of the third glycosylation site were discussed to be a target for autopolysialylation. However, we never observed a peptide signal corresponding to the de-N-glycosylated glycosylation site 3.
Interestingly, polysialylated NCAM and ST8SiaII were present in the postacrosomal region of mammalian sperm. To determine the origin of polySia, tissue sections of testis and epididymis were investigated demonstrating that polySia was expressed by epithelial cells of the caput. It is well known that epithelial cells in all parts of the epididymis release so-called epididymosomes containing a number of different glycoproteins, which are integrated into the membrane of sperm during maturation (53–56). Epididymosomes contain proteins, for example, that are necessary to prevent a killing of sperm by the innate and adaptive immune system of females. Many studies indicated that insemination leads to a recruitment of immune cells (57–59). Mammalian seminal plasma and sperm contain different components to counteract this activation of the female immune system. For example, N-glycans in semen containing the Lewisx and Lewisy epitope are discussed to inhibit the innate and adaptive immune system of women (60). Intriguingly, α2,8-linked polySia influences also the immune system. For example, tissue macrophages express Siglec-11 that is able to bind α2,8-linked polySia (61). In this way, polySia suppresses the immune response of tissue macrophages in the brain (microglia) after lipopolysaccharide (LPS) stimulation (62). Thus, secreted and partially integrated polySia carriers may also contribute to the immune suppression.
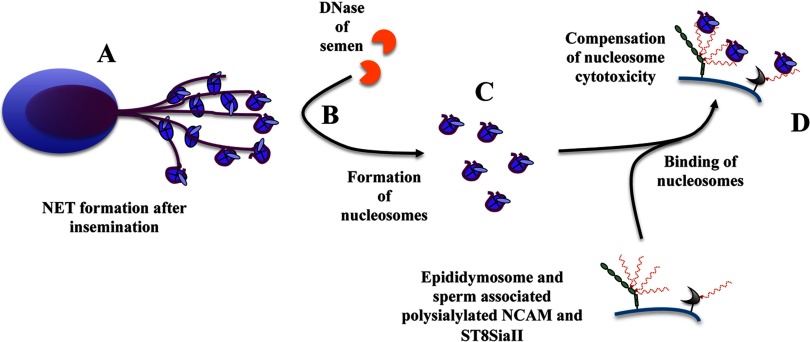
The main population of invading immune cells after insemination consists of neutrophils. One mechanism of neutrophils against invading microorganisms is the formation of neutrophil extracellular traps (NET). Therefore, neutrophils sacrifice themselves, and histone-containing DNA forms together with anti-microbial enzymes and peptides a meshwork to capture and kill pathogens (63, 64). After insemination, neutrophils generate NET, and sperm entangle in this network of DNA and histones (65). DNase of the seminal plasma, however, degrades DNA, and spermatozoa can escape from this system. Interestingly, high levels of DNase in seminal plasma have been associated with higher fertility in bovine systems (66, 67). The most cytotoxic entities of NET for endogenous cells, however, are the extracellular histones (68). Consequently, sperm and epithelial cells would be negatively influenced by exaggerated NET formation and the formation of nucleosomes after degradation of DNA. Recently, we could show that α2,8-linked polySia of bacteria and humans can diminish the cytotoxicity of extracellular histones as well as nucleosomes and therefore may represent a cytoprotective component (68–70). Thus, polySia attached to NCAM and ST8SiaII as the content of mammalian ejaculates may act in the same manner in the female reproductive tract increasing the number of surviving sperm as illustrated in Fig. 7. In terms of the declining semen quality in men (38, 41), the administration of polySia during insemination could represent a useful strategy to increase the number of sperm that are able to escape the innate immune system of women.
FIGURE 7.
Proposed model for a putative cytoprotective function of polySia attached to ST8SiaII and NCAM. A, after insemination, neutrophils are activated, and NET formation is induced. B, DNase of semen degrades DNA. C, cytotoxic nucleosomes are formed. D, polySia chains of NCAM and ST8SiaII localized on sperm as well as epididymosomes bind nucleosomes counteracting nucleosome-mediated cytotoxicity.
