Background: The role of the amyloid precursor protein (APP) in neural stem/progenitor cell (NSPC) proliferation is poorly understood.
Results: Immunodepletion of cystatin C from NSPC conditioned medium abrogated an effect of APP on NSPC proliferation.
Conclusion: Cystatin C mediates APP-induced NSPC proliferation.
Significance: The results increase understanding of mechanisms promoting NSPC survival and differentiation.
Keywords: Amyloid, Amyloid Precursor Protein, Neural Stem Cell, Neurogenesis, Proliferation, Cystatin C
Abstract
The amyloid precursor protein (APP) is well studied for its role in Alzheimer disease. However, little is known about its normal function. In this study, we examined the role of APP in neural stem/progenitor cell (NSPC) proliferation. NSPCs derived from APP-overexpressing Tg2576 transgenic mice proliferated more rapidly than NSPCs from the corresponding background strain (C57Bl/6xSJL) wild-type mice. In contrast, NSPCs from APP knock-out (APP-KO) mice had reduced proliferation rates when compared with NSPCs from the corresponding background strain (C57Bl/6). A secreted factor, identified as cystatin C, was found to be responsible for this effect. Levels of cystatin C were higher in the Tg2576 conditioned medium and lower in the APP-KO conditioned medium. Furthermore, immunodepletion of cystatin C from the conditioned medium completely removed the ability of the conditioned medium to increase NSPC proliferation. The results demonstrate that APP expression stimulates NSPC proliferation and that this effect is mediated via an increase in cystatin C secretion.
Introduction
Neural stem cells are self-renewing, multipotent cells that can produce all of the major cellular phenotypes in the nervous system (1, 2). Neural stem cells are important, not only because they produce the entire complement of neuronal and glial cells of the mature nervous system and continue to generate new neurons throughout life, but also because they may be useful for the therapeutic replacement of cells in neurodegenerative diseases. The mechanisms that promote neural stem or progenitor cell (NSPC)4 proliferation are only partially understood. Both epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) are well studied for their roles in stimulating NSPC proliferation in vitro (2, 3). However, other autocrine growth factors produced by the NSPCs themselves may also be necessary for optimum growth.
The β-amyloid precursor protein (APP) is a 110–130-kDa integral type I transmembrane glycoprotein that has been extensively studied for its role in Alzheimer disease (AD) (4). Despite the very large number of published studies on APP, the normal function of APP has remained a mystery. APP is encoded by a single gene located on chromosome 21 (5). APP is post-translationally glycosylated and phosphorylated and can be cleaved by two major proteolytic pathways. In one pathway, sequential cleavage of APP by α- and γ-secretase generates a large ectodomain fragment (sAPPα), which is secreted into the extracellular milieu, and a small C-terminal fragment (the APP intracellular domain or AICD), which may have a role in regulating gene expression. In the other pathway, cleavage of APP by β- and γ-secretase generates a different ectodomain fragment (sAPPβ), as well as the AICD peptide. Cleavage of APP via this second pathway also generates the β-amyloid protein (Aβ) of AD (6).
Although the normal function of APP is poorly understood, the pattern of expression of APP suggests that it may be important for neuronal growth and differentiation, not only in the developing brain but also in the mature or aging nervous system. The expression of APP has been shown to increase as the nervous system matures (7). APP expression increases as NSPCs mature into neurons, and soluble APP has been reported to promote neural differentiation (8, 9). APP may also play a role in later stages of neuronal development. For example, soluble APP is reported to stimulate neurite outgrowth in a variety of cell systems (10–14). Our studies have shown that APP expression is increased in the olfactory neuroepithelium at the developmental stage when neurogenesis and neurite outgrowth begin (15). Similarly, APP has been reported to regulate a number of developmental functions including neuronal migration (16) and cell growth (17, 18). A role for APP in cell growth is supported by the rapid up-regulation of APP that occurs in response to axonal injury (19–21). Dystrophic neurites found around amyloid plaques are highly immunoreactive for APP (22–24), consistent with the possibility that APP may play a role in neural repair.
Neurogenesis is reported to be increased in transgenic mice that overexpress APP. For example, Jin et al. (25) reported a 2-fold increase in BrdU-labeled cells in PDGF-APPsw,Ind mice at 3 months of age. More recently, López-Toledano and Shelanski (26) reported similar findings. The increase in neural precursor proliferation was attributed either to a compensatory mechanism resulting from disease pathology in the mice (25) or to a direct effect of Aβ (26).
To address the role of APP in NSPC proliferation and neurogenesis, we have examined the growth and proliferation in culture of NSPCs derived from APP transgenic mice (Tg2576) and from APP knock-out (APP-KO) mice. We report that the proliferation rate of NSPCs from APP-overexpressing cells is increased and that the proliferation of NSPCs from APP-KO cells is decreased when compared with the corresponding background strain NSPCs. Furthermore, we report that this effect is mediated by a secreted factor. Despite previous suggestions that sAPPα can influence the growth of neural stem cells (9, 27, 28), we did not find any evidence that the effect on NSPCs is mediated by sAPPα. Instead, we demonstrate that APP-induced NSPC proliferation is mediated, at least in part, by secreted cystatin C.
EXPERIMENTAL PROCEDURES
Materials
Synthetic human sequence Aβ peptides (>95% pure) were obtained from the Keck Biotechnology Resource Laboratory (New Haven, CT). Dulbecco's modified Eagle's medium (DMEM), B27 supplement, and poly-l-lysine were from Life Technologies Australia Pty. Ltd. (Mulgrave, Australia). Penicillin, streptomycin, human recombinant sAPPα, and human recombinant EGF were all obtained from Sigma-Aldrich Pty. Ltd. (Castle Hill, Australia). Human recombinant bFGF was from PeproTech (Rocky Hill, NJ). The anti-Aβ monoclonal antibody (mAb) 6E10 was from Covance Pty. Ltd. (North Ryde, New South Wales, Australia). Mouse recombinant cystatin C, anti-mouse cystatin C antibody, affinity-purified polyclonal goat immunoglobulin G, and normal goat immunoglobulin G were from R&D Systems Inc. (Minneapolis, MN). Anti-βIII tubulin mAb was from Promega Australia (Alexandria, Australia). Normal mouse immunoglobulin G, anti-goat horseradish peroxidase-conjugated secondary antibody, and anti-mouse horseradish peroxidase-conjugated secondary antibody were from Dako Australia Pty. Ltd. (Campbellfield, Australia). Newborn pups (postnatal day 0) of APP-KO mice (from The Jackson Laboratory, Bar Harbor, ME) and their corresponding wild-type background strain controls (C57Bl/6), as well as human APP-overexpressing mice (APPSW Tg2576, from Taconic Farms Inc., Hudson, NY) and their corresponding background strain controls (C57Bl/6×SJL), were used for this study. Mice were housed in the animal facility at the University of Tasmania. All experiments were approved by the University of Tasmania Animal Ethics Committee.
Neurosphere and Isolated NSPC Cultures
Primary neurosphere cultures derived from cerebral cortices of newborn mice (postnatal day 0) were prepared according to the procedure of Millet et al. (29). Brain cortices were cleared of meninges and hippocampus and then incubated in 1×TrypLETM Express with EDTA (Life Technologies Australia) for 10 min at 37 °C. Tissue was disrupted mechanically with a 1000-μl fine tip, and then the tissue was passed through a 40-μm cell strainer (BD Biosciences, North Ryde, Australia) to remove undissociated cells. The single neurospheres were prepared by growing cells in suspension in T75 cell culture flasks at a density of 20,000 cells/ml in proliferation medium (DMEM supplemented with 2% B27, penicillin (100 units/ml), streptomycin (100 units/ml), human bFGF (20 ng/ml), and human EGF (20 ng/ml). After 7 days in culture, neurospheres were dissociated mechanically with 200-μl fine tips, and cells were counted in a hemocytometer and then either reseeded as suspension cultures or replated as adherent cultures in 200 μl per well of proliferation medium on poly-l-lysine-coated 96-well plates at a density of 2000 cells/well. All cultures were incubated in a humidified incubator at 37 °C with 5% CO2.
