Background: How damaged mitochondria are removed by mitophagy is not fully described.
Results: Ischemia and reoxygenation (I/R)-induced injury triggers mitochondria association of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and mitophagy, and protein kinase Cδ (PKCδ) activation inhibits it.
Conclusion: PKCδ-mediated phosphorylation of GAPDH inhibits mitophagy.
Significance: GAPDH/PKCδ is a signaling switch, which is activated during ischemic injury to regulate the balance between cell survival by mitophagy and cell death by apoptosis.
Keywords: Heart, Ischemia, Lysosomes, Mitochondria, Protein Kinase C (PKC), PKCδ, GAPDH, Mitophagy
Abstract
After cardiac ischemia and reperfusion or reoxygenation (I/R), damaged mitochondria propagate tissue injury by promoting cell death. One possible mechanism to protect from I/R-induced injury is the elimination of damaged mitochondria by mitophagy. Here we identify new molecular events that lead to mitophagy using a cell culture model and whole hearts subjected to I/R. We found that I/R induces glyceraldehyde-3-phosphate dehydrogenase (GAPDH) association with mitochondria and promotes direct uptake of damaged mitochondria into multiorganellar lysosomal-like (LL) structures for elimination independently of the macroautophagy pathway. We also found that protein kinase C δ (PKCδ) inhibits GAPDH-driven mitophagy by phosphorylating the mitochondrially associated GAPDH at threonine 246 following I/R. Phosphorylated GAPDH promotes the accumulation of mitochondria at the periphery of LL structures, which coincides with increased mitochondrial permeability. Either inhibition of PKCδ or expression of a phosphorylation-defective GAPDH mutant during I/R promotes a reduction in mitochondrial mass and apoptosis, thus indicating rescued mitophagy. Taken together, we identified a GAPDH/PKCδ signaling switch, which is activated during oxidative stress to regulate the balance between cell survival by mitophagy and cell death due to accumulation of damaged mitochondria.
Introduction
Following myocardial infarction and injury induced by ischemia, the return of blood flow to the heart and subsequent reoxygenation produces further oxidative damage to the heart (1). This ischemia-reoxygenation or reperfusion (I/R)5-induced injury is mediated by reactive oxygen species, generated primarily by damaged mitochondria, which leads to opening of the mitochondrial permeability transition pore and the onset of cell death by apoptosis and necrosis (1, 2). Hence, ensuring proper elimination of damaged mitochondria is imperative to cell survival.
One possible way to promote cardiomyocyte survival during I/R-induced injury is to eliminate damaged mitochondria by targeting them for lysosomal degradation by autophagy, a process often referred to as mitophagy (3). One major form of mitophagy is macromitophagy, where mitochondria are targeted to lysosomes after sequestration into autophagosomes and fusion of the autophagosomes with the lysosome (4, 5). A number of proteins, including a ubiquitin ligase (Parkin), a serine threonine kinase (Ulk1), and a BH3 family member (NIX), have been shown to be required for macromitophagy (6–8). Several recent studies in yeast have reported that mitochondria can also be degraded by micromitophagy, where the lysosomes in mammals or vacuoles in plant and fungi directly engulf mitochondria (9–13). Macroautophagy-independent mitophagy has also been observed in hepatocytes following photoirradiation in the presence of the macroautophagy inhibitor, 3-methylalanine (3MA) (14, 15). The molecular mechanisms of macromitophagy have been well described (16–18). However, little is known about the molecular regulation of micromitophagy and its role in cell fate after an ischemic insult.
We have previously shown in mouse, rat, and porcine models of myocardial infarction that translocation and activation of protein kinase Cδ (PKCδ) from the cytosol to the mitochondria at the onset of reoxygenation promotes irreversible damage to the myocardium by impairing multiple mitochondrion-mediated processes (19–22). I/R-induced translocation and activation of mitochondrial PKCδ is associated with reduced mitochondrial ATP production, increased mitochondrion-mediated apoptosis, and coincides with the appearance of mitochondria that are swollen and structurally in disarray (19, 20, 23, 24). In contrast, inhibition of PKCδ translocation to mitochondria by a selective PKCδ peptide inhibitor, δV1–1, during I/R inhibits mitochondrial dysfunction (20, 23, 24). Here, we identified a pathway of mitochondrial elimination following I/R-induced injury and the role of PKCδ in the regulation of this process using isolated whole hearts and cells in culture.
EXPERIMENTAL PROCEDURES
Cell Cultures
The murine HL1 cardiomyocyte cell line was kindly obtained from Dr. William Claycomb (Louisiana State University Health Sciences Center) and expanded in Claycomb medium (Sigma), supplemented with 10% (v/v) fetal bovine serum (FBS), 4 mm l-glutamine, 100 μm norepinephrine and antibiotics. HL1 cells were subcultured at high density (typically a 1:3 split) on 10-cm2 plates or glass coverslips pre-coated with 0.02% gelatin and 25 μg/ml of fibronectin (Sigma). HL1 cell culture medium was replaced daily to maintain their differentiated cardiomyocyte phenotype and contractile activity. Atg5−/− and Atg5+/+ mouse embryonic fibroblasts (MEFs) were kindly obtained from Dr. Noboru Mizushima and maintained in Dulbecco's modified Eagle's medium, supplemented with 10% (v/v) FBS and antibiotics.
Simulation of I/R-induced Injury in Cultured Cells
HL1 or Atg5 cells were grown to confluence in 10- or 25-cm2 dishes, or to 90% subconfluence on glass coverslips in 6-well plates and I/R-induced injury was performed as described (25) Briefly, cells were rinsed twice with phosphate-buffered saline (PBS), followed by the addition of ischemia mimetic solution (125 mm NaCl, 8 mm KCl, 1.2 mm KH2PO4, 1.25 mm MgSO4, 1.2 mm CaCl2, 6.25 mm NaHCO3, 5 mm sodium lactate, 20 mm HEPES, pH 6.6) and placing the cells in hypoxic pouches (GasPak EZ Anaerobe Gas Generating Pouch System with indicator, BD Biosciences). After 2.5 h of simulated ischemia, reperfusion injury was initiated by removing the ischemia buffer and immediately replacing with pre-warmed normoxic Krebs-Henseleit solution (110 mm NaCl, 4.7 mm KCl, 1.2 mm KH2PO4, 1.25 mm MgSO4, 1.2 mm CaCl2, 25 mm NaHCO3, 15 mm glucose, 20 mm HEPES, pH 7.4) and incubated at 95% room air, 5% CO2 for 30 min or as indicated. Control groups were incubated for the equivalent amount of time in normoxic Krebs-Henseleit solution.
Peptide-mediated Inhibition of PKCδ Translocation
PKCδ isozyme translocation to mitochondria was inhibited by the isozyme-specific translocation inhibitor peptide, δV1–1 (amino acid residues 14–21 of PKCδ). The peptide was designed as previously described (21, 26). The peptide was dissolved in sterile water and used at a final concentration of 1 μm in cultured cells throughout I/R-induced injury.
Expression of Recombinant Active (GAPDH-V5) and Inactive GAPDH (iGAPDH-V5)
Catalytically active and inactive V5-tagged human GAPDH cDNAs were kindly obtained from Dr. Douglas Green (St. Jude Children's Research Hospital) (27). Retrovirus production in 293T cells was performed by co-transfecting 293T cells with cDNA encoding GAPDH-V5 or iGAPDH-V5, the VSVG envelope protein, and GAG-POL polyprotein and collecting the virus-containing supernatant after 48 h. HL1 cells or Atg5−/− MEFs were transduced with freshly harvested GAPDH-V5 or iGAPDH-V5 virus-containing supernatant for 16 h in the presence of 8 μg/ml of Polybrene and analyzed 48 h later.
Expression of Recombinant WT iGAPDH and Mutant T246A iGAPDH
The catalytically inactive V5-tagged human GAPDH cDNA was kindly obtained from Dr. Douglas Green (St. Jude Children's Research Hospital) (27). Threonine at position 246 was mutated to alanine (T246A) to inhibit phosphorylation using a QuikChange site-directed mutagenesis kit (Stratagene). The following primers were used: Ala-246 sense, 5′-ccc act gcc aac gtg tca gtg gtg gac ctg gcc tgc cgt cta gaa aaa cct gcc aaa tat-3′; Ala-246 antisense, 5′-ata ttt ggc agg ttt ttc tag gca gca ggc cag gtc cac cac tga cac gtt ggc agt ggg-3′. Mutants were sequenced to ensure that no additional mutations were introduced and then transfected into 293T cells using FuGENE6 transfection reagent (Roche Applied Science). Retrovirus production was performed as described above.
