Abstract
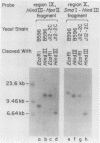
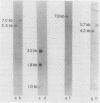
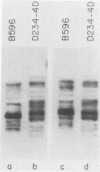
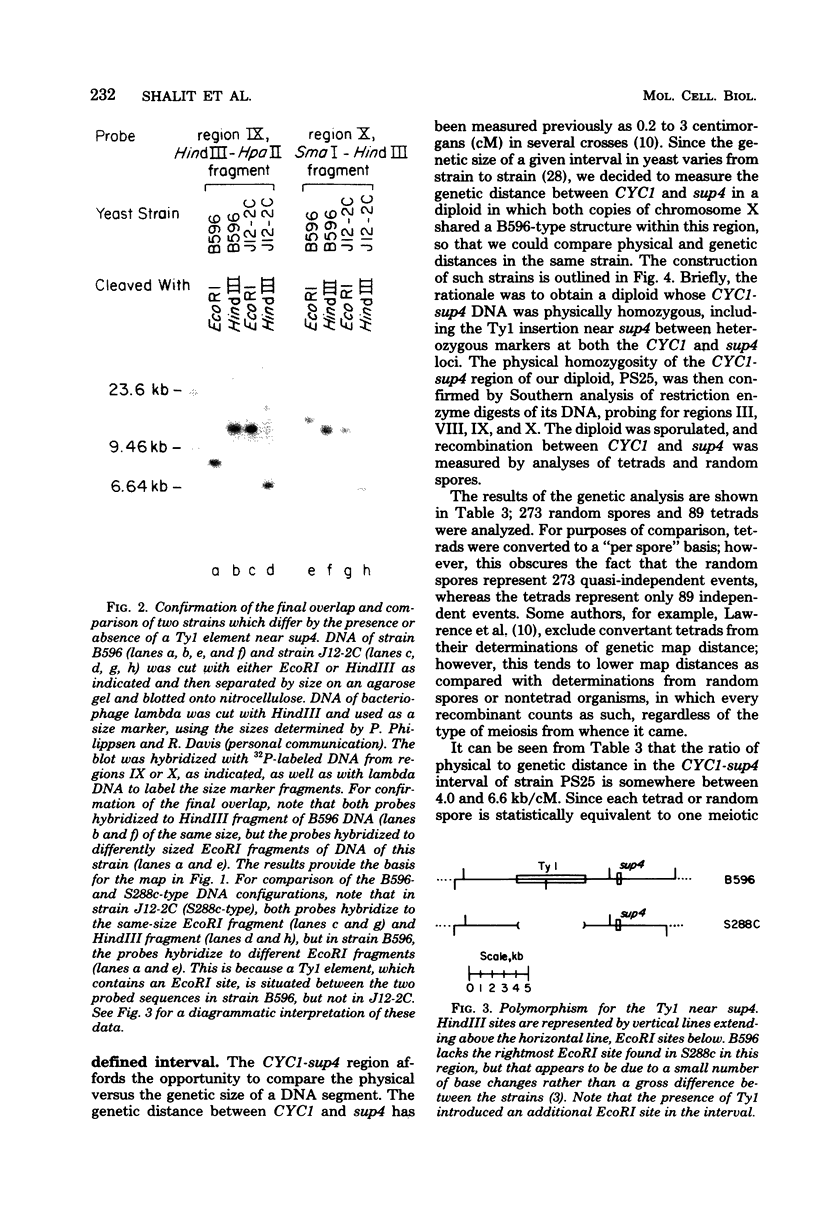
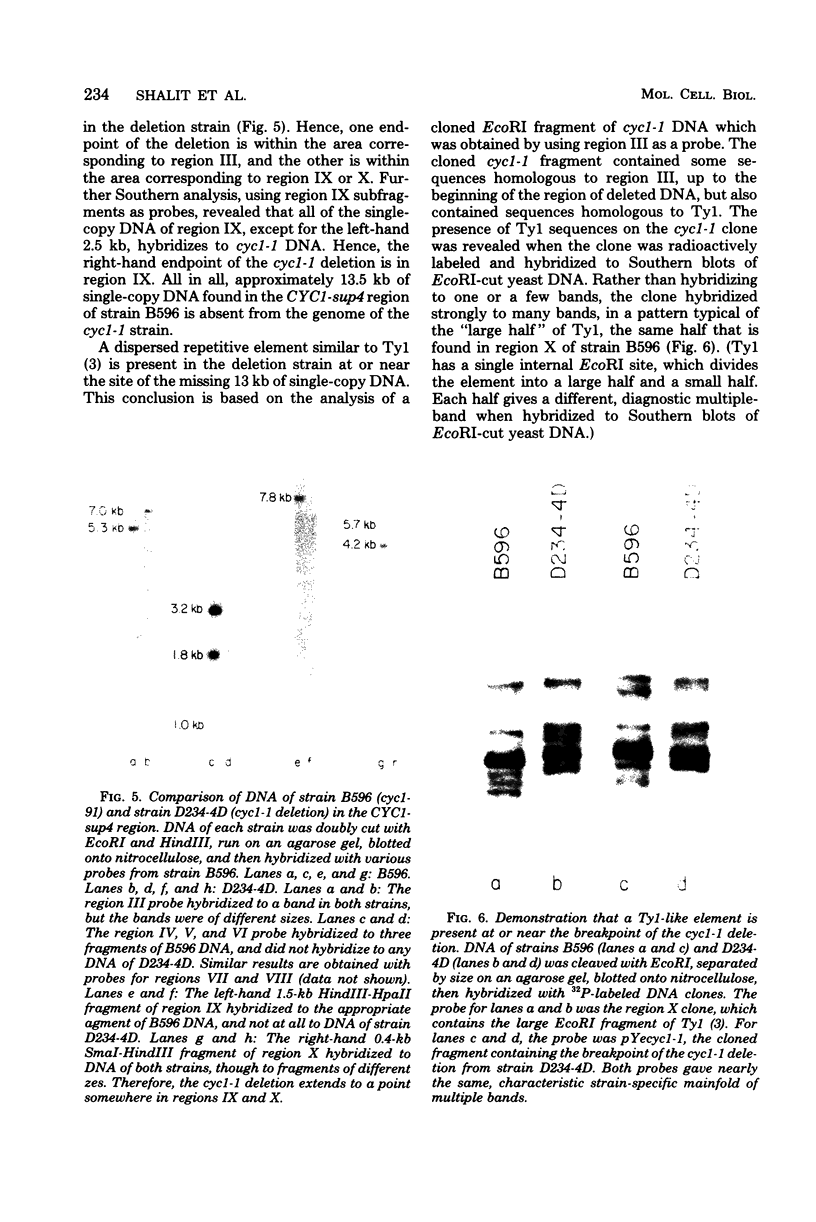
CYC1 and sup4 are part of a tightly linked cluster of genes on chromosome X in the yeast Saccharomyces cerevisiae. Using as probes previously cloned fragments containing the CYC1 and sup4 genes, we have identified and cloned the deoxyribonucleic acid (DNA) present between these genes in one strain of yeast. We find that the CYC1 and sup4 genes are approximately 21 kilobases apart. In the same strain, the meiotic map distance is approximately 3.7 centimorgans, for a ratio of 5.6 kilobases per centimorgan in this interval. The physical mapping has allowed unambiguous determination of the orientation of CYC1 and sup4 relative to each other, the centromere, and a nearby transfer ribonucleic acid (tRNA(2Ser)) gene. The spontaneous mutation cyc1-1 inactivates the CYC1 gene as well as the neighboring loci OSM1 and RAD7. We have determined that a cyc1-1-bearing strain lacks approximately 13 kilobases of single-copy DNA from the CYC1-sup4 region, including all of the CYC1 coding information. There is a sequence homologous to the middle-repetitive element Ty1 at or near the breakpoint of the cyc1-1 deletion. We discuss the possibility that Ty elements play a role in the formation of such large, spontaneous deletions, which occur frequently in this region of chromosome X in certain yeast strains.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Cameron J. R., Loh E. Y., Davis R. W. Evidence for transposition of dispersed repetitive DNA families in yeast. Cell. 1979 Apr;16(4):739–751. doi: 10.1016/0092-8674(79)90090-4. [DOI] [PubMed] [Google Scholar]
- Chinault A. C., Carbon J. Overlap hybridization screening: isolation and characterization of overlapping DNA fragments surrounding the leu2 gene on yeast chromosome III. Gene. 1979 Feb;5(2):111–126. doi: 10.1016/0378-1119(79)90097-0. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., Olson M. V., Hall B. D. Nucleotide sequence of a mutant eukaryotic gene: the yeast tyrosine-inserting ochre suppressor SUP4-o. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5453–5457. doi: 10.1073/pnas.74.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- Kurjan J., Hall B. D., Gillam S., Smith M. Mutations at the yeast SUP4 tRNATyr locus: DNA sequence changes in mutants lacking suppressor activity. Cell. 1980 Jul;20(3):701–709. doi: 10.1016/0092-8674(80)90316-5. [DOI] [PubMed] [Google Scholar]