In Vitro Assays of Cell Number and Proliferation
Cell number was measured by alamarBlue assay (28). Dissociated cells cultured adherently on poly-l-lysine-coated 96-well plates were incubated for up to 6 days, and then 20 μl of alamarBlue reagent (Life Technologies Australia) was added into each well, and the cells were incubated for a further 4 h. The fluorescence intensity was determined using a FLUOstar Optima microplate fluorescence plate reader at an excitation wavelength of 540 nm and an emission wavelength of 590 nm. Cell number was expressed as the relative fluorescence intensity.
Cell proliferation was measured by EdU incorporation. After 4 days in proliferation medium, cells were incubated with EdU for 8 h as described previously (30).
Analysis of Differentiation of NSPCs
Neurospheres were mechanically dissociated, and then isolated cells were plated at a density of 105 cells/well in 24-well plate. The cells were grown in a differentiation medium (DMEM containing 1% fetal calf serum (FCS), 2% B27 supplement, 100 units/ml penicillin, and 100 units/ml streptomycin) for 5 days at 37 °C in an atmosphere containing 5% CO2. The cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) (8 g/liter NaCl, 0.2 g/liter KCl, 1.44 g/liter Na2HPO4, and 0.24 g/liter KH2PO4, pH 7.2) for 15 min, permeabilized with 0.03% (v/v) Triton X-100 in PBS for 5 min, and then blocked in 4% goat serum in PBS for 20 min. Fixed cells were stained with mouse anti-βIII tubulin antibody (1:1000 diluted in 2% goat serum in PBS) and then incubated with a goat anti-mouse IgG conjugated to Alexa Fluor 488 (1:1000 diluted in 2% goat serum in PBS) and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) at 1:7000 dilution.
Effect of Conditioned Medium on NSPC Proliferation
Conditioned medium was collected from neurosphere cultures that had been grown over a period of 7 days. To examine the effect of conditioned medium on NSPC proliferation, dissociated cells from neurospheres were cultured adherently on poly-l-lysine-coated 96-well plates in 100 μl/well of proliferation medium. Sixteen hours after plating, 100 μl of conditioned medium or normal proliferation medium (control) was added, and the cells were incubated for 3 or 5 days, after which proliferation was measured using the alamarBlue assay.
Immunoblotting
The level of sAPPα and cystatin C in conditioned medium was determined by Western blotting. The volume per well of conditioned medium that was analyzed was adjusted so that it represented the same number of viable cells, as determined using the alamarBlue assay. Routinely, ∼10–30 μl was loaded into each gel lane for analysis. For the analysis of intracellular cystatin C, cells were washed with PBS and then lysed as described previously (31) prior to analysis by Western blotting.
Proteins were separated on 8% (sAPPα) or 12% (cystatin C) sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gels before being transferred electrophoretically onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked for 1 h with 5% skim milk powder in 50 mm Tris-buffered saline (pH 8) containing 0.05% Tween 20 (TBS-Tween) and incubated overnight at 4 °C with either anti-APP antibody (22C11 at 1:1000 dilution or 6E10 at 1:1000 dilution) or anti-cystatin C antibody (1:1000 dilution). Chemiluminescence reactions were monitored using a CHEMI-SMART 5000, and images were collected using Chemi-Capt 50001. For quantification of immunoreactivity, images of blots were analyzed using ImageJ version 1.46.
Immunoprecipitation
For depletion of sAPPα or cystatin C from conditioned medium, mAb 6E10, anti-mouse cystatin C antibody, or goat immunoglobulin G (35 μg) was incubated with 500 μl of protein G agarose gel (Roche Products Pty. Ltd., Dee Why, Australia) overnight at 4 °C in 5 ml of PBS. The gel was then washed three times with 5 ml PBS, after which the gel was incubated with 5.5 ml of conditioned medium for 3 h at 4 °C. Finally, the gel slurry containing conditioned medium was centrifuged (10,000 × g), and the resulting supernatant fraction was assayed by Western blotting or used for cell proliferation experiments.
Real-time PCR
RNA was extracted from the neurospheres of n = 6 independent mouse cohorts using an RNeasy mini kit (Qiagen Pty. Ltd., Chadstone, Australia) as described by the manufacturer. Each preparation of neurospheres contained ∼106 cells. Six independent RNA extracts were obtained from each neurosphere preparation. cDNA was obtained from 400 ng of RNA with an RT2 First Strand kit (Qiagen) as described by the manufacturer. The cystatin C primers (Cst3) were from Qiagen, and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were from Geneworks Pty. Ltd. (Hindmarsh, Australia). GAPDH was used as an internal control. All primers were used at a concentration of 10 μm. All samples were diluted 1:10 and analyzed in triplicate. Standard curves for Cst3 and GAPDH with concentrations 1, 0.5, 0.25, and 0.125 μg were used to quantify Cst3 mRNA using SYBR master mix (Qiagen). The results were analyzed using a LightCycler 480 (Roche Diagnostics Australia Pty. Ltd., Castle Hill, Australia).
Statistical Analysis
Statistical analysis was performed with GraphPad Prism software, version 5.04. Data were tested by one-way ANOVA or Student's t test. Post hoc comparisons were analyzed using Tukey's test. Differences were considered statistically significant when p < 0.05. Data values are presented as the means ± S.E. All results were derived from at least three independent experiments in which cells were derived from at least three different mice of the same strain.
RESULTS
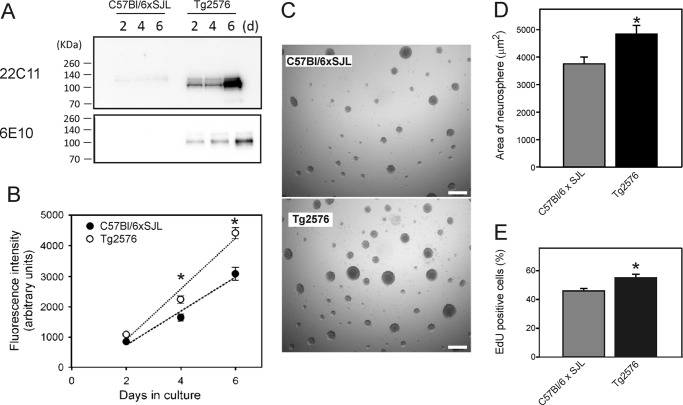
We first examined the level of APP in neurosphere cultures to confirm that the level of APP expression was higher in the Tg2576 cultures than in the background strain (C57Bl/6xSJL) littermate control cultures. At 2, 4, and 6 days after plating, conditioned medium was analyzed for APP by Western blotting with mAb 22C11,which recognizes both mouse and human APP and the APP homologue amyloid protein-like protein-2, and with mAb 6E10, which recognizes human sAPPα. The results confirmed that APP levels were much higher in the medium of Tg2576 neurosphere cultures than in the background strain cultures (Fig. 1A). A major band of 100–110 kDa was detected in the medium, corresponding to the molecular mass of sAPPα.
FIGURE 1.
Analysis of APP levels and proliferation of NSPCs derived from Tg2576 mice and background strain C57Bl/6xSJL mice. A, Western blot analysis of the level of APP in the conditioned medium of neurosphere cultures. Blots were stained with mAb 22C11, which recognizes both human and mouse APP, and mAb 6E10, which is specific for human-sequence APP. B, analysis of the proliferation of NSPCs. Neurospheres were dissociated, and the isolated cells were seeded on poly-l-lysine-coated 96-well plates at a density of 2000 cells/well. After 2, 4, or 6 days in culture, the relative number of cells was estimated using the alamarBlue assay. Fluorescence intensity was taken as an index of cell number. Values are means ± S.E. (n = 3 wells). C, phase-contrast microscopy of neurospheres after 7 days in culture. Scale bar = 200 μm. D, quantitation of the area of neurospheres from the experiment shown in C. Values are means ± S.E. (n = 200 neurospheres). E, EdU incorporation assay of cell proliferation. NSPCs were cultured in proliferation medium for 4 days prior to incubation for 8 h with EdU (30). The graph shows the mean values (± S.E.) of the percentage of cells incorporating EdU (n = 30 incubations). * = significantly different from corresponding values for the background strain C57BL/6xSJL cells (p < 0.05, experiment in panel B, one-way ANOVA with post hoc Tukey's test; experiment in panel D, Student's t test).