In summary, we were able to show that polysialylated ST8SiaII as well as NCAM are partially integrated in the postacrosomal region of a subfraction of sperm. Based on previous findings, these polySia carriers may represent a cytoprotective and/or immune modulatory element of mammalian ejaculates. However, the precise role of polySia and, in particular, the reason for the polysialylation of ST8SiaII in addition to NCAM need to be further investigated.
Supplementary Material
Acknowledgments
We thank Christina Galuska for critical remarks on the manuscript and Sandra Frank for the preparation of figures and proofreading. In addition, we acknowledge Christina Ulm and Caroline Feuerstacke for training the involved medical doctoral candidates and Werner Mink and Siegfried Kühnhardt for expert technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft Grant GA 1755/1-1 (to S. P. G.).

This article contains supplemental Figs. S1 and S2.
- polySia
- polysialic acid
- DMB
- 1,2-diamino-4,5-methylenedioxybenzene
- endoN
- endoneuraminidase
- Neu5Ac
- N-acetylneuraminic acid
- NCAM
- neural cell adhesion molecule
- NET
- neutrophil extracellular traps
- PNGaseF
- peptide-N-glycosidase F.
REFERENCES
- 1. Rutishauser U. (2008) Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 9, 26–35 [DOI] [PubMed] [Google Scholar]
- 2. Galuska S. P., Oltmann-Norden I., Geyer H., Weinhold B., Kuchelmeister K., Hildebrandt H., Gerardy-Schahn R., Geyer R., Mühlenhoff M. (2006) Polysialic acid profiles of mice expressing variant allelic combinations of the polysialyltransferases ST8SiaII and ST8SiaIV. J. Biol. Chem. 281, 31605–31615 [DOI] [PubMed] [Google Scholar]
- 3. Inoue S., Inoue Y. (2001) A challenge to the ultrasensitive chemical method for the analysis of oligo- and polysialic acids at a nanogram level of colominic acid and a milligram level of brain tissues. Biochimie 83, 605–613 [DOI] [PubMed] [Google Scholar]
- 4. Inoue S., Inoue Y. (2001) Developmental profile of neural cell adhesion molecule glycoforms with a varying degree of polymerization of polysialic acid chains. J. Biol. Chem. 276, 31863–31870 [DOI] [PubMed] [Google Scholar]
- 5. Inoue S., Lin S. L., Inoue Y. (2000) Chemical analysis of the developmental pattern of polysialylation in chicken brain. Expression of only an extended form of polysialyl chains during embryogenesis and the presence of disialyl residues in both embryonic and adult chicken brains. J. Biol. Chem. 275, 29968–29979 [DOI] [PubMed] [Google Scholar]
- 6. Nakata D., Troy F. A., 2nd (2005) Degree of polymerization (DP) of polysialic acid (polySia) on neural cell adhesion molecules (N-CAMS): development and application of a new strategy to accurately determine the DP of polySia chains on N-CAMS. J. Biol. Chem. 280, 38305–38316 [DOI] [PubMed] [Google Scholar]
- 7. Finne J. (1982) Occurrence of unique polysialosyl carbohydrate units in glycoproteins of developing brain. J. Biol. Chem. 257, 11966–11970 [PubMed] [Google Scholar]
- 8. Angata K., Suzuki M., Fukuda M. (1998) Differential and cooperative polysialylation of the neural cell adhesion molecule by two polysialyltransferases, PST and STX. J. Biol. Chem. 273, 28524–28532 [DOI] [PubMed] [Google Scholar]
- 9. Nelson R. W., Bates P. A., Rutishauser U. (1995) Protein determinants for specific polysialylation of the neural cell adhesion molecule. J. Biol. Chem. 270, 17171–17179 [DOI] [PubMed] [Google Scholar]