Mitochondria Enrichment by Differential Centrifugation
Mitochondria were isolated from HL1 or Atg5−/− cells by differential centrifugation. Confluent monolayers of cells were rinsed with PBS and then scraped from plates using mannitol-sucrose buffer (MS; 210 mm mannitol, 70 mm sucrose, 5 mm MOPS, 1 mm EDTA, pH 7.4). Cells were triturated 16 times on ice using a 27-gauge needle, followed by microcentrifugation at 800 × g to pellet nuclei and cell debri. The postnuclear supernatant was microcentrifuged at 10,000 × g for 15 min to obtain a mitochondrial pellet, which was surface washed three times with MS buffer prior to downstream applications. It should be noted that the amount of mitochondria removed by the 800 × g spin or the amount of mitochondria obtained in the 10,000 × g spin was unaffected by I/R treatments (data not shown).
Mitochondria and Lysosomal Enrichment Using Density Gradients
In a parallel approach, density gradients were employed to enrich for mitochondria and lysosomes. Cells were subjected to hypobaric shock 5 times, pelleted at 500 × g to remove nuclei, and the released organelles were separated on density gradients by ultracentrifugation using a lysosome enrichment kit for tissues and cultured cells (Thermo Scientific). Fractions were collected from each gradient from the top to the bottom, combined with 2 volumes of PBS, and pelleted at 10,000 × g for 30 min. The organelle pellets were surface washed twice with buffer A (supplied in the kit) prior to downstream applications.
Immunofluorescence
HL1 cells or Atg5−/− cells, exponentially growing on glass coverslips, were fixed with freshly prepared PBS containing 4% (w/v) paraformaldehyde for 15 min at 4 °C, permeabilized with PBS containing 3% (w/v) BSA and 0.1% (v/v) Triton X-100 at room temperature for 15 min, and then blocked overnight at 4 °C with PBS containing 3% (w/v) BSA (blocking buffer). In some instances, cells were labeled with 0.5 μm LysoTracker Red DND-99 (Molecular Probes) for 30 min as recommended by the supplier, prior to fixation. Fixed cells were then incubated with primary antibodies diluted in blocking buffer for 3 h at room temperature in a humidified chamber followed by 1 h of incubation with Alexa Fluor 488-conjugated or Alexa Fluor 568-conjugated secondary antibodies (Molecular Probes). Samples were washed with PBS and counterstained with Hoechst (Molecular Probes) to visualize nuclei and analyzed using a DeltaVision OMX SR wide field deconvolution fluorescence microscope (Applied Precision Inc). Consecutive images were acquired at ×100 magnification using the oil objective with the red, green, and blue channels, pseudo colored, and overlaid using SoftWoRx 3.4.5 software (Applied Precision).
Transmission Electron Microscopy (TEM)
Isolated organelles or intact HL1 and Atg5−/− MEFs growing on glass coverslips were fixed in Karnovsky's fixative (2% (v/v) glutaraldehyde and 4% (v/v) formaldehyde in 0.1 m sodium cacodylate, pH 7.4) for 1 h at room temperature. Sections were postfixed in 1% osmium tetroxide for 1 h at room temperature, washed with ultrafiltered water, and stained for 2 h at room temperature. Samples were then dehydrated in a series of ethanol washes for 15 min at 4 °C beginning at 50, 70, and 95% ethanol in water. Samples were then equilibrated to room temperature and washed with 100% ethanol twice, then propylene oxide (PO) for 15 min. Samples were infiltrated with EMbed-812 resin (EMD) that was mixed with PO. Samples were placed into EMbed-812 for 2 to 4 h, then placed into molds with fresh resin, and incubated overnight at 65 °C. Sections were cut at 75- and 90-nm intervals, picked up on formvar/carbon-coated slot copper grids, and stained for 15 to 20 min in 1:1 saturated uranyl acetate (∼7.7%) and 100% ethanol followed by staining in 0.2% lead citrate for 3 to 4 min. Images were acquired using a JEOL1230 Gatan 967 CCD transmission electron microscope at 80 kV and a Gatan Orius 4k × 4k digital camera. Images were acquired at ×1000, 3000, or 10,000 magnifications from cells of approximately the same size.
Determination of GAPDH Activity
GAPDH activity was determined using a KDalert GAPDH assay kit as instructed by the manufacturer (Applied Biosystems). Briefly, samples were diluted in KDalert lysis buffer along with a series of GAPDH enzyme dilutions for the standard curve. After 20 min of incubation on ice, samples were combined with the KDalert master mixture in 96-well plates and read at 615 nm using a UV-visible plate reader. GAPDH activity was calculated and expressed as units of GAPDH/mg of total protein.
RESULTS
PKCδ Translocates to HL1 Mitochondria during I/R-induced Injury and Inhibits Mitochondrial Elimination
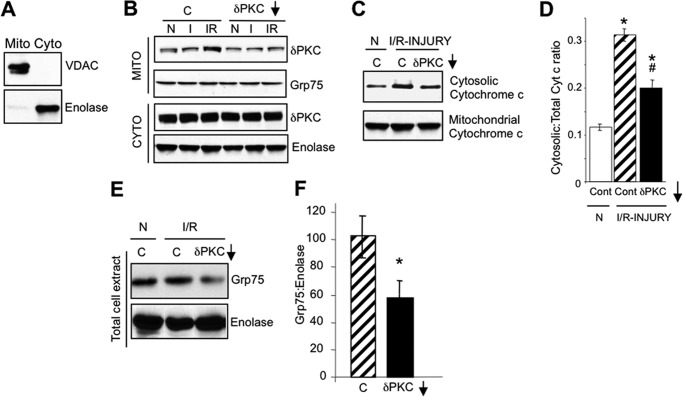
We have previously shown that PKCδ translocates to mitochondria during I/R-induced injury and mediates mitochondrial dysfunction by inhibiting ATP production and promoting the release of cytochrome c to the cytosol (20, 23, 24). Here we studied the effects of PKCδ on mitochondrial elimination. I/R was modeled in HL1 cardiomyocytes using a modified method of Gottlieb and collaborators (25), where cells were subjected to 2.5 h of ischemia followed by a short 30-min reoxygenation. I/R-induced injury promoted a robust translocation of PKCδ to HL1 mitochondria, whereas ischemia alone (I) did not (Fig. 1B). As expected, PKCδ translocation to mitochondria during I/R-induced injury was inhibited by the selective inhibitor of PKCδ translocation and function, δV1–1 (Fig. 1B) (21). Furthermore, as previously shown in ex vivo and in vivo models of rat, mouse, and porcine of I/R-induced injury (20, 23, 24), PKCδ translocation to HL1 mitochondria coincided with increased cytosolic levels of cytochrome c, which were reduced in the presence of δV1–1 (Fig. 1, C and D). Together, these results show that I/R-induced injury in HL1 cardiomyocytes recapitulates the translocation of PKCδ to mitochondria and the onset of apoptosis seen in other models of I/R.
FIGURE 1.
PKCδ translocates to HL1 mitochondria during I/R-induced injury and inhibits mitochondrial elimination. A, Western blots with antibodies to VDAC, a mitochondrial protein, and enolase, a cytosolic protein, showing enrichment of mitochondrial (Mito) and cytosolic (Cyto) fractions from HL1 cells. B, representative PKCδ Western blots of HL1 mitochondrion (Mito)- and cytosol (Cyto)-enriched fractions from HL1 cells subjected to normoxia (N), 2.5 h of ischemia alone (I), or 2.5 h of ischemia followed by 30 min of reoxygenation (I/R) in the absence or presence of a control peptide (C) or the PKCδ translocation inhibitor, δV1–1 (δPKC↓). Blots were stripped and re-probed with anti-Grp75 and anti-enolase as a loading control. C, representative Western blots of anti-cytochrome c in the cytosol- and mitochondrion-enriched fractions from HL1 cells subjected to normoxia or I/R in the presence of a control peptide (C) or the PKCδ translocation inhibitor (δV1–1). D, quantification of Western blots shown in C. Significant differences (p < 0.05) to the normoxic control group (*) and I/R control group (#) were observed. E, representative Western blot showing a reduction of mitochondrial mass (Grp75) in total cell extracts from HL1 cells subjected to normoxia or I/R in the presence of a control peptide (C) or the PKCδ translocation inhibitor, δV1–1 (δPKC↓). The blot was stripped and re-probed with anti-enolase. F, mitochondrial mass following I/R-induced injury was determined by quantifying Western blots described in E, presenting the ratio of Grp75 protein levels to enolase protein levels. Significant differences (*, p < 0.05) to the I/R control group were observed.