Next, to examine the role of APP in NSPC proliferation, we compared the proliferation of cells derived from Tg2576 mice with that of the background strain controls. Neurosphere cultures were dissociated into a single cell suspension on day 7, and then cells were cultured adherently on poly-l-lysine-coated 96-well plates. The proliferation of the cells was measured using an alamarBlue assay. Fluorescence intensity in an alamarBlue assay was taken as an index of the number of viable cells. Overall, the growth rate of the cells derived from the Tg2576 mice was significantly greater (p < 0.05, one-way ANOVA with post hoc Tukey's test) than that of the cells derived from the background strain mice (Fig. 1B).
We also examined the growth of neurospheres to determine whether the proliferation of NSPCs was increased in the Tg2576 cultures. Neurospheres were cultured for a period of 7 days, after which they were examined under phase-contrast microscopy (Fig. 1, C and D). Neurospheres derived from Tg2576 mice were on average greater in size than the neurospheres from the background strain mice, confirming that the growth of NSPCs in Tg2576 cultures was greater than that of the background strain cultures.
To confirm that the increased growth of the Tg2576 NSPC was due to a higher proliferation rate, we measured proliferation using an EdU uptake assay (30). The percentage of EdU-positive cells in the Tg2576 cultures was ∼20% higher than the cultures derived from background strain mice (Fig. 1E). These results clearly supported the view that the increased growth observed using the alamarBlue assay and in the neurosphere cultures was due to an increase in the amount of NSPC proliferation.
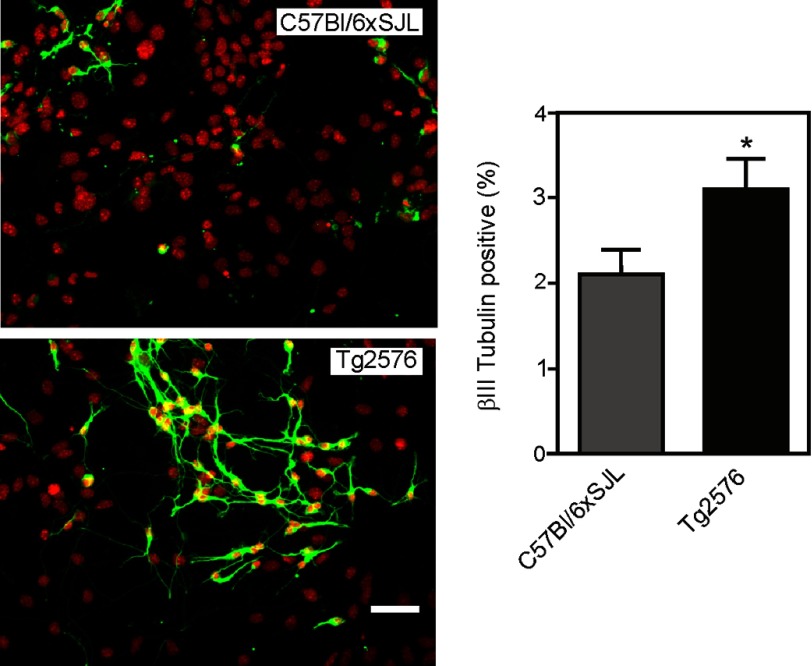
To determine whether the higher proliferation rate of the Tg2576 cells was also associated with a higher potential for neuronal differentiation, isolated NSPCs, grown adherently, were transferred to a differentiation medium containing 1% FCS but lacking EGF and bFGF. The cells were incubated over 5 days and then immunostained for the neuronal marker βIII-tubulin (Fig. 2). The results showed that there was an increased proportion of neurons in the Tg2576 cell population when compared with the cells derived from the background strain (C57Bl/6xSJL) mice (Fig. 2). Tg2576 cells also possessed longer, more extensive neurite networks than the background strain cells.
FIGURE 2.
Neuronal differentiation of NSPCs derived from C57Bl/6xSJL and Tg2576 mice. Neurospheres were dissociated, and the resulting cells were cultured adherently on poly-l-lysine for 5 days in differentiation medium. The cells were then stained for βIII-tubulin (green) and counterstained with DAPI (red) to visualize cell nuclei. Scale bar = 50 μm. The figure also shows quantitation of the percentage of βIII tubulin-positive cells for both groups. Values are means ± S.E. Twenty fields were counted for each group. Each field contained ∼100 DAPI-positive cells. * = significantly different from C57Bl/6xSJL group (p < 0.05, Student's t test).
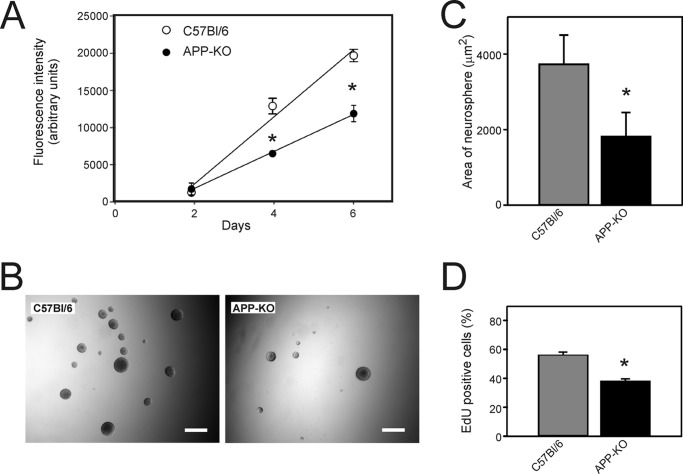
To examine whether expression of endogenous mouse APP influences NSPC proliferation and to rule out the possibility that the increase in proliferation observed in Tg2576 cultures was due to a factor unrelated to APP overexpression, we also examined the proliferation rate of NSPCs from APP-KO mice. We found that NSPC proliferation was decreased in APP-KO cultures when compared with the corresponding background strain (C57Bl/6) cultures (Fig. 3). The growth rate of the APP-KO cells, as assessed by an alamarBlue assay, was ∼60% of that of the cells derived from background strain mice (Fig. 3A). Furthermore, neurospheres from APP-KO mice were smaller and less numerous than the C57Bl/6 neurospheres (Fig. 3, B and C). In addition, the percentage of proliferating EdU-positive cells was also significantly decreased. These experiments clearly demonstrated that endogenous APP was also involved in the regulation of NSPC proliferation.
FIGURE 3.
Analysis of proliferation of NSPCs derived from APP knock-out and from C57Bl/6 (corresponding background strain) mice. A, neurospheres were dissociated, and the isolated cells were seeded on poly-l-lysine-coated 96-well plates at a density of 2000 cells/well. After 2, 4, or 6 days in culture, the relative number of cells was estimated using the alamarBlue assay. Fluorescence intensity was measured as an index of cell number. Values are means ± S.E. (n = 3 wells). * = significantly different from corresponding values for the background strain C57Bl/6 cells (p < 0.05, one-way ANOVA). B, phase-contrast microscopy of neurospheres after 7 days in culture. Scale bar = 200 μm. C, quantitation of the area of neurospheres from the experiment shown in B. Values are means ± S.E. (n = 200 neurospheres). D, EdU incorporation assay of cell proliferation. The experiment was performed as described in the legend for Fig. 1. In panels C and D, the asterisk shows values that are significantly different from C57Bl/6 cultures (p < 0.05, Student's t test).