- Lauer G. D., Roberts T. M., Klotz L. C. Determination of the nuclear DNA content of Saccharomyces cerevisiae and implications for the organization of DNA in yeast chromosomes. J Mol Biol. 1977 Aug 25;114(4):507–526. doi: 10.1016/0022-2836(77)90175-9. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Sherman F., Jackson M., Gilmore R. A. Mapping and gene conversion studies with the structural gene for iso-1-cytochrome C in yeast. Genetics. 1975 Dec;81(4):615–629. doi: 10.1093/genetics/81.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman S. W., Singh A., Sherman F. A mutator affecting the region of the iso-1-cytochrome c gene in yeast. Genetics. 1979 Jul;92(3):783–802. doi: 10.1093/genetics/92.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley D. L., Sandler L. The genetic analysis of meiosis in female Drosophila melanogaster. Philos Trans R Soc Lond B Biol Sci. 1977 Mar 21;277(955):295–312. doi: 10.1098/rstb.1977.0019. [DOI] [PubMed] [Google Scholar]
- Montgomery D. L., Hall B. D., Gillam S., Smith M. Identification and isolation of the yeast cytochrome c gene. Cell. 1978 Jul;14(3):673–680. doi: 10.1016/0092-8674(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in yeast. Methods Cell Biol. 1975;11:221–233. doi: 10.1016/s0091-679x(08)60325-8. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Olson M. V., Hall B. D., Cameron J. R., Davis R. W. Cloning of the yeast tyrosine transfer RNA genes in bacteriophage lambda. J Mol Biol. 1979 Jan 25;127(3):285–295. doi: 10.1016/0022-2836(79)90330-9. [DOI] [PubMed] [Google Scholar]
- Olson M. V., Loughney K., Hall B. D. Identification of the yeast DNA sequences that correspond to specific tyrosine-inserting nonsense suppressor loci. J Mol Biol. 1979 Aug 15;132(3):387–410. doi: 10.1016/0022-2836(79)90267-5. [DOI] [PubMed] [Google Scholar]
- Roeder G. S., Fink G. R. DNA rearrangements associated with a transposable element in yeast. Cell. 1980 Aug;21(1):239–249. doi: 10.1016/0092-8674(80)90131-2. [DOI] [PubMed] [Google Scholar]
- Ross D. G., Swan J., Kleckner N. Physical structures of Tn10-promoted deletions and inversions: role of 1400 bp inverted repetitions. Cell. 1979 Apr;16(4):721–731. doi: 10.1016/0092-8674(79)90088-6. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J., Esposito R. E., Esposito M. S. The effect of ochre suppression on meiosis and ascospore formation in Saccharomyces. Genetics. 1977 Jan;85(1):35–54. doi: 10.1093/genetics/85.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. Deletions of a tyrosine tRNA gene in S. cerevisiae. Cell. 1979 May;17(1):185–190. doi: 10.1016/0092-8674(79)90306-4. [DOI] [PubMed] [Google Scholar]