Interestingly, we observed that I/R-induced injury in the presence of δV1–1 significantly reduced the amount of the mitochondrial matrix protein, Grp75, in total cell extracts, whereas I/R-induced injury alone did not (Fig. 1, E and F). These data suggest that PKCδ translocation to mitochondria is potentially involved in the pathway leading to mitochondrial elimination during I/R-induced injury.
I/R-induced Injury Triggers the Formation of Lysosomal-like (LL) Structures
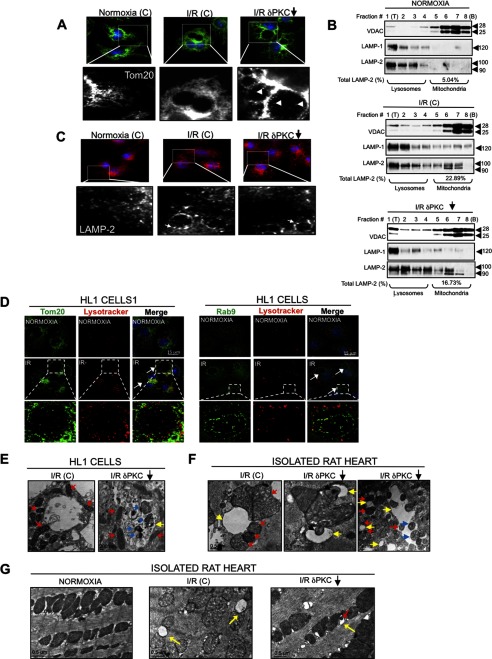
To assess whether PKCδ contributes to cell death by inhibiting the elimination of damaged mitochondria, HL1 cells were subjected to I/R-induced injury, and analyzed by immunofluorescence using the mitochondrial marker, Tom20. We noticed that I/R-induced injury triggers the formation of vacuolated structures of varying size (Fig. 2A). Furthermore, inhibition of PKCδ translocation by δV1–1 during I/R-induced injury promoted the appearance of Tom20-positive (Tom20+) material inside the lumen of these vacuoles, suggesting mitochondrial internalization into the vacuolated structures when PKCδ is inhibited (Fig. 2A, right panel).
FIGURE 2.
I/R-induced injury triggers the formation of LL structures. A, HL1 cells were treated with a control peptide (C) and the PKCδ translocation inhibitor peptide (δV1–1, indicated by a downward arrow) and subjected to normoxia (N) or I/R. The cells were fixed and analyzed by immunofluorescence (×100 magnification) using an antibody for the mitochondrial marker, Tom20 (green channel). An indicated portion of each image is expanded to illustrate the uptake of mitochondria (Tom20+, white arrowheads) into the large vacuolated structures in the presence of δV1–1 (white arrowheads). B, HL1 cells subjected to normoxia or I/R with a control peptide (C), or I/R with δV1–1 were fractionated on density gradients. Fractions were collected from the top (T) to the bottom (B) of each gradient. Organelles were pelleted and analyzed by Western blotting for the presence of lysosomes, mitochondria, and late endosomes using anti-LAMP-1, anti-VDAC, and anti-LAMP-2 antibodies, respectively. LAMP-2 levels in the mitochondrion-enriched fractions (at the bottom of each gradient) are indicated as a percentage of LAMP-2 levels present in the entire gradient. C, HL1 cells subjected to normoxia or I/R with a control peptide (C), or I/R with δV1–1 were fixed and analyzed by immunofluorescence microscopy using anti-LAMP-2 antibody (×100 magnification). White arrows indicate LAMP-2+ late endosome structures. D, HL1 cells subjected to normoxia or I/R were labeled with LysoTracker Red, fixed, and analyzed by immunofluorescence with a deconvolution microscope using anti-Tom20 or anti-Rab9 to visualize mitochondria and late endosomal membranes, respectively. Images were acquired at ×100 magnification and represent maximum intensity projection of image stacks. White arrows indicate LL structures. The boxed portion in each I/R group is expanded, showing representative LL structures. E and F, TEM analysis of intact HL1 cells (E) or isolated rat hearts (F) following I/R-induced injury in the absence or presence of the PKCδ translocation inhibitor, δV1–1. Yellow arrows indicate the LL structures, red arrows indicate the peri-LL-associated mitochondria, and blue arrows the internalized mitochondria. G, TEM images of normoxic control rat hearts (left panel) or hearts subjected to 30 min of ischemia followed by 30 min of reperfusion in the presence of a control peptide (middle panel) or δV1–1 (right panel). Direct engulfment of mitochondrial material into a vacuolated structure is shown in the right panel. Yellow arrows indicate the I/R-induced appearance of vacuolated structures, in the vicinity of mitochondria following I/R injury. Red arrows indicate segregated portions of mitochondria engulfed by vacuolated structures.
The primary organelle thought to be responsible for uptake and elimination of mitochondria in mammalian cells is the lysosome (4, 28). Thus, we reasoned that I/R-induced injury triggers a rapid expansion of the lysosomes seen as large vacuolated structures to facilitate the elimination of damaged cellular components (Fig. 2A). To test this hypothesis directly, organelles were isolated by density-gradient centrifugation. Under normoxic conditions, mitochondria were found at the bottom of density gradients, as indicated by the presence of the mitochondrial protein, VDAC (voltage-dependent anion channel) in that fraction. Lysosomes were present at the top of gradients, as evidenced by the presence of the lysosomal protein, LAMP-1 (Fig. 2B). We noticed that I/R-induced injury in the absence or presence of δV1–1 promoted only a modest expansion of the lysosomal system as determined by comparing the relative amounts of LAMP-1 (Fig. 2B, middle and bottom panels as compared with the top panel). LAMP-2 has been known to be predominantly associated with lysosomes (29, 30), but recently it was detected in a late endosomal compartment of dendritic cells that mediates microautophagy of specific cytosolic proteins, by invaginations of the endosomal membrane (31). As such, under normoxic conditions or following I/R in the absence or presence of δV1–1, LAMP-2 was detected at the top of gradients along with LAMP-1 lysosomes. Interestingly, I/R-induced injury resulted in a 3–4-fold increase in lysosomal LAMP-2 protein in the mitochondrial fraction as indicated by co-fractionation with the mitochondrial marker protein, VDAC. The increased amount of the lysosomal marker, LAMP-2, following I/R likely indicates expansion of the lysosomal machinery for degradation of damaged mitochondria. Consistently, immunofluorescence studies showed that LAMP-2-positive material is partially localized to the vacuolated structures triggered by I/R-induced injury in the absence or presence of δV1–1 (Fig. 2C). Additional immunofluorescence studies confirmed that these structures were also positive for LysoTracker Red and Rab9, markers used to detect acidic organelles, and lysosome and late endosomes, respectively (Fig. 2D). Taken together, our studies suggest that the vacuolated structures observed after I/R-induced injury are of a late endosomal and lysosomal origin and that they internalize mitochondria during I/R-induced injury in the presence of δV1–1.
We further examined these organelles by TEM. We observed that these vacuolated structures contained internalized mitochondria in intact HL1 cells and in isolated rat hearts following I/R in the presence of δV1–1 (Fig. 2, E–G), which coincides with a reduction in mitochondrial mass that we observed (Fig. 1E). In addition, I/R-induced injury promoted the accumulation of mitochondria containing fragmented cristae and amorphous dense bodies around the vacuolated structures, whereas the mitochondrial morphology was noticeably improved in the presence of δV1–1 (Fig. 2G). These data suggest roles for the vacuolated structures and PKCδ in mitochondrial elimination following I/R-induced injury. Based on our observations, we termed these distinct vacuolated structures as LL structures.