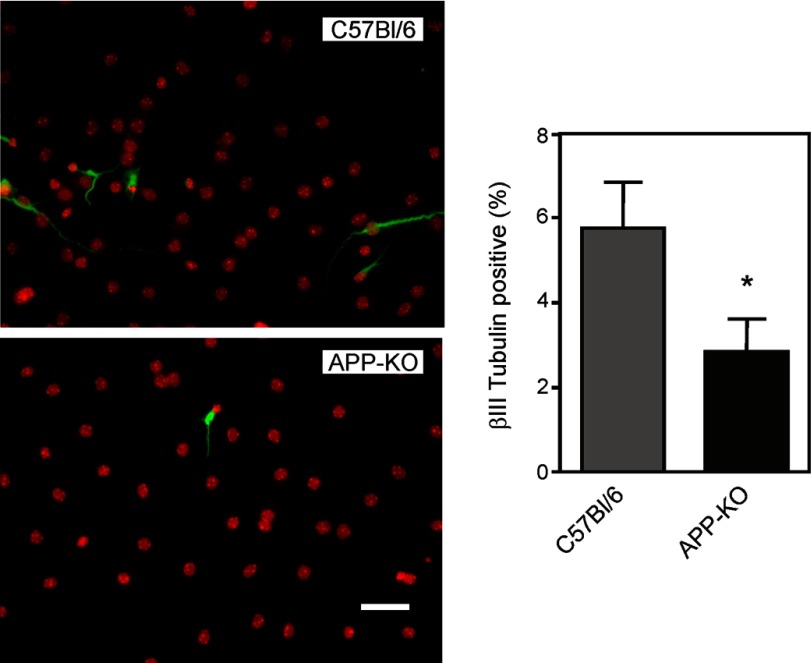
We also examined the capacity of APP-KO NSPCs to differentiate using the same procedure as described previously for the Tg2576 cells. The APP-KO cells were compared with the corresponding C57Bl/6 background strain cells. The total number of βIII-tubulin-positive cells was lower in the APP-KO cultures than in the background strain cultures (Fig. 4).
FIGURE 4.
Neuronal differentiation of NSPCs derived from C57Bl/6 and APP-KO mice. Neurospheres were dissociated, and the resulting cells were cultured adherently on poly-l-lysine for 5 days in differentiation medium. The cells were then stained for βIII-tubulin (green) and counterstained with DAPI (red) to visualize cell nuclei. Scale bar = 50 μm. The figure also shows quantitation of the percentage of βIII tubulin-positive cells for both groups. Values are means ± S.E. For each group, 24–27 fields were counted. Each field contained ∼100 DAPI-positive cells. * = significantly different from C57Bl/6 group (p < 0.05, Student's t test).
We next examined whether the effect of APP on proliferation was mediated by a factor that was secreted into the culture medium. Conditioned medium was prepared over 7 days from Tg2576 and the corresponding background strain (C57Bl/6xSJL) neurosphere cultures. In parallel, dissociated C57Bl/6xSJL NSPCs were plated and incubated for 16 h. Conditioned medium from the neurosphere cultures or unconditioned proliferation medium was then added to the dissociated cell cultures. The amount of proliferation was measured after 5 days of incubation. There was a higher rate of proliferation in cultures containing conditioned medium (whether from C57Bl/6xSJL or Tg2576 cells) than in cultures containing unconditioned medium (Fig. 5A). Importantly, the conditioned medium from Tg2576 cell cultures stimulated cell proliferation more than the conditioned medium from the C57Bl/6xSJL cultures, supporting the view that the increased proliferation in the Tg2576 cultures was due to the secretion of a factor into the conditioned medium.
FIGURE 5.
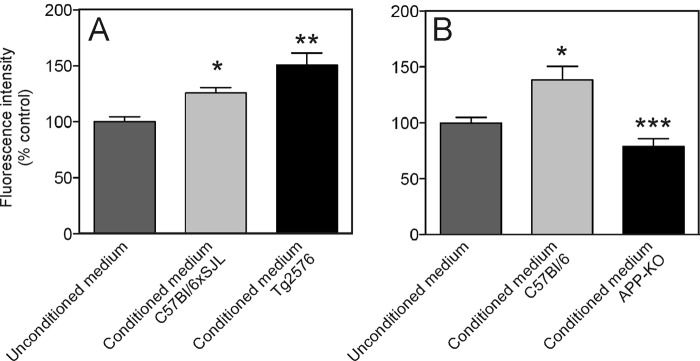
Effect of neurosphere conditioned medium on NSPC proliferation. A, effect of unconditioned medium, conditioned medium from Tg2576 neurosphere cultures (CM-Tg2576), and conditioned medium from C57Bl/6xSJL neurosphere cultures (CM-C57Bl/6xSJL) on the growth of C57Bl/6xSJL NSPCs. Medium was conditioned for 7 days prior to adding to cultures to test for the effect on proliferation over a period of 5 days. Cell number is shown as a the percentage of the fluorescence intensity measured in an alamarBlue assay when compared with control (unconditioned medium). Values are means + S.E. * = significantly different from unconditioned medium (control) (p < 0.05). ** = significantly different from unconditioned (control) medium and from CM-C57Bl/6xSJL (p < 0.05). B, effect of unconditioned medium, conditioned medium from APP knock-out neurosphere cultures (CM-APP-KO), and conditioned medium from C57Bl/6 neurosphere cultures (CM-C57Bl/6) on the growth of wild-type (C57Bl/6) NSPCs. Cell number is represented by the percentage of the fluorescence intensity measured in an alamarBlue assay when compared with control (unconditioned medium). Values are means + S.E. * = significantly different from unconditioned (control) medium (p < 0.05). *** = significantly different from unconditioned (control) medium and from CM-C57Bl/6 (p < 0.05).
To test this hypothesis further, we examined the effect of conditioned medium from APP-KO cell cultures on NSPC proliferation. This time, isolated NSPCs grown adherently were incubated with unconditioned medium (control), conditioned medium from APP-KO neurosphere cultures, or conditioned medium from the corresponding background strain C57Bl/6 neurosphere cultures (Fig. 5B). In contrast to the results with Tg2576 cultures, the APP-KO conditioned medium was significantly less potent in stimulating proliferation than the background strain conditioned medium. This result again supported the view that there was a factor secreted into the medium of APP-expressing cells that increased cell proliferation.
As it has been reported that sAPPα can stimulate neural stem cell proliferation or differentiation (9, 27, 28), we examined the effect of recombinant human sAPPα on proliferation in our cultures. However, despite repeated experiments aimed at determining whether sAPPα can stimulate NSPC proliferation, we were unable to demonstrate any effect of sAPPα over a range of different concentrations (50–2000 ng/ml) (Fig. 6A). Despite suggestions that Aβ amyloid may stimulate stem cell proliferation (26), we were also unable to find any effect of Aβ1–40 or Aβ1–42 on proliferation (Fig. 6A).
FIGURE 6.
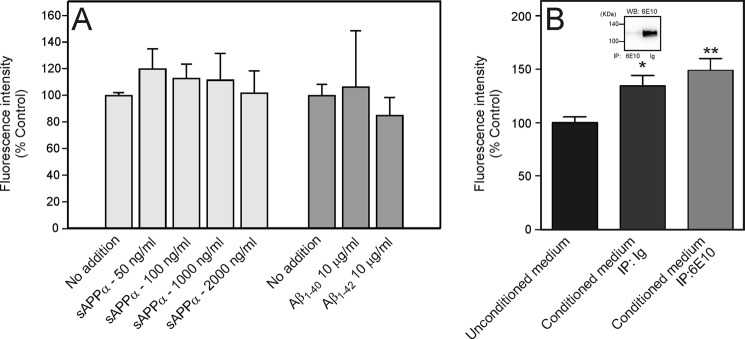
Effect of sAPPα and Aβ peptides on NSPC proliferation. Isolated NSPCs were prepared from C57Bl/6xSJL mice and cultured adherently on poly-l-lysine. The figure shows cell number as represented by the percentage of the fluorescence intensity measured in an alamarBlue assay. Values are means ± S.E. A, effect of recombinant sAPPα, Aβ1–40, and Aβ1–42 on NSPC proliferation. B, immunoprecipitation of >98% of the sAPPα from Tg2576 conditioned medium did not decrease the ability of the conditioned medium to increase NSPC proliferation. The figure shows the effect of Tg2576 conditioned medium after immunoprecipitation with an immunoglobulin fraction (IP: Ig) and after immunoprecipitation with mAb 6E10 (IP: 6E10). The inset shows Western blot (WB) analysis of the conditioned medium from both fractions. * = significantly different from control incubation. ** = significantly different from control incubation, but not significantly different from incubation of immunoglobulin fraction.