- 10. Liedtke S., Geyer H., Wuhrer M., Geyer R., Frank G., Gerardy-Schahn R., Zähringer U., Schachner M. (2001) Characterization of N-glycans from mouse brain neural cell adhesion molecule. Glycobiology 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 11. von Der Ohe M., Wheeler S. F., Wuhrer M., Harvey D. J., Liedtke S., Mühlenhoff M., Gerardy-Schahn R., Geyer H., Dwek R. A., Geyer R., Wing D. R., Schachner M. (2002) Localization and characterization of polysialic acid-containing N-linked glycans from bovine NCAM. Glycobiology 12, 47–63 [DOI] [PubMed] [Google Scholar]
- 12. Brusés J. L., Rutishauser U. (2001) Roles, regulation, and mechanism of polysialic acid function during neural development. Biochimie 83, 635–643 [DOI] [PubMed] [Google Scholar]
- 13. Fujimoto I., Bruses J. L., Rutishauser U. (2001) Regulation of cell adhesion by polysialic acid. Effects on cadherin, immunoglobulin cell adhesion molecule, and integrin function and independence from neural cell adhesion molecule binding or signaling activity. J. Biol. Chem. 276, 31745–31751 [DOI] [PubMed] [Google Scholar]
- 14. Rutishauser U. (1992) NCAM and its polysialic acid moiety: a mechanism for pull/push regulation of cell interactions during development? Dev. Suppl. 116, 99–104 [PubMed] [Google Scholar]
- 15. Rutishauser U. (1996) Polysialic acid and the regulation of cell interactions. Curr. Opin. Cell Biol. 8, 679–684 [DOI] [PubMed] [Google Scholar]
- 16. Tang J., Landmesser L., Rutishauser U. (1992) Polysialic acid influences specific pathfinding by avian motoneurons. Neuron 8, 1031–1044 [DOI] [PubMed] [Google Scholar]
- 17. Walsh F. S., Doherty P. (1997) Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annu. Rev. Cell Dev. Biol. 13, 425–456 [DOI] [PubMed] [Google Scholar]
- 18. Zuber C., Lackie P. M., Catterall W. A., Roth J. (1992) Polysialic acid is associated with sodium channels and the neural cell adhesion molecule N-CAM in adult rat brain. J. Biol. Chem. 267, 9965–9971 [PubMed] [Google Scholar]
- 19. Yabe U., Sato C., Matsuda T., Kitajima K. (2003) Polysialic acid in human milk. CD36 is a new member of mammalian polysialic acid-containing glycoprotein. J. Biol. Chem. 278, 13875–13880 [DOI] [PubMed] [Google Scholar]
- 20. Curreli S., Arany Z., Gerardy-Schahn R., Mann D., Stamatos N. M. (2007) Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J. Biol. Chem. 282, 30346–30356 [DOI] [PubMed] [Google Scholar]
- 21. Galuska S. P., Rollenhagen M., Kaup M., Eggers K., Oltmann-Norden I., Schiff M., Hartmann M., Weinhold B., Hildebrandt H., Geyer R., Mühlenhoff M., Geyer H. (2010) Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc. Natl. Acad. Sci. U.S.A. 107, 10250–10255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lackie P. M., Zuber C., Roth J. (1990) Polysialic acid and N-CAM localisation in embryonic rat kidney: mesenchymal and epithelial elements show different patterns of expression. Development 110, 933–947 [DOI] [PubMed] [Google Scholar]
- 23. Lackie P. M., Zuber C., Roth J. (1991) Expression of polysialylated N-CAM during rat heart development. Differentiation 47, 85–98 [DOI] [PubMed] [Google Scholar]
- 24. Lackie P. M., Zuber C., Roth J. (1994) Polysialic acid of the neural cell adhesion molecule (N-CAM) is widely expressed during organogenesis in mesodermal and endodermal derivatives. Differentiation 57, 119–131 [DOI] [PubMed] [Google Scholar]