Catalytically Inactive GAPDH (iGAPDH) Associates with Mitochondria during I/R-induced Injury
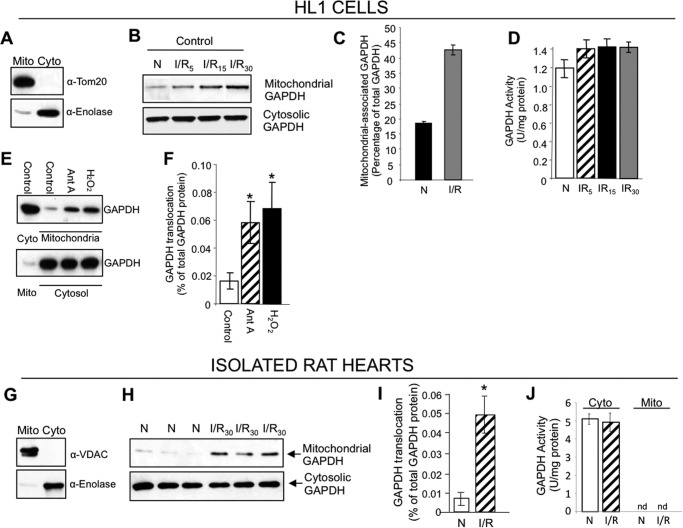
We next focused on the molecular mechanism by which I/R-induced mitophagy occurs. We often used glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a loading control on Western blots but consistently observed a time-dependent increased level of GAPDH accumulating in the mitochondrially enriched fraction from HL1 cells following I/R-induced injury (Fig. 3, B and C). We also found that the mitochondrially damaging agents, antimycin A and hydrogen peroxide (H2O2), promoted the rapid translocation of GAPDH to mitochondria in HL1 cells, suggesting that the mitochondrion-mediated damage triggers the association of GAPDH with mitochondria (Fig. 3, E and F). Cytosolic GAPDH catalyzes the sixth step of glycolysis, where glucose is converted to pyruvate. In addition to its crucial role in glycolysis, GAPDH has also been shown to be involved in several nonglycolytic cellular processes, including DNA repair, membrane fusion and transport, cytoskeletal dynamics, and cell death (32). Furthermore, both the nuclear and cytosolic forms of overexpressed GAPDH are implicated in the clearance of mitochondria by macroautophagy (27). Based on these previously reported functions of GAPDH in macroautophagy and our observation of GAPDH accumulation in the mitochondrial fraction during I/R, we set out to determine whether GAPDH contributes to the regulation of I/R-induced mitophagy.
FIGURE 3.
Catalytically inactive GAPDH associates with mitochondria during I/R-induced injury. A, Western blot showing the enrichment of mitochondrial (Mito) and cytosolic (Cyto) fractions from HL1 cells using antibodies to the mitochondrial marker, Tom20, and cytosolic marker, enolase. B, HL1 cells were subjected to 2.5 h of ischemia followed by 30 min of reoxygenation for the indicated times. The mitochondrion- and cytosol-enriched fractions obtained by differential centrifugation were pooled and analyzed for GAPDH protein levels by Western blotting. C, the relative amount of mitochondrially associated GAPDH present was calculated using ImageJ software. D, measurement of GAPDH activity in total extract (n = 3). There was no significant increase in GAPDH enzymatic activity. E, GAPDH Western blots of mitochondrion- and cytosol-enriched fractions from HL1 cells incubated with DMSO (control), antimycin A (Ant A), or H2O2 for 30 min. F, quantification of Western blots shown in E (n = 3 per group, *, p < 0.05 versus control group). G, Western blot showing the enrichment of mitochondrial (Mito) and cytosolic (Cyto) fractions from isolated rat hearts using antibodies to the mitochondrial marker, VDAC, and cytosolic marker, enolase. H, isolated rat hearts were subjected to normoxia or 30 min of ischemia, followed by 30 min of reoxygenation (I/R) ex vivo. The mitochondrion- and cytosol-enriched fractions were pooled and GAPDH protein levels were determined by Western blotting. Shown are analyses of three cardiac extracts per treatment. I, quantification of mitochondrial GAPDH translocation described in H (*, p < 0.05). J, measurement of GAPDH activity in the mitochondrial (Mito) and cytosolic (Cyto) fractions described in H. There was no detectable (nd) enzymatic activity in the mitochondrial fraction (n = 6 per group).
Interestingly, despite a noticeable I/R-induced increase in the mitochondrially associated GAPDH protein in HL1 cells, there was no significant increase in GAPDH enzymatic activity (Fig. 3D). We also observed an increase in GAPDH protein accumulating in the mitochondrially enriched fraction from isolated rat hearts subjected to I/R-induced injury without an increase in GAPDH enzymatic activity in the mitochondrial fraction (Fig. 3, H–J). These data suggest that I/R-induced injury promotes the mitochondrial accumulation of GAPDH and that the catalytic activity of the enzyme is not required for its mitochondrial role.
Exogenously Expressed iGAPDH Associates with Mitochondria and Induces the Formation of LL Structures
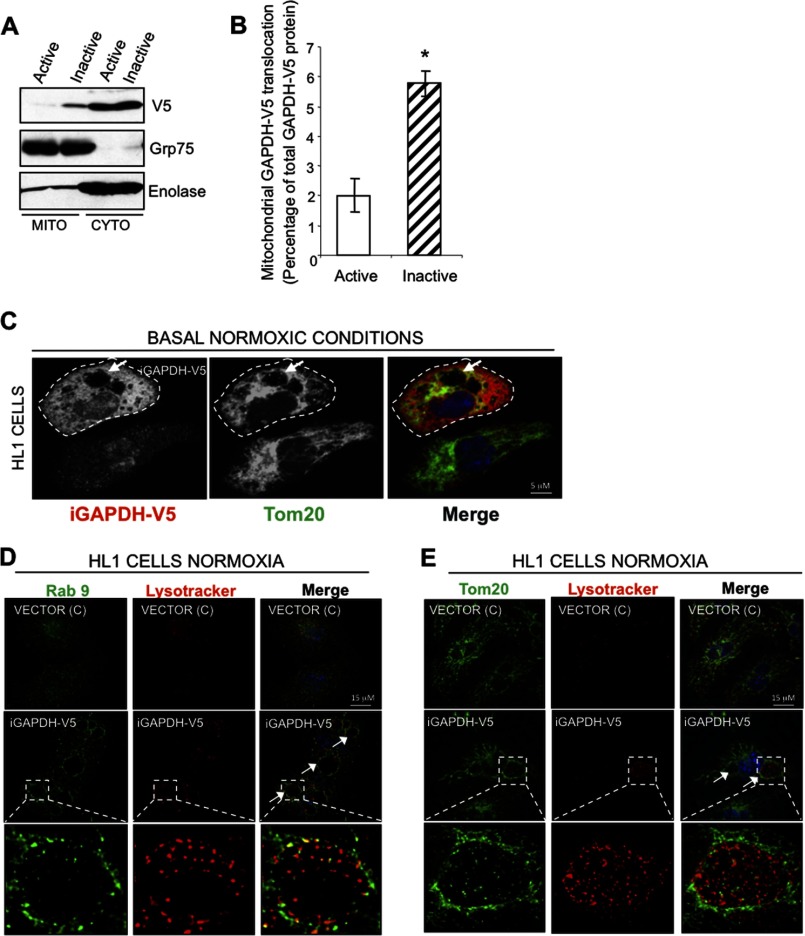
To determine whether indeed catalytically inactive GAPDH is involved in the formation of LL structures for mitochondrial elimination, we expressed both catalytically active and inactive GAPDH tagged with V5 sequence (GAPDH-V5 and iGAPDH-V5) in HL1 cells. We observed association of iGAPDH with the mitochondrial fraction under normoxic conditions; mitochondrial association of iGAPDH was much greater as compared with active GAPDH (Fig. 4, A and B). Because catalytically inactive GAPDH preferentially associates with mitochondria under basal conditions, we further characterized the morphology of cells expressing iGAPDH by immunofluorescence.