To rule out the possibility that the lack of effect of recombinant sAPPα might be due to the fact that the protein was not in a native conformation, we also tested the effect of removing endogenous sAPPα from the conditioned medium (Fig. 6B). More than 98% of the endogenously expressed sAPPα in the conditioned medium was removed by immunoabsorption with mAb 6E10 when compared with immunoabsorption with an immunoglobulin (Ig) control (Fig. 6B, inset). However, despite removal of most of the sAPPα, there was no decrease in the ability of the conditioned medium to stimulate proliferation. On the basis of these experiments, we concluded that neither sAPPα nor Aβ (both of which are fragments of full-length APP that can be released into the culture medium) was the secreted factor that mediated the effect of APP overexpression on NSPC proliferation.
As several studies have suggested that cystatin C is an important autocrine regulator of neural stem cell proliferation (32, 33), we examined the possibility that cystatin C was the mediator of APP-induced NSPC proliferation. Analysis of the conditioned medium from both Tg2576 cultures and APP-KO cultures by Western blotting showed that cystatin C levels correlated with the effect on proliferation (Fig. 7). In addition to a major 14-kDa cystatin C band, an additional 16-kDa immunoreactive band was also present in the conditioned medium. This higher molecular mass band may represent a post-translationally modified form of cystatin C (32). Consistent with the view that the effect on proliferation was due to cystatin C, we found that cystatin C immunoreactivity was elevated in the Tg2576 conditioned medium (Fig. 7A), and lower in the APP-KO cell conditioned medium (Fig. 7B).
FIGURE 7.
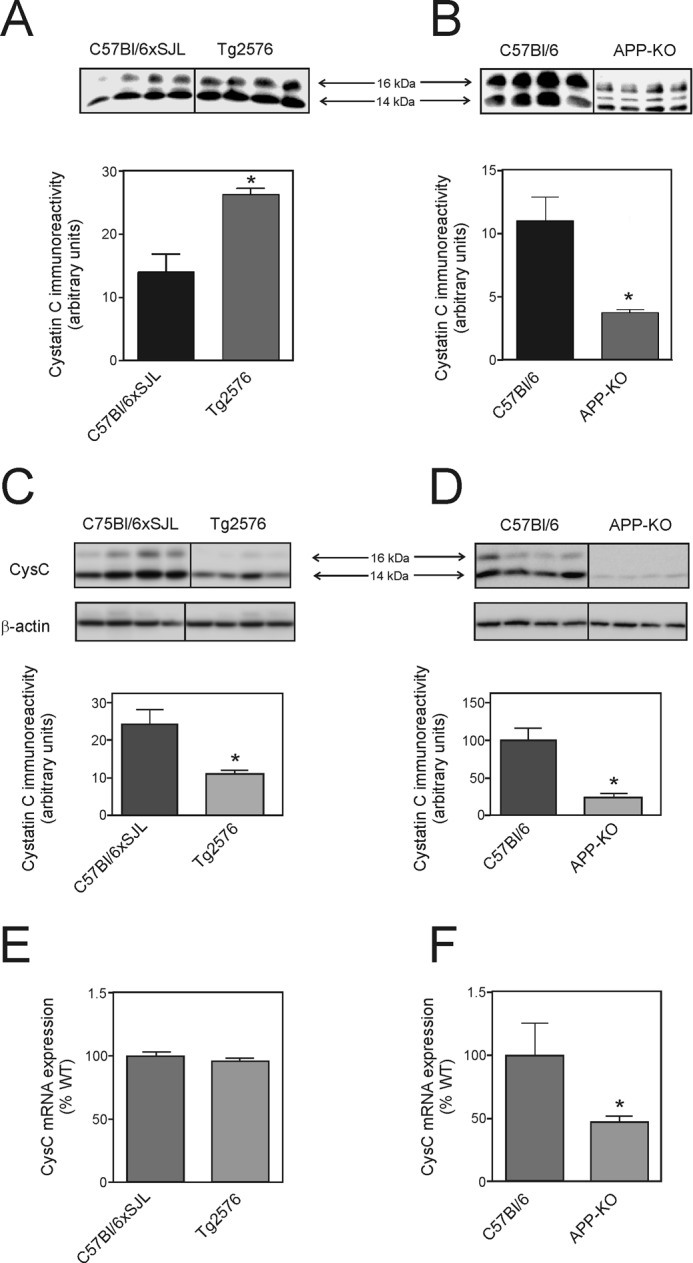
Levels of cystatin C and expression of cystatin C mRNA. The figure shows representative Western blots and quantification of cystatin C immunoreactivity by image capture analysis (A–D). Values are means ± S.E. (n = 4). A and C, Western blotting analysis of cystatin C (CysC) in conditioned medium (A) and cell lysate (C) of Tg2576 mouse NSPC cultures and the corresponding background strain (C57Bl/6xSJL) cultures. Each lane represents a sample from a different line of NSPCs. B and D, Western blotting analysis of cystatin C in conditioned medium (B) and cell lysate (D) of APP-KO mouse NSPC cultures and the corresponding background strain (C57Bl/6) cultures. Each lane represents a sample from a different line of NSPCs. E and F, cystatin C mRNA expression determined by real-time PCR. Values are means ± S.E. (n = 6). * = significantly different (p < 0.05) from corresponding background strain control cultures (Student's t test).
To determine whether the changes in secreted extracellular cystatin C reflected changes in the intracellular pools, we also examined the level of cell-associated cystatin C (Fig. 7, C and D). Surprisingly, the level of cystatin C in the cell lysates was lower in both Tg2576 cultures and APP-KO cultures when compared with the corresponding background strain cells for each group. We also analyzed the level of cystatin C mRNA by real-time PCR (Fig. 7, E and F). These experiments showed that although the level of cystatin C mRNA expression was lower in the APP-KO cells, there was no significant difference in expression in the Tg2576 cells.
Similar to previous studies (32, 33), cystatin C increased NSPC proliferation in a concentration-dependent manner (50–100 ng/ml cystatin C) (Fig. 8A). As the level of cystatin C in the conditioned medium of background strain cells was higher than this concentration range (Fig. 8B, panel i), this indicated that the concentration of cystatin C in the conditioned medium in both wild-type and background strain cells was sufficiently high to influence NSPC proliferation. Therefore, we tested whether the endogenous secreted factor in the conditioned medium was identical to cystatin C. We immunoprecipitated cystatin C from the conditioned medium of both C57Bl/6xSJL and Tg2576 cultures (Fig. 8B, panel ii) and measured the effect of the immunodepleted medium on cell proliferation. Immunodepletion of cystatin C from the conditioned medium completely removed the APP-associated increase in NSPC proliferation (Fig. 8C). Proliferation was not significantly decreased when background strain NSPC conditioned medium was immunodepleted of cystatin C. However, after immunodepletion of cystatin C from the Tg2576 conditioned medium, the level of NSPC proliferation was significantly lower than the corresponding incubation in which the conditioned medium was preabsorbed with Ig.
FIGURE 8.