- 25. Weinhold B., Seidenfaden R., Röckle I., Mühlenhoff M., Schertzinger F., Conzelmann S., Marth J. D., Gerardy-Schahn R., Hildebrandt H. (2005) Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J. Biol. Chem. 280, 42971–42977 [DOI] [PubMed] [Google Scholar]
- 26. Close B. E., Colley K. J. (1998) In vivo autopolysialylation and localization of the polysialyltransferases PST and STX. J. Biol. Chem. 273, 34586–34593 [DOI] [PubMed] [Google Scholar]
- 27. Close B. E., Mendiratta S. S., Geiger K. M., Broom L. J., Ho L. L., Colley K. J. (2003) The minimal structural domains required for neural cell adhesion molecule polysialylation by PST/ST8Sia IV and STX/ST8Sia II. J. Biol. Chem. 278, 30796–30805 [DOI] [PubMed] [Google Scholar]
- 28. Mühlenhoff M., Eckhardt M., Bethe A., Frosch M., Gerardy-Schahn R. (1996) Autocatalytic polysialylation of polysialyltransferase-1. EMBO J. 15, 6943–6950 [PMC free article] [PubMed] [Google Scholar]
- 29. Troy F. A., 2nd (1992) Polysialylation: from bacteria to brains. Glycobiology 2, 5–23 [DOI] [PubMed] [Google Scholar]
- 30. Miyata S., Sato C., Kitajima K. (2007) Glycobiology of polysialic acid on sea urchin gametes. Trends Glycosci. Glyc. 19, 85–98 [Google Scholar]
- 31. Miyata S., Sato C., Kumita H., Toriyama M., Vacquier V. D., Kitajima K. (2006) Flagellasialin: a novel sulfated α2,9-linked polysialic acid glycoprotein of sea urchin sperm flagella. Glycobiology 16, 1229–1241 [DOI] [PubMed] [Google Scholar]
- 32. Gerardy-Schahn R., Eckhardt M., Ledermann J., Kemshead J. T. (1994) Topography of NCAM antigenic epitopes recognized by SCLC-cluster-1 antibodies. A consensus view. Int. J. Cancer Suppl. 8, 27–29 [DOI] [PubMed] [Google Scholar]
- 33. Moolenaar C. E., Muller E. J., Schol D. J., Figdor C. G., Bock E., Bitter-Suermann D., Michalides R. J. (1990) Expression of neural cell adhesion molecule-related sialoglycoprotein in small cell lung cancer and neuroblastoma cell lines H69 and CHP-212. Cancer Res. 50, 1102–1106 [PubMed] [Google Scholar]
- 34. Frosch M., Görgen I., Boulnois G. J., Timmis K. N., Bitter-Suermann D. (1985) NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc. Natl. Acad. Sci. U.S.A. 82, 1194–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stummeyer K., Dickmanns A., Mühlenhoff M., Gerardy-Schahn R., Ficner R. (2005) Crystal structure of the polysialic acid-degrading endosialidase of bacteriophage K1F. Nat. Struct. Mol. Biol. 12, 90–96 [DOI] [PubMed] [Google Scholar]
- 36. Sato C., Inoue S., Matsuda T., Kitajima K. (1998) Development of a highly sensitive chemical method for detecting α2–8-linked oligo/polysialic acid residues in glycoproteins blotted on the membrane. Anal. Biochem. 261, 191–197 [DOI] [PubMed] [Google Scholar]
- 37. Galuska S. P., Geyer H., Mink W., Kaese P., Kühnhardt S., Schäfer B., Mühlenhoff M., Freiberger F., Gerardy-Schahn R., Geyer R. (2012) Glycomic strategy for efficient linkage analysis of di-, oligo-, and polysialic acids. J. Proteomics 75, 5266–5278 [DOI] [PubMed] [Google Scholar]
- 38. Rolland M., Le Moal J., Wagner V., Royère D., De Mouzon J. (2013) Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum. Reprod. 28, 462–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inoue S., Lin S. L., Lee Y. C., Inoue Y. (2001) An ultrasensitive chemical method for polysialic acid analysis. Glycobiology 11, 759–767 [DOI] [PubMed] [Google Scholar]
- 40. Roepstorff P., Fohlman J. (1984) Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed. Mass Spectrom. 11, 601. [DOI] [PubMed] [Google Scholar]