FIGURE 4.
Exogenously expressed iGAPDH associates with mitochondria and induces the formation of LL structures under normoxic condition. A, representative Western blot showing that inactive GAPDH preferentially associates with mitochondria. HL1 cells transiently expressing GAPDH-V5 (active) or iGAPDH-V5 (inactive) under normoxic conditions were fractionated for mitochondrion (Mito)- and cytosol (Cyto)-enriched fractions and analyzed by Western blotting with anti-GAPDH. Grp75 and enolase were used as markers of mitochondrial and cytosolic fractions, respectively. B, quantification of GAPDH translocation experiments described in A, using ImageJ software (n = 4, *, p < 0.05). C, HL1 cells transiently expressing iGAPDH-V5 were maintained under normoxic conditions. Cells were fixed and stained with anti-V5 (as a marker of transfected GAPDH) and anti-Tom20 antibodies (as a marker of mitochondria). Confocal images were obtained at ×100 magnification. Arrows indicate the presence of LL structures. D and E, HL1 cells transiently expressing iGAPDH-V5 or control vector (c) were labeled with LysoTracker Red, fixed, and analyzed by immunofluorescence with anti-Rab9 (D) and with anti-Tom20 (E). Images were acquired at ×100 magnification. LL structures are indicated by white arrows.
We observed that iGAPDH expression under normoxic conditions was sufficient to promote the formation of large vacuolated structures, some of which contained Tom20+ material (Fig. 4C), suggestive of mitochondrial uptake. These organelles in iGAPDH-V5-expressing cells were also positive for a marker labeling acidic compartments, LysoTracker, and for a lysosomal and late endosomal marker, Rab9 (Fig. 4, D and E), suggesting that these vacuolated structures are LL structures that we observed in HL1 cells during I/R-induced injury. Therefore, these results suggest that exogenous iGAPDH expression is sufficient to promote the formation of LL structures under normoxic conditions. Furthermore, iGAPDH expression coincides with the recruitment of mitochondria into these structures.
Mitochondrially Associated GAPDH Is Phosphorylated by PKCδ during I/R-induced Injury
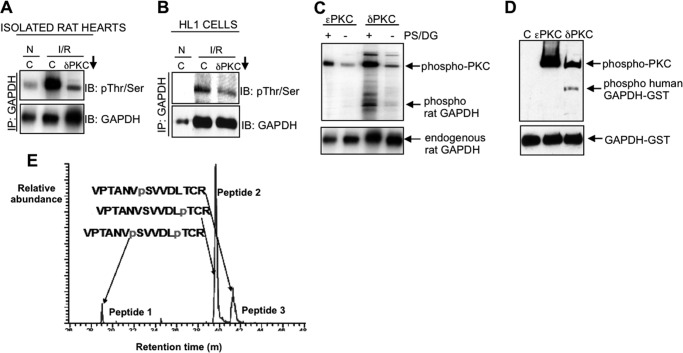
Because both PKCδ and GAPDH associate with the mitochondria following I/R, we next determined whether the mitochondrially associated GAPDH is affected by PKCδ activity. We observed that I/R-induced injury promoted the phosphorylation of the mitochondrially associated iGAPDH in isolated rat hearts and HL1 cells, which was reduced in the presence of the PKCδ translocation inhibitor, δV1–1 (Fig. 5, A and B, upper blots). These results suggested that the mitochondrially associated GAPDH is phosphorylated following I/R, either directly or indirectly by PKCδ.
FIGURE 5.
Mitochondrially associated GAPDH is phosphorylated by PKCδ during I/R-induced injury. A and B, isolated rat hearts (A) or HL1 cells (B) were subjected to normoxia (N) or I/R in the presence of the PKCδ translocation inhibitor (δV1–1, indicated by a downward arrow) or control peptide (C). The mitochondrial fraction was immunoprecipitated with a polyclonal antibody to GAPDH and immunoblotted with a mixture of antibodies to phosphorylated serine and threonine, stripped, and re-probed with anti-GAPDH. C and D, in vitro kinase assays containing immunoprecipitated GAPDH from isolated rat hearts (C) or purified recombinant GAPDH-GST alone or in combination with recombinant PKCϵ or recombinant PKCδ (D) were carried out, and the products were immunoblotted with a combination of phosphorylated serine and threonine antibodies (top) or with anti-GAPDH (bottom). PS and DG, cofactors of PKCδ, are abbreviations of phosphatidylserine and diacylglycerol. E, phosphopeptide mapping of the products of the kinase assays described in D. Three phosphorylated peptides were detected in the kinase reaction containing GAPDH-GST and PKCδ only; GAPDH phosphorylated at Ser-241 and Thr-246 (peptide 1), GAPDH phosphorylated at Thr-246 alone (peptide 2), and GAPDH phosphorylated at Ser-241 only (peptide 3). Peptide 2 (Thr-246 alone) comprised over 70% of the entire phosphorylated material detected. No phosphorylation of GST was detected.
In vitro kinase assays using endogenous GAPDH immunoprecipitated from isolated rat hearts (Fig. 5C), or purified GAPDH-GST (Fig. 5D) confirmed that GAPDH is directly phosphorylated by PKCδ, but not by PKCϵ (Fig. 5, C and D), a related PKC isozyme that is also activated in the myocardium during I/R-induced injury (21). Phosphopeptide mapping of the products of the kinase assays shown in Fig. 5D identified three phosphorylation species: phosphorylation(s) at Ser-241 and Thr-246 (peptide 1), Thr-246 alone (peptide 2), and Ser-241 alone (peptide 3), but peptide 2 comprised ∼70% of the entire phosphorylated material detected (Fig. 5E). Importantly, Thr-246 (SVVDLT246CRLE) is within the consensus sequence for PKC substrates and is evolutionary conserved in GAPDH from a variety of species, including human, rat, and mouse (data not shown). Collectively, these results suggest that PKCδ translocates to mitochondria following I/R and phosphorylates GAPDH, predominantly at Thr-246.
Inhibition of PKCδ-mediated iGAPDH Phosphorylation during I/R Promotes Mitochondrial Uptake into LL Structures, Which Coincides with Reduced Mitochondrial Mass
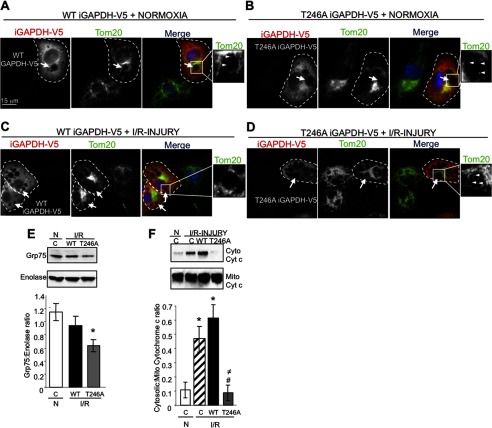
To determine the effects of GAPDH phosphorylation on GAPDH-driven mitophagy during I/R-induced injury, we mutated threonine at position 246 of catalytically inactive human iGAPDH-V5 (WT iGAPDH-V5) to an alanine (T246A iGAPDH-V5) and expressed both proteins in HL1 cells.
Using confocal microscopy, we found that expression of WT iGAPDH-V5 (Fig. 6A, cell outlined in dashed line) or T246A iGAPDH-V5 (Fig. 6B, cell outlined in dashed line) under normoxic conditions promoted the appearance of large LL structures, which were surrounded by Tom20+ mitochondria and varied in size and number per cell (Fig. 6, A and B, arrows). Furthermore, these vacuolated structures also contained Tom20+ material inside their lumen, suggestive of mitochondrial uptake (Fig. 6, A and B, arrowheads). Following I/R, Tom20+ staining increased inside the LL structures of T246A iGAPDH-V5 expressing cells, but not in WT iGAPDH-V5 expressing cells (Fig. 6, C and D, respectively). These results suggest that increased phosphorylation of the mitochondrially associated iGAPDH-V5 during I/R prevents the uptake of mitochondria into LL structures.
FIGURE 6.