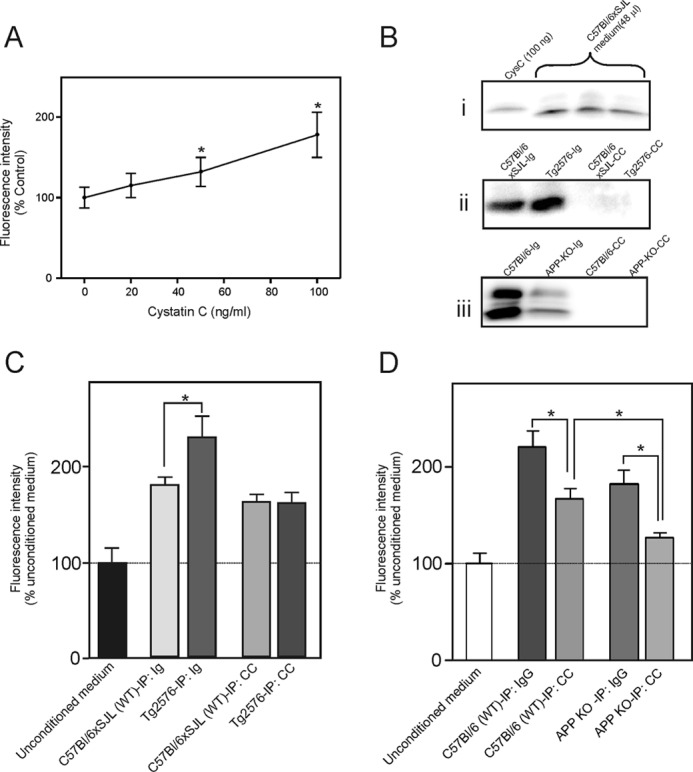
Role of secreted cystatin C in stimulating NSPC proliferation. The number of viable cells was calculated from the fluorescence intensity in an alamarBlue assay. A, effect of cystatin C on NSPC proliferation. Dissociated neurosphere-derived cells were plated on poly-l-lysine-coated plates and then incubated for 5 days in proliferation medium containing various concentrations of cystatin C. Values are means ± S.E., (n = 4) and shown as the percentage of the control value (no added cystatin C). * = significantly different from the control group (no cystatin C) (p < 0.05, ANOVA with post hoc Tukey's test). B, Western blot analysis of cystatin C (CysC) immunoreactivity in cell culture medium. Panel i, comparison of the level of cystatin C in 48 μl of C57Bl/6xSJL cell medium when compared with 100 ng of recombinant cystatin C. Panel ii, Western blot stained for cystatin C showing efficiency of immunodepletion of cystatin C from the C57Bl/6xSJL and Tg2576 conditioned medium. Ig = medium after immunodepletion with control immunoglobulin; CC = medium after immunodepletion with cystatin C antibody. Panel iii, Western blot stained for cystatin C showing efficiency of immunoprecipitation of cystatin C from the C57Bl/6 and APP-KO cell medium. C and D, immunoprecipitation of cystatin C removes the APP-induced growth factor that stimulates proliferation. C, C57Bl/6xSJL neurosphere-derived cells were incubated with unconditioned medium or conditioned medium from C57Bl/6xSJL cultures or Tg2576 cultures that was previously immunoabsorbed with a nonspecific Ig fraction (IP: Ig) or with an anti-cystatin C antibody (IP: CC). D, APP-KO neurosphere-derived cells were incubated with unconditioned medium or conditioned medium from C57Bl/6 cultures or APP-KO cultures that was previously immunoabsorbed with a nonspecific Ig fraction (IP: Ig) or with an anti-cystatin C antibody (IP: CC). Values in panels C and D are means ± S.E. (n = 4) and shown as the percentage of the mean of the values for unconditioned medium. * = significantly different (p < 0.05, ANOVA with post hoc Tukey's test).
Similar results were obtained in separate experiments using conditioned medium from C57Bl/6 and APP-KO cultures. In these experiments, the immunodepleted conditioned medium was tested on cultures of APP-KO cells (Fig. 8D). Immunodepletion of cystatin C (Fig. 8B, panel iii) resulted in a significant decrease in proliferation of NSPCs when compared with the corresponding incubations in which the conditioned medium was preabsorbed with Ig. Taken together, these results clearly indicated that cystatin C was a major contributor to the ability of APP to increase NSPC proliferation.
DISCUSSION
The present study demonstrates that APP expression regulates the proliferation of NSPCs and that this effect is mediated, at least in part, by an APP-stimulated increase in cystatin C secretion. The results increase our understanding of both the normal function of APP and the mechanisms involved in neural stem cell proliferation and differentiation.
Our findings also provide an explanation for previous studies demonstrating that neural stem cell proliferation is increased in transgenic mice that overexpress APP (25, 26). Studies by Jin et al. (25) reported that neural stem cell proliferation was increased in PDGF-APPsw,Ind mice. López-Toledano and Shelanski (26) confirmed and extended this observation in their studies. In both studies, no clear demonstration of the mechanism of increased proliferation was provided, although it was suggested that the stimulation of proliferation may have been due to Aβ accumulation or some aspect of the associated Aβ pathology (25, 26). Our studies clearly support the view that NSPC proliferation is directly influenced by the expression of APP. Although we cannot rule out the possibility that a product of APP metabolism may be responsible for this effect, we did not find any direct evidence that this effect was influenced by sAPPα or Aβ peptides.
Interestingly, although neural stem cell proliferation is increased in APP-overexpressing mice, there is no obvious phenotype resulting from this increase. For example, prior to the onset of AD-type pathology, the brains of young APP transgenic mice appear relatively normal. Nevertheless, subtle abnormalities may be present in these mice. For example, overexpression of APP in transgenic mice is reported to lead to changes in synaptic density (34), and Rodgers et al. (35) report that APP transgenic mice exhibit persistent locomotor hyperactivity. Whether these abnormalities are due to an increase in the number of specific neuronal populations is unclear. In the case of APP knock-out mice, there are clear abnormalities, most notably agenesis of the corpus callosum (36). However, whether this phenotype is due to a neural stem cell proliferation deficit is also unclear.
A surprising finding to emerge out of these studies was that the effect of APP overexpression was not mediated through sAPPα. A large number of studies have suggested that sAPPα has trophic properties on neural stem cells (9, 27, 28, 37, 38). In our studies, recombinant human sAPPα had no effect on proliferation, nor did immunoprecipitation of endogenous secreted mouse sAPPα inhibit proliferation. Instead, we found that immunoprecipitation of cystatin C from the culture medium decreased the APP-induced stimulation, clearly demonstrating that some of the effect on proliferation was due to cystatin C. Moreover, cystatin C stimulated NSPC proliferation, and levels in the culture medium were correlated with APP expression, clearly supporting this idea.
Interestingly, in experiments with APP-KO cells, we found that there was a significant residual effect of APP, even after removal of most of the cystatin C. Cystatin C-immunodepleted C57Bl/6 conditioned medium was still significantly more potent in stimulating the proliferation of APP-KO cells than the corresponding immunodepleted APP-KO conditioned medium (Fig. 8D). This suggests the possibility that there is an additional, as yet unidentified, secreted molecule that also contributes to APP-induced NSPC proliferation. This effect was not seen in experiments where we tested the effect of conditioned medium on C57Bl6xSJL cells (Fig. 8C). The reason for this difference is unclear, but it could relate to the fact that the APP-KO cells may be more sensitive to this unidentified factor.
We also found that a greater proportion of Tg2576 cells expressed βIII-tubulin when NSPCs were incubated in a medium that promotes differentiation. This effect on neurogenesis could have been mediated by sAPPα, similar to some previous studies (9, 27, 38), or by cystatin C.