- Royal A., Garapin A., Cami B., Perrin F., Mandel J. L., LeMeur M., Brégégègre F., Gannon F., LePennec J. P., Chambon P. The ovalbumin gene region: common features in the organisation of three genes expressed in chicken oviduct under hormonal control. Nature. 1979 May 10;279(5709):125–132. doi: 10.1038/279125a0. [DOI] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., Jackson M., Liebman S. W., Schweingruber A. M., Stewart J. W. A deletion map of cyc1 mutants and its correspondence to mutationally altered iso-1-cytochromes c of yeast. Genetics. 1975 Sep;81(1):51–73. doi: 10.1093/genetics/81.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., Stewart J. W., Jackson M., Gilmore R. A., Parker J. H. Mutants of yeast defective in iso-1-cytochrome c. Genetics. 1974 Jun;77(2):255–284. doi: 10.1093/genetics/77.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., Stewart J. W., Parker J. H., Inhaber E., Shipman N. A., Putterman G. J., Gardisky R. L., Margoliash E. The mutational alteration of the primary structure of yeast iso-1-cytochrome c. J Biol Chem. 1968 Oct 25;243(20):5446–5456. [PubMed] [Google Scholar]
- Simchen G., Ball N., Nachshon I. Fine control of genetic recombination in yeast. Heredity (Edinb) 1971 Feb;26(1):137–140. doi: 10.1038/hdy.1971.12. [DOI] [PubMed] [Google Scholar]
- Singh A., Sherman F. Deletions of the iso-1-cytochrome c and adjacent genes of yeast: discovery of the OSM1 gene controlling osmotic sensitivity. Genetics. 1978 Aug;89(4):653–665. doi: 10.1093/genetics/89.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Leung D. W., Gillam S., Astell C. R., Montgomery D. L., Hall B. D. Sequence of the gene for iso-1-cytochrome c in Saccharomyces cerevisiae. Cell. 1979 Apr;16(4):753–761. doi: 10.1016/0092-8674(79)90091-6. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Newlon C. S., Herskowitz I., Hicks J. B. Isolation of a circular derivative of yeast chromosome III: implications for the mechanism of mating type interconversion. Cell. 1979 Oct;18(2):309–319. doi: 10.1016/0092-8674(79)90050-3. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Zitomer R. S., Montgomery D. L., Nichols D. L., Hall B. D. Transcriptional regulation of the yeast cytochrome c gene. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3627–3631. doi: 10.1073/pnas.76.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]