Expression of phosphorylation defective iGAPDH (T246A) during I/R promotes mitochondrial uptake into LL structures, which coincides with reduced mitochondrial mass. A–D, HL1 cells transiently expressing WT iGAPDH-V5 and T246A iGAPDH-V5 were maintained under normoxic (N) conditions or subjected to I/R injury. Cells were fixed and stained with anti-V5 (red channel) and anti-Tom20 (green channel) antibodies. Confocal images were acquired at ×100 magnification. Arrows indicate the presence of LL structures in a GAPDH-V5-expressing cell (outlined with a dashed line). Arrowheads in the expanded images indicate the presence of Tom20+ material inside the expanded LL structures. E, representative anti-Grp75 Western blot of total cell lysates prepared from control HL1 cells or HL1 cells transiently expressing WT iGAPDH-V5 and T246A iGAPDH-V5 and subjected to normoxic (N) or I/R injury. Blots were stripped and re-probed for enolase. Quantification of Grp75 levels in three independent experiments to determine mitochondrial mass is provided (expressed as a ratio of Grp75: enolase, *, p < 0.05). F, Western blotting for cytochrome c levels in mitochondrion (Mito)- and cytosol (Cyto)-enriched fractions isolated from control vector-transfected HL1 cells (C) or HL1 cells transiently expressing WT iGAPDH-V5 or T246A iGAPDH-V5 and subjected to normoxia or I/R injury. Quantification of cytosolic cytochrome c levels in three independent experiments is provided. Significant differences (p < 0.05) to normoxic control group (*), I/R control group (#), and I/R WT iGAPDH-V5 group (≠).
We hence reasoned that the I/R-induced recruitment and uptake of mitochondria into LL structures act as a mechanism to rapidly eliminate damaged mitochondria during I/R. Indeed, the mitochondrial mass in HL1 cells expressing the phosphorylation-defective T246A iGAPDH-V5 mutant during I/R-induced injury was lower relative to WT iGAPDH-V5, as demonstrated by a decline in the mitochondrial matrix protein, Grp75, in total cell extracts (Fig. 6E). Furthermore, the increased levels of cytosolic cytochrome c were detected in I/R-injured HL1 cells expressing WT iGAPDH-V5, as compared with normoxic control cells. In contrast, expression of the phosphorylation-defective T246A iGAPDH-V5 mutant inhibited I/R-induced release of cytochrome c (Fig. 6F).
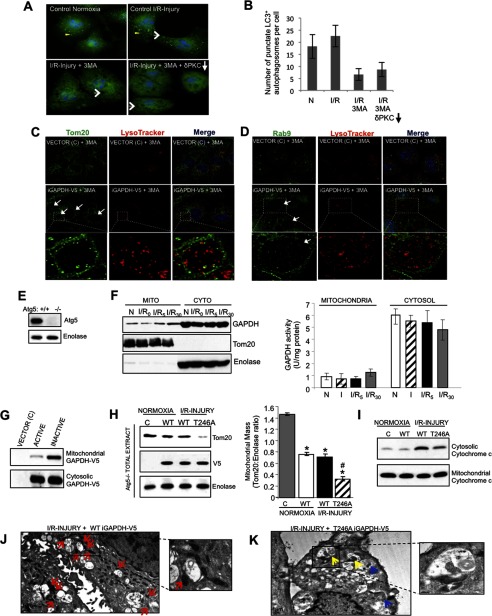
GAPDH-induced Mitophagy Occurs Independently of the Macroautophagy Pathway
We next used 3MA, an inhibitor of macroautophagy, to determine whether mitochondrial elimination induced by the mitochondrially associated GAPDH is dependent on macroautophagy. In line with Hamacher-Brady et al. (25), 3MA treatment of HL1 cells prevented the formation of LC3+ autophagosomes (Fig. 7, A and B, indicated by yellow arrowheads). However, in HL1 cells that transiently express iGAPDH-V5, inhibition of macroautophagy with 3MA did not prevent the formation of LL structures (Fig. 7, A, C, and D, indicated by white arrows); the vacuolated structures contained the late endosome and lysosomal markers, Rab9 and LysoTracker, within their lumen. These results suggest that iGAPDH promotes a redistribution of the lysosomal system into distinct degradation centers in the cell and does so independently of macroautophagy. Furthermore, the presence of Tom20+ material within LL structures of 3MA-treated iGAPDH expressing cells suggests that mitochondria are recruited to these structures independently of macroautophagy (Fig. 7C). To further examine a potential role for macroautophagy in GAPDH-mediated mitophagy, we also used macroautophagy-deficient, Atg5−/− MEF cells (33). I/R-induced injury in these macroautophagy-deficient cells also promoted a time-dependent translocation of inactivated and endogenous GAPDH to mitochondria (Fig. 7F). Furthermore, as in HL1 cells, iGAPDH preferentially associated with the mitochondrial fraction (Fig. 7G) and the phosphorylation-defective T246A iGAPDH-V5 mutant showed increased mitochondrial elimination and reduced cytochrome c release relative to WT iGAPDH-V5 under I/R-induced injury (Fig. 7, H and I). These data suggest that GAPDH-mediated mitophagy is independent of the macroautophagy process. In support of this, we observed by TEM many instances of mitochondria being directly engulfed by LL structures in T246A iGAPDH-V5 expressing Atg5−/− MEF cells during I/R but not in WT iGAPDH-V5 Atg5−/− MEF cells (Fig. 7, J and K).
FIGURE 7.
GAPDH-induced mitophagy occurs independently of the macroautophagy pathway. A, HL1 cells maintained under normoxic conditions (left, top panel) or subjected to I/R-induced injury (right, top panel), I/R-induced injury in the presence 100 μm 3MA (left, bottom panel), or I/R-induced injury in the presence of 100 μm 3MA and the PKCδ translocation inhibitor, δV1–1 (right, bottom panel), were fixed and analyzed by immunofluorescence using a rabbit anti-LC3 antibody (green channel). Cells were counterstained with Hoechst (blue channel) to visualize nuclei. Yellow arrowheads indicate LC+ autophagosomes and white arrows indicate the formation of LL structures. Images were acquired at ×100 magnification. B, the number of punctate LC3+ autophagosomes per cell was scored. Results are expressed as the mean ± S.D. (n = 40). C and D, HL1 cells transiently expressing iGAPDH-V5 or control vector (C) were incubated with 3MA for 4 h to inhibit macroautophagy. During the last 30 min, cells were labeled with LysoTracker Red, fixed, and analyzed by immunofluorescence with a deconvolution microscope using anti-Tom20 (A, green channel) or anti-Rab9 (B, green channel) antibodies. LL structures are indicated within white arrows. Images were acquired at ×100 magnification and represent maximum intensity projection of image stacks. The boxed area is expanded in the lower panels and representative LL structures are indicated by white arrows. E, Atg5 Western blot of Atg5+/+ and Atg5−/− MEF cells. F, representative GAPDH Western blot of mitochondrion (Mito)- and cytosol (Cyto)-enriched fractions from Atg5−/− MEFs subjected to normoxia (N) or 2.5 h of ischemia, followed by 0, 5, or 30 min of reoxygenation (I/R). The blot was stripped and re-probed with anti-Tom20 and anti-enolase as indicators of mitochondrial and cytosolic enrichment, respectively. GAPDH activity in mitochondrial and cytosolic fractions was measured (n = 4 per group). There was no significant enzymatic activity of GAPDH in mitochondrial fractions. G, representative Western blot showing that inactive GAPDH (iGAPDH-V5) preferentially associates with mitochondria in Atg5−/− MEFs. Atg5−/− MEFs transiently expressing control vector (C), GAPDH-V5 (active), or iGAPDH-V5 (inactive) under normoxic conditions were enriched for mitochondria and cytosol and analyzed by Western blotting with anti-GAPDH. H, Atg5−/− MEFs transiently expressing control vector (C), iGAPDH-V5, or T246A iGAPDH-V5 were subjected to normoxia or I/R injury. Total cell extracts were analyzed by Western blotting with anti-Tom20. The blot was re-probed for enolase and V5. Quantification of the Western blots is provided. Significant differences (p < 0.05) to the normoxic control group (*) and WT I/R group (#) are indicated. I, Western blotting for cytochrome c levels in the mitochondrion- and cytosol-enriched fractions isolated from mock transfected Atg5−/− MEFs (C) or Atg5−/− MEFs transiently expressing WT iGAPDH-V5 or T246A iGAPDH-V5 and subjected to normoxia or I/R injury. J and K, TEM images of Atg5−/− MEFs transiently expressing WT iGAPDH-V5 (J) and T246A iGAPDH-V5 (K). The cells were subjected to I/R-induced injury. Images were acquired at ×3,000 magnification. Red arrows indicate mitochondria accumulated around LL structures. Yellow arrows indicate perivacuole-associated mitochondria in the process of being directly engulfed, whereas blue arrows indicate mitochondria internalized into LL structures.