The mechanism of the APP-stimulated increase in cystatin C and NSPC proliferation is also not yet known. Our experiments suggest that there may be two mechanisms involved. Real-time PCR experiments showed that cystatin C expression was decreased in APP-KO cells when compared with the corresponding background strain cells. As the extracellular domain of APP (i.e. sAPPα) was not found to stimulate proliferation, this suggests that the APP intracellular domain (AICD) may be involved in mediating this effect. Indeed, based on an analogy with the notch intracellular domain (NICD), which is also released by γ-secretase (39), a number of studies suggest that the AICD may regulate gene expression (40). Whether AICD regulates the expression of cystatin C is not yet known and will require further studies.
However, cystatin C expression was not increased in Tg2576 cells when compared with the corresponding background strain cells. Indeed, a surprising finding was that although cystatin C secretion was higher in the Tg2576 conditioned medium (Fig. 7A), levels were lower in the cell lysate (Fig. 7C). This suggests the possibility that the higher levels of cystatin C in the Tg2576 culture medium may be due to an increased rate of cystatin C secretion with a concomitant decrease in intracellular cystatin C. The idea that increased APP may result in increased secretion is consistent with published studies. For example, Lee et al. (41) reported that APP overexpression can increase vesicle exocytosis in PC12 cells. Furthermore, the cytoplasmic domain of APP can interact with proteins associated with synaptic vesicle release such as synaptotagmin-1 (42). Thus, we speculate that the cytoplasmic domain of APP may possess two different functions: 1) it may be translocated to the nucleus to alter gene expression or 2) it may interact with proteins on the cytoplasmic leaflet of the plasma membrane to alter events such as exocytosis. The balance of these two functions could conceivably be regulated by the level of expression of APP and the degree of saturation of binding to different adaptor proteins.
Finally, our study may have implications for understanding the role of cystatin C in the pathogenesis of AD. It is interesting to note that a polymorphism (G73A) in cystatin C has been linked to AD (43). Furthermore, cystatin C is increased in regions around Aβ deposits in the AD brain (44, 45), suggesting that it may play a role in pathogenesis or in response to Aβ pathology. Indeed, two groups, Mi et al. (46) and Kaeser et al. (47), found that cystatin C may have a protective effect as APP transgenic mice that had higher cystatin C expression were found to have diminished Aβ deposition. As APP is also increased in dystrophic neurites around amyloid plaques, it is tempting to speculate that increased cystatin C may be due to an increase in local APP expression. Further studies on the role of APP in regulating cystatin C expression in the AD brain may help to identify new targets for drug development in AD.
This work was supported by project grants from the National Health and Medical Research Council of Australia.
- NSPC
- neural stem/progenitor cell
- APP
- β-amyloid precursor protein
- sAPP
- soluble APP
- APP-KO
- APP knock-out
- Aβ
- β-amyloid protein
- AICD
- APP intracellular domain
- bFGF
- basic fibroblast growth factor
- AD
- Alzheimer disease
- EdU
- 5-ethynyl-2′-deoxyuridine
- ANOVA
- analysis of variance.
REFERENCES
- 1. Reynolds B. A., Weiss S. (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255, 1707–1710 [DOI] [PubMed] [Google Scholar]
- 2. Richards L. J., Kilpatrick T. J., Bartlett P. F. (1992) De novo generation of neuronal cells from the adult mouse brain. Proc. Natl. Acad. Sci. U.S.A. 89, 8591–8595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moyse E., Segura S., Liard O., Mahaut S., Mechawar N. (2008) Microenvironmental determinants of adult neural stem cell proliferation and lineage commitment in the healthy and injured central nervous system. Curr. Stem Cell Res. Ther. 3, 163–184 [DOI] [PubMed] [Google Scholar]
- 4. Small D. H., Mok S. S., Bornstein J. C. (2001) Alzheimer's disease and Aβ toxicity: from top to bottom. Nat. Rev. Neurosci. 2, 595–598 [DOI] [PubMed] [Google Scholar]
- 5. Patterson D., Gardiner K., Kao F. T., Tanzi R., Watkins P., Gusella J. F. (1988) Mapping of the gene encoding the β-amyloid precursor protein and its relationship to the Down syndrome region of chromosome 21. Proc. Natl. Acad. Sci. U.S.A. 85, 8266–8270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nunan J., Small D. H. (2000) Regulation of APP cleavage by α-, β-, and γ-secretases. FEBS Lett. 483, 6–10 [DOI] [PubMed] [Google Scholar]
- 7. Small D. H., Nurcombe V., Moir R., Michaelson S., Monard D., Beyreuther K., Masters C. L. (1992) Association and release of the amyloid protein precursor of Alzheimer's disease from chick brain extracellular matrix. J. Neurosci. 12, 4143–4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee J. A., Cole G. J. (2007) Generation of transgenic zebrafish expressing green fluorescent protein under control of zebrafish amyloid precursor protein gene regulatory elements. Zebrafish 4, 277–286 [DOI] [PubMed] [Google Scholar]
- 9. Freude K. K., Penjwini M., Davis J. L., LaFerla F. M., Blurton-Jones M. (2011) Soluble amyloid precursor protein induces rapid neural differentiation of human embryonic stem cells. J. Biol. Chem. 286, 24264–24274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Milward E. A., Papadopoulos R., Fuller S. J., Moir R. D., Small D., Beyreuther K., Masters C. L. (1992) The amyloid protein precursor of Alzheimer's disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron 9, 129–137 [DOI] [PubMed] [Google Scholar]
- 11. Mattson M. P. (1994) Secreted forms of β-amyloid precursor protein modulate dendrite outgrowth and calcium responses to glutamate in cultured embryonic hippocampal neurons. J. Neurobiol. 25, 439–450 [DOI] [PubMed] [Google Scholar]
- 12. Small D. H., Nurcombe V., Reed G., Clarris H., Moir R., Beyreuther K., Masters C. L. (1994) A heparin-binding domain in the amyloid protein precursor of Alzheimer's disease is involved in the regulation of neurite outgrowth. J. Neurosci. 14, 2117–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gakhar-Koppole N., Hundeshagen P., Mandl C., Weyer S. W., Allinquant B., Müller U., Ciccolini F. (2008) Activity requires soluble amyloid precursor protein α to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur. J. Neurosci. 28, 871–882 [DOI] [PubMed] [Google Scholar]
- 14. Chasseigneaux S., Dinc L., Rose C., Chabret C., Coulpier F., Topilko P., Mauger G., Allinquant B. (2011) Secreted amyloid precursor protein β and secreted amyloid precursor protein α induce axon outgrowth in vitro through Egr1 signaling pathway. PLoS One 6, e16301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clarris H. J., Key B., Beyreuther K., Masters C. L., Small D. H. (1995) Expression of the amyloid protein precursor of Alzheimer's disease in the developing rat olfactory system. Brain Res. Dev. Brain Res. 88, 87–95 [DOI] [PubMed] [Google Scholar]