- 41. Swan S. H., Elkin E. P., Fenster L. (2000) The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ. Health Perspect. 108, 961–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Galuska S. P., Geyer R., Gerardy-Schahn R., Mühlenhoff M., Geyer H. (2008) Enzyme-dependent variations in the polysialylation of the neural cell adhesion molecule (NCAM) in vivo. J. Biol. Chem. 283, 17–28 [DOI] [PubMed] [Google Scholar]
- 43. Haselhorst T., Stummeyer K., Mühlenhoff M., Schaper W., Gerardy-Schahn R., von Itzstein M. (2006) Endosialidase NF appears to bind polySia DP5 in a helical conformation. ChemBioChem. 7, 1875–1877 [DOI] [PubMed] [Google Scholar]
- 44. Gatti J. L., Castella S., Dacheux F., Ecroyd H., Métayer S., Thimon V., Dacheux J. L. (2004) Post-testicular sperm environment and fertility. Anim. Reprod. Sci. 82, 321–339 [DOI] [PubMed] [Google Scholar]
- 45. Sullivan R., Frenette G., Girouard J. (2007) Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J. Androl. 9, 483–491 [DOI] [PubMed] [Google Scholar]
- 46. Drake P. M., Nathan J. K., Stock C. M., Chang P. V., Muench M. O., Nakata D., Reader J. R., Gip P., Golden K. P., Weinhold B., Gerardy-Schahn R., Troy F. A., 2nd, Bertozzi C. R. (2008) Polysialic acid, a glycan with highly restricted expression, is found on human and murine leukocytes and modulates immune responses. J. Immunol. 181, 6850–6858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Drake P. M., Stock C. M., Nathan J. K., Gip P., Golden K. P., Weinhold B., Gerardy-Schahn R., Bertozzi C. R. (2009) Polysialic acid governs T-cell development by regulating progenitor access to the thymus. Proc. Natl. Acad. Sci. U.S.A. 106, 11995–12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bax M., van Vliet S. J., Litjens M., García-Vallejo J. J., van Kooyk Y. (2009) Interaction of polysialic acid with CCL21 regulates the migratory capacity of human dendritic cells. PLoS One 4, e6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rey-Gallardo A., Delgado-Martín C., Gerardy-Schahn R., Rodríguez-Fernández J. L., Vega M. A. (2011) Polysialic acid is required for neuropilin-2a/b-mediated control of CCL21-driven chemotaxis of mature dendritic cells and for their migration in vivo. Glycobiology 21, 655–662 [DOI] [PubMed] [Google Scholar]
- 50. Close B. E., Tao K., Colley K. J. (2000) Polysialyltransferase-1 autopolysialylation is not requisite for polysialylation of neural cell adhesion molecule. J. Biol. Chem. 275, 4484–4491 [DOI] [PubMed] [Google Scholar]
- 51. Close B. E., Wilkinson J. M., Bohrer T. J., Goodwin C. P., Broom L. J., Colley K. J. (2001) The polysialyltransferase ST8Sia II/STX: post-translational processing and role of autopolysialylation in the polysialylation of neural cell adhesion molecule. Glycobiology 11, 997–1008 [DOI] [PubMed] [Google Scholar]
- 52. Mühlenhoff M., Manegold A., Windfuhr M., Gotza B., Gerardy-Schahn R. (2001) The impact of N-glycosylation on the functions of polysialyltransferases. J. Biol. Chem. 276, 34066–34073 [DOI] [PubMed] [Google Scholar]
- 53. Saez F., Frenette G., Sullivan R. (2003) Epididymosomes and prostasomes: their roles in post-testicular maturation of the sperm cells. J. Androl. 24, 149–154 [DOI] [PubMed] [Google Scholar]
- 54. Aitken R. J., Nixon B., Lin M., Koppers A. J., Lee Y. H., Baker M. A. (2007) Proteomic changes in mammalian spermatozoa during epididymal maturation. Asian J. Androl. 9, 554–564 [DOI] [PubMed] [Google Scholar]