DISCUSSION
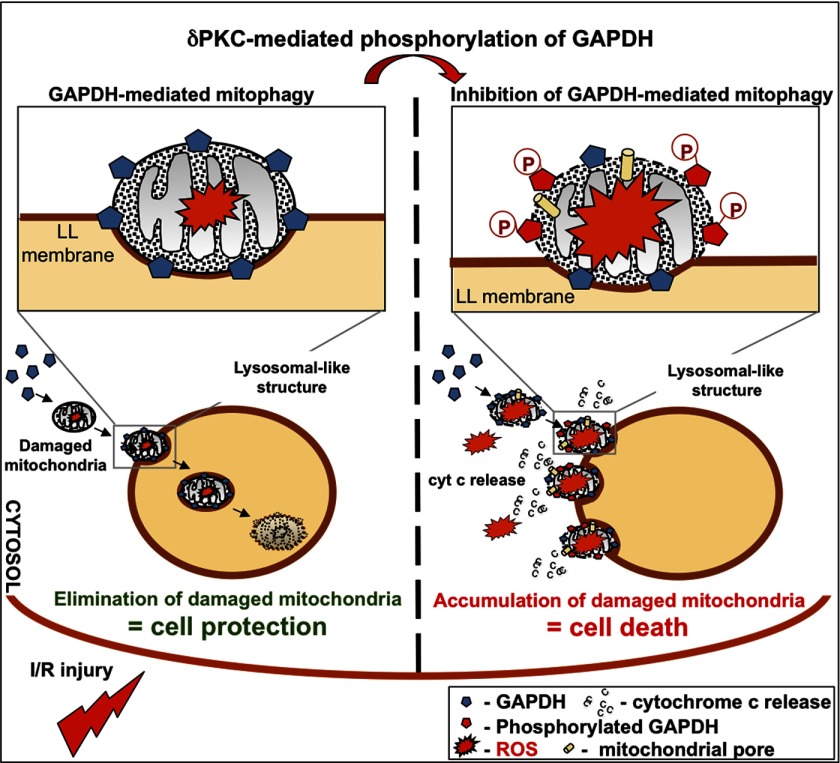
In this study, we identified a rapidly mounted mitophagy process that is mediated by mitochondrial association of GAPDH but is negatively regulated by PKCδ during I/R-induced injury (see the proposed model in Fig. 8). We show that mitochondrial uptake by LL structures is regulated by the association of GAPDH with mitochondria during I/R-induced injury. This, in turn, mediates direct recruitment and uptake of damaged mitochondria into LL structures. We also show that mitochondrially associated GAPDH is a direct substrate for PKCδ, a known mediator of mitochondrial dysfunction and cell death in cardiomyocytes (19, 20, 23, 24). As I/R-induced injury increases, PKCδ translocation to mitochondria may reach a critical threshold, where it phosphorylates enough mitochondrial GAPDH at Thr-246 to inhibit GAPDH-driven mitophagy and instead, promotes the peri-LL accumulation of damaged mitochondria, the cytosolic release of cytochrome c from these mitochondria, and the onset of mitochondrion-mediated cell death by apoptosis and likely also by necrosis.
FIGURE 8.
Proposed model for GAPDH-dependent, PKCδ-modulated mitophagy by LL structures. GAPDH associates with mitochondria during I/R-induced injury and targets these organelles to expanded LL structures for elimination. As I/R-induced oxidative injury increases, more GAPDH is phosphorylated by the mitochondrially associated PKCδ. Phosphorylation of GAPDH inhibits GAPDH-driven mitophagy by LL structures, resulting in cytosolic accumulation of damaged mitochondria, leakage of cytochrome c, and the onset of mitochondrially mediated cell death. Accordingly, either inhibition of PKCδ translocation to mitochondria or expression of a phosphorylation defective T246A GAPDH mutant during I/R-induced injury correlates with reduced cytochrome c release and cell injury.
PKCδ-mediated phosphorylation of GAPDH mostly occurred at Thr-246, with a smaller amount of phosphorylation occurring at Ser-241. It is possible that either site or both can be phosphorylated also by other kinases such as casein kinase II or Ca2+/camodulin-dependent protein kinase II (34, 35), although the exact amino acid modified by them is unknown. Nonetheless, Thr-246 substituted by alanine (T246A) was sufficient to abolish PKCδ-mediated inhibition of mitophagy during I/R-induced injury, which coincided with a reduction in mitochondrial mass. These results suggest that GAPDH phosphorylation at Thr-246 by PKCδ is a critical regulator of GAPDH-driven mitophagy by LL structures. Phosphorylation at Thr-246 by other kinases may propagate other signaling cascades. Additionally, we noticed that the catalytic activity of GAPDH is not required for mitochondrial elimination by mitophagy following I/R.
To date, several molecular machineries have been identified for the elimination of mitochondria, including autophagy-related 32 (Atg32) in yeast, and NIP1-like protein X (NIX) during red blood cell differentiation, unc51-like kinase (Ulk1), and p62/SQSTM1-ubiquitinated protein complexes in mammalian cells (6, 7, 36, 37). Phosphatase and tensin homolog-induced putative kinase protein 1 (PINK1) and Parkin have been found to be involved in mitophagy as well (6, 38). In this model, VDACs and mitofusin 2 (Mfn2) were very recently identified as mitochondrial docking sites for recruitment of Parkin from cytosol to mitochondria (39, 40). Although most of these studies focus on macroautophagy, our studies suggest that GAPDH-induced mitophagy occurs by what appears to be a micromitophagy process and is independent of macroautophagy. This is consistent with recent studies showing that photodamage-induced mitophagy in hepatocytes can occur in the presence of 3MA, suggesting that mitochondrial elimination in mammalian cells can also occur independently of macroautophagy (14, 15).
GAPDH-induced mitophagy described here was initiated by oxidative stress during I/R-induced injury. Under oxidative stress conditions, many proteins can undergo oxidative modifications, which may further modulate their activities. It has been reported that oxidative stress by H2O2 induces tyrosine phosphorylation of PKC isozymes, including PKCδ, by tyrosine kinases such as Lck and Syk, thereby activating PKC (41–45). Additionally, oxidative stress results in translocation of PKCδ to mitochondria where it promotes cytochrome c release and apoptosis (19, 46). GAPDH was also reported to be modified under oxidative stress conditions. Specifically, a cysteine residue in the active site of GAPDH was oxidized to cysteic acid, which makes this enzyme inactive (47, 48). Although the precise mechanism by which GAPDH is translocated to mitochondria has not yet been determined, oxidative stress in I/R potentially regulates the onset of GAPDH and PKCδ-mediated mitophagy.
How mitochondrially associated GAPDH promotes the formation of LL structures and the uptake of mitochondria into these structures still needs to be investigated. However, based on previous studies, it is possible that GAPDH can act as a fusogen, promoting the association and fusion of negatively charged phospholipids, in particular plasmenylethanolamine and phosphatidylserine (PS) (49, 50). Although PS is present in all organelle membrane, it is likely to confer a negative charge to organelles with a single limiting membrane, such as plasma membrane, endosomes, and lysosomes, because PS present in mitochondria, Golgi, and ER is confined to their luminal leaflets (51). It is therefore possible that GAPDH may promote fusion of the outer mitochondrial membrane with the LL membrane, thereby facilitating direct uptake of damaged mitochondria during I/R-induced injury. In support of this possibility, TEM analysis in isolated rat hearts subjected to I/R injury shows the presence of mitochondria, with their outer membrane directly associated and in some cases, fused with LL membranes. Another possibility is that GAPDH-induced mitophagy under oxidative stress conditions may depend on chaperone-mediated autophagy and Hsc70. GAPDH contains a KFERQ motif that is selectively recognized by this chaperone heat shock cognate protein (52). This interaction targets the complex to the lysosomal membrane, where it binds to the LAMP-2 originated LL structures, which acts as a receptor for this pathway (53).