- 16. Young-Pearse T. L., Bai J., Chang R., Zheng J. B., LoTurco J. J., Selkoe D. J. (2007) A critical function for β-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J. Neurosci. 27, 14459–14469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hornsten A., Lieberthal J., Fadia S., Malins R., Ha L., Xu X., Daigle I., Markowitz M., O'Connor G., Plasterk R., Li C. (2007) APL-1, a Caenorhabditis elegans protein related to the human β-amyloid precursor protein, is essential for viability. Proc. Natl. Acad. Sci. U.S.A. 104, 1971–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joshi P., Liang J. O., DiMonte K., Sullivan J., Pimplikar S. W. (2009) Amyloid precursor protein is required for convergent-extension movements during Zebrafish development. Dev. Biol. 335, 1–11 [DOI] [PubMed] [Google Scholar]
- 19. Gentleman S. M., Graham D. I., Roberts G. W. (1993) Molecular pathology of head trauma: altered β APP metabolism and the aetiology of Alzheimer's disease. Prog. Brain Res. 96, 237–246 [DOI] [PubMed] [Google Scholar]
- 20. Blumbergs P. C., Scott G., Manavis J., Wainwright H., Simpson D. A., McLean A. J. (1995) Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J. Neurotrauma. 12, 565–572 [DOI] [PubMed] [Google Scholar]
- 21. Itoh T., Satou T., Nishida S., Tsubaki M., Hashimoto S., Ito H. (2009) Expression of amyloid precursor protein after rat traumatic brain injury. Neurol Res. 31, 103–109 [DOI] [PubMed] [Google Scholar]
- 22. Cras P., Kawai M., Lowery D., Gonzalez-DeWhitt P., Greenberg B., Perry G. (1991) Senile plaque neurites in Alzheimer disease accumulate amyloid precursor protein. Proc. Natl. Acad. Sci. U.S.A. 88, 7552–7556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cochran E., Bacci B., Chen Y., Patton A., Gambetti P., Autilio-Gambetti L. (1991) Amyloid precursor protein and ubiquitin immunoreactivity in dystrophic axons is not unique to Alzheimer's disease. Am. J. Pathol. 139, 485–489 [PMC free article] [PubMed] [Google Scholar]
- 24. Tabaton M., Cammarata S., Mandybur T., Richey P., Kawai M., Perry G., Gambetti P. (1992) Senile plaques in cerebral amyloid angiopathy show accumulation of amyloid precursor protein without cytoskeletal abnormalities. Brain Res. 593, 299–303 [DOI] [PubMed] [Google Scholar]
- 25. Jin K., Galvan V., Xie L., Mao X. O., Gorostiza O. F., Bredesen D. E., Greenberg D. A. (2004) Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw,Ind) mice. Proc. Natl. Acad. Sci. U.S.A. 101, 13363–13367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. López-Toledano M. A., Shelanski M. L. (2007) Increased neurogenesis in young transgenic mice overexpressing human APPSw,Ind. J. Alzheimers Dis. 12, 229–240 [DOI] [PubMed] [Google Scholar]
- 27. Lazarov O., Demars M. P. (2012) All in the family: How the APPs regulate neurogenesis. Front. Neurosci. 6, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hayashi Y., Kashiwagi K., Ohta J., Nakajima M., Kawashima T., Yoshikawa K. (1994) Alzheimer amyloid protein precursor enhances proliferation of neural stem cells from fetal rat brain. Biochem. Biophys. Res. Commun. 205, 936–943 [DOI] [PubMed] [Google Scholar]
- 29. Millet P., Lages C. S., Haïk S., Nowak E., Allemand I., Granotier C., Boussin F. D. (2005) Amyloid-β peptide triggers Fas-independent apoptosis and differentiation of neural progenitor cells. Neurobiol. Dis. 19, 57–65 [DOI] [PubMed] [Google Scholar]
- 30. Young K. M., Psachoulia K., Tripathi R. B., Dunn S. J., Cossell L., Attwell D., Tohyama K., Richardson W. D. (2013) Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cui H., Hung A. C., Klaver D. W., Suzuki T., Freeman C., Narkowicz C., Jacobson G. A., Small D. H. (2011) Effects of heparin and enoxaparin on APP processing and Aβ production in primary cortical neurons from Tg2576 mice. PLoS One 6, e23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taupin P., Ray J., Fischer W. H., Suhr S. T., Hakansson K., Grubb A., Gage F. H. (2000) FGF-2-responsive neural stem cell proliferation requires CCg, a novel autocrine/paracrine cofactor. Neuron 28, 385–397 [DOI] [PubMed] [Google Scholar]
- 33. Kato T., Heike T., Okawa K., Haruyama M., Shiraishi K., Yoshimoto M., Nagato M., Shibata M., Kumada T., Yamanaka Y., Hattori H., Nakahata T. (2006) A neurosphere-derived factor, cystatin C, supports differentiation of ES cells into neural stem cells. Proc. Natl. Acad. Sci. U.S.A. 103, 6019–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mucke L., Masliah E., Johnson W. B., Ruppe M. D., Alford M., Rockenstein E. M., Forss-Petter S., Pietropaolo M., Mallory M., Abraham C. R. (1994) Synaptotrophic effects of human amyloid β protein precursors in the cortex of transgenic mice. Brain Res 666, 151–167 [DOI] [PubMed] [Google Scholar]
- 35. Rodgers S. P., Born H. A., Das P., Jankowsky J. L. (2012) Transgenic APP expression during postnatal development causes persistent locomotor hyperactivity in the adult. Mol. Neurodegener. 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Müller U., Cristina N., Li Z. W., Wolfer D. P., Lipp H. P., Rülicke T., Brandner S., Aguzzi A., Weissmann C. (1994) Behavioral and anatomical deficits in mice homozygous for a modified β-amyloid precursor protein gene. Cell 79, 755–765 [DOI] [PubMed] [Google Scholar]
- 37. Kwak Y. D., Brannen C. L., Qu T., Kim H. M., Dong X., Soba P., Majumdar A., Kaplan A., Beyreuther K., Sugaya K. (2006) Amyloid precursor protein regulates differentiation of human neural stem cells. Stem Cells Dev. 15, 381–389 [DOI] [PubMed] [Google Scholar]
- 38. Zhou Z. D., Chan C. H., Ma Q. H., Xu X. H., Xiao Z. C., Tan E. K. (2011) The roles of amyloid precursor protein (APP) in neurogenesis: Implications to pathogenesis and therapy of Alzheimer disease. Cell Adh. Migr. 5, 280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guruharsha K. G., Kankel M. W., Artavanis-Tsakonas S. (2012) The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 13, 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pardossi-Piquard R., Checler F. (2012) The physiology of the β-amyloid precursor protein intracellular domain AICD. J. Neurochem. 120, Suppl. 1, 109–124 [DOI] [PubMed] [Google Scholar]
- 41. Lee H. W., Park J. W., Sandagsuren E. U., Kim K. B., Yoo J. J., Chung S. H. (2008) Overexpression of APP stimulates basal and constitutive exocytosis in PC12 cells. Neurosci. Lett. 436, 245–249 [DOI] [PubMed] [Google Scholar]
- 42. Kohli B. M., Pflieger D., Mueller L. N., Carbonetti G., Aebersold R., Nitsch R. M., Konietzko U. (2012) Interactome of the amyloid precursor protein APP in brain reveals a protein network involved in synaptic vesicle turnover and a close association with synaptotagmin-1. J. Proteome Res. 11, 4075–4090 [DOI] [PubMed] [Google Scholar]
- 43. Hua Y., Zhao H., Lu X., Kong Y., Jin H. (2012) Meta-analysis of the cystatin C(CST3) gene G73A polymorphism and susceptibility to Alzheimer's disease. Int. J. Neurosci. 122, 431–438 [DOI] [PubMed] [Google Scholar]
- 44. Steinhoff T., Moritz E., Wollmer M. A., Mohajeri M. H., Kins S., Nitsch R. M. (2001) Increased cystatin C in astrocytes of transgenic mice expressing the K670N-M671L mutation of the amyloid precursor protein and deposition in brain amyloid plaques. Neurobiol. Dis. 8, 647–654 [DOI] [PubMed] [Google Scholar]
- 45. Kaur G., Levy E. (2012) Cystatin C in Alzheimer's disease. Front. Mol. Neurosci. 5, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mi W., Pawlik M., Sastre M., Jung S. S., Radvinsky D. S., Klein A. M., Sommer J., Schmidt S. D., Nixon R. A., Mathews P. M., Levy E. (2007) Cystatin C inhibits amyloid-β deposition in Alzheimer's disease mouse models. Nat. Genet. 39, 1440–1442 [DOI] [PubMed] [Google Scholar]
- 47. Kaeser S. A., Herzig M. C., Coomaraswamy J., Kilger E., Selenica M. L., Winkler D. T., Staufenbiel M., Levy E., Grubb A., Jucker M. (2007) Cystatin C modulates cerebral β-amyloidosis. Nat. Genet. 39, 1437–1439 [DOI] [PubMed] [Google Scholar]