- 55. Thimon V., Frenette G., Saez F., Thabet M., Sullivan R. (2008) Protein composition of human epididymosomes collected during surgical vasectomy reversal: a proteomic and genomic approach. Hum. Reprod. 23, 1698–1707 [DOI] [PubMed] [Google Scholar]
- 56. Marengo S. R. (2008) Maturing the sperm: unique mechanisms for modifying integral proteins in the sperm plasma membrane. Anim. Reprod. Sci. 105, 52–63 [DOI] [PubMed] [Google Scholar]
- 57. Drobnis E. Z., Overstreet J. W. (1992) Natural history of mammalian spermatozoa in the female reproductive tract. Oxf. Rev. Reprod. Biol. 14, 1–45 [PubMed] [Google Scholar]
- 58. Pandya I. J., Cohen J. (1985) The leukocytic reaction of the human uterine cervix to spermatozoa. Fertil. Steril. 43, 417–421 [DOI] [PubMed] [Google Scholar]
- 59. Thompson L. A., Barratt C. L., Bolton A. E., Cooke I. D. (1992) The leukocytic reaction of the human uterine cervix. Am. J. Reprod. Immunol. 28, 85–89 [DOI] [PubMed] [Google Scholar]
- 60. Pang P. C., Tissot B., Drobnis E. Z., Sutovsky P., Morris H. R., Clark G. F., Dell A. (2007) Expression of bisecting type and Lewisx/Lewisy terminated N-glycans on human sperm. J. Biol. Chem. 282, 36593–36602 [DOI] [PubMed] [Google Scholar]
- 61. Wang X., Chow R., Deng L., Anderson D., Weidner N., Godwin A. K., Bewtra C., Zlotnik A., Bui J., Varki A., Varki N. (2011) Expression of Siglec-11 by human and chimpanzee ovarian stromal cells, with uniquely human ligands: implications for human ovarian physiology and pathology. Glycobiology 21, 1038–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang Y., Neumann H. (2010) Alleviation of neurotoxicity by microglial human Siglec-11. J. Neurosci. 30, 3482–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A. (2004) Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 [DOI] [PubMed] [Google Scholar]
- 64. Brinkmann V., Zychlinsky A. (2007) Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 5, 577–582 [DOI] [PubMed] [Google Scholar]
- 65. Alghamdi A. S., Foster D. N. (2005) Seminal DNase frees spermatozoa entangled in neutrophil extracellular traps. Biol. Reprod. 73, 1174–1181 [DOI] [PubMed] [Google Scholar]
- 66. McCauley T. C., Zhang H., Bellin M. E., Ax R. L. (1999) Purification and characterization of fertility-associated antigen (FAA) in bovine seminal fluid. Mol. Reprod. Dev. 54, 145–153 [DOI] [PubMed] [Google Scholar]
- 67. Bellin M. E., Oyarzo J. N., Hawkins H. E., Zhang H., Smith R. G., Forrest D. W., Sprott L. R., Ax R. L. (1998) Fertility-associated antigen on bull sperm indicates fertility potential. J. Anim. Sci. 76, 2032–2039 [DOI] [PubMed] [Google Scholar]
- 68. Saffarzadeh M., Juenemann C., Queisser M. A., Lochnit G., Barreto G., Galuska S. P., Lohmeyer J., Preissner K. T. (2012) Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 7, e32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Saffarzadeh M., Preissner K. T. (2013) Fighting against the dark side of neutrophil extracellular traps in disease: manoeuvres for host protection. Curr. Opin. Hematol. 20, 3–9 [DOI] [PubMed] [Google Scholar]
- 70. Ulm C., Saffarzadeh M., Mahavadi P., Müller S., Prem G., Saboor F., Simon P., Middendorff R., Geyer H., Henneke I., Bayer N., Rinne S., Lütteke T., Böttcher-Friebertshauser E., Gerardy-Schahn R., Schwarzer D., Mühlenhoff M., Preissner K. T., Günther A., Geyer R., Galuska S. P. (2013) Soluble polysialylated NCAM: a novel player of the innate immune system in the lung. Cell. Mol. Life Sci., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.