What is the relative contribution and importance of mitochondrial clearance by GAPDH-driven mitophagy and macroautophagy in cardiac cell survival after I/R-induced injury? Cell-based and animal-based studies have shown that macroautophagy is down-regulated in cardiomyocytes during I/R-induced injury and that pharmacological up-regulation of this process ensures maximal recovery from cardiac I/R-induced injury (28). Therefore, it appears that cardiomyocytes deploy two complimentary autophagy responses to cull damaged mitochondria and both of these are attenuated during I/R-induced injury. We show that a GAPDH-driven mitophagy response is rapidly blunted by PKCδ activation and phosphorylation of GAPDH. A macroautophagy response can also be deployed to remove damaged mitochondria, along with other cytosolic components in cardiomyocytes. However, like GAPDH-driven mitophagy, macroautophagy is rapidly inactivated upon I/R-induced injury in cardiomyocytes (25). Therefore, pharmacological up-regulation of GAPDH-driven mitophagy (e.g. by inhibiting PKCδ translocation to mitochondria) or macroautophagy (e.g. with rapamycin or chloramphenicol succinate (25, 54)) would promote the clearance of damaged mitochondria and therefore prevent the onset of mitochondrially mediated cell death following I/R. Altogether, these two independent autophagy systems could contribute to the removal of damaged mitochondria and the preservation of a healthy mitochondrial population. In summary, we report that mitochondrially associated, inactive GAPDH promotes cell protective mitophagy by LL structures in cardiomyocytes. This is a tightly regulated process, which can be readily switched to mitochondrially mediated cell death by PKCδ-mediated phosphorylation of mitochondrially associated, inactive GAPDH and inhibition of the cytoprotective mechanism of damaged mitochondrial elimination by mitophagy (Fig. 8).
Acknowledgments
We thank Drs. Jianbo Dong and Eric Churchill for initial observations on GAPDH subcellular distribution, Dr. Marie-Helene Disatnik for helpful discussions and guidance with experiments, Dr. William Claycomb for the HL1 cell line, Dr. Noboru Mizushima for the Atg5−/− MEFs, Dr. Douglas Green for the GAPDH cDNA construct, Dr. Ron Kopito for the LC3 antibody, Dr. John Perrino for help with TEM, and Dr. Jon Mulholland for help with deconvolution microscopy.
This work was supported, in whole or in part, by National Institutes of Health Grant HL052141 (to D. M. R.).
- I/R
- ischemia and reoxygenation or reperfusion
- iGAPDH
- inactive glyceraldehyde-3-phosphate dehydrogenase
- VDAC
- voltage-dependent anion channel
- LAMP-1 (or LAMP-2)
- lysosome-associated membrane proteins 1 (or 2)
- LC3
- microtubule-associated protein 1 light chain 3
- TEM
- transmission electron microscopy
- 3MA
- 3-methylalanine
- MEF
- mouse embryonic fibroblast
- PO
- propylene oxide
- LL
- lysosomal-like
- PS
- phosphatidylserine.
REFERENCES
- 1. Prasad A., Stone G. W., Holmes D. R., Gersh B. (2009) Reperfusion injury, microvascular dysfunction, and cardioprotection. The “dark side” of reperfusion. Circulation 120, 2105–2112 [DOI] [PubMed] [Google Scholar]
- 2. Honda H. M., Ping P. (2006) Mitochondrial permeability transition in cardiac cell injury and death. Cardiovasc. Drugs Ther. 20, 425–432 [DOI] [PubMed] [Google Scholar]
- 3. Youle R. J., Narendra D. P. (2011) Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klionsky D. J. (2007) Autophagy. From phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 8, 931–937 [DOI] [PubMed] [Google Scholar]
- 5. Rabinowitz J. D., White E. (2010) Autophagy and metabolism. Science 330, 1344–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wild P., Dikic I. (2010) Mitochondria get a Parkin' ticket. Nat. Cell Biol. 12, 104–106 [DOI] [PubMed] [Google Scholar]
- 7. Kundu M., Lindsten T., Yang C. Y., Wu J., Zhao F., Zhang J., Selak M. A., Ney P. A., Thompson C. B. (2008) Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 112, 1493–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanki T. (2010) Nix, a receptor protein for mitophagy in mammals. Autophagy 6, 433–435 [DOI] [PubMed] [Google Scholar]
- 9. Li W. W., Li J., Bao J. K. (2012) Microautophagy. Lesser-known self-eating. Cell. Mol. Life Sci. 69, 1125–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mijaljica D., Prescott M., Devenish R. J. (2011) Microautophagy in mammalian cells. Revisiting a 40-year-old conundrum. Autophagy 7, 673–682 [DOI] [PubMed] [Google Scholar]
- 11. Kanki T., Klionsky D. J. (2008) Mitophagy in yeast occurs through a selective mechanism. J. Biol. Chem. 283, 32386–32393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farré J. C., Krick R., Subramani S., Thumm M. (2009) Turnover of organelles by autophagy in yeast. Curr. Opin. Cell Biol. 21, 522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tolkovsky A. M. (2009) Mitophagy. Biochim. Biophys. Acta 1793, 1508–1515 [DOI] [PubMed] [Google Scholar]
- 14. Kim I., Lemasters J. J. (2011) Mitophagy selectively degrades individual damaged mitochondria after photoirradiation. Antioxid. Redox Signal. 14, 1919–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim I., Rodriguez-Enriquez S., Lemasters J. J. (2007) Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 462, 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gottlieb R. A., Mentzer R. M., Jr., Linton P. J. (2011) Impaired mitophagy at the heart of injury. Autophagy 7, 1573–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang C., Andres A. M., Ratliff E. P., Hernandez G., Lee P., Gottlieb R. A. (2011) Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS One 6, e20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dorn G. W., 2nd. (2010) Mitochondrial pruning by Nix and BNip3. An essential function for cardiac-expressed death factors. J. Cardiovasc. Transl. Res. 3, 374–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Churchill E. N., Murriel C. L., Chen C. H., Mochly-Rosen D., Szweda L. I. (2005) Reperfusion-induced translocation of PKCδ to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circ. Res. 97, 78–85 [DOI] [PubMed] [Google Scholar]
- 20. Inagaki K., Chen L., Ikeno F., Lee F. H., Imahashi K., Bouley D. M., Rezaee M., Yock P. G., Murphy E., Mochly-Rosen D. (2003) Inhibition of protein kinase Cδ protects against reperfusion injury of the ischemic heart in vivo. Circulation 108, 2304–2307 [DOI] [PubMed] [Google Scholar]
- 21. Chen L., Hahn H., Wu G., Chen C. H., Liron T., Schechtman D., Cavallaro G., Banci L., Guo Y., Bolli R., Dorn G. W., 2nd, Mochly-Rosen D. (2001) Opposing cardioprotective actions and parallel hypertrophic effects of PKCδ and PKCϵ. Proc. Natl. Acad. Sci. U.S.A. 98, 11114–11119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Churchill E. N., Szweda L. I. (2005) Translocation of PKCδ to mitochondria during cardiac reperfusion enhances superoxide anion production and induces loss in mitochondrial function. Arch. Biochem. Biophys. 439, 194–199 [DOI] [PubMed] [Google Scholar]
- 23. Murriel C. L., Churchill E., Inagaki K., Szweda L. I., Mochly-Rosen D. (2004) Protein kinase Cδ activation induces apoptosis in response to cardiac ischemia and reperfusion damage. A mechanism involving BAD and the mitochondria. J. Biol. Chem. 279, 47985–47991 [DOI] [PubMed] [Google Scholar]
- 24. Ikeno F., Inagaki K., Rezaee M., Mochly-Rosen D. (2007) Impaired perfusion after myocardial infarction is due to reperfusion-induced PKCδ-mediated myocardial damage. Cardiovasc. Res. 73, 699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamacher-Brady A., Brady N. R., Gottlieb R. A. (2006) Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J. Biol. Chem. 281, 29776–29787 [DOI] [PubMed] [Google Scholar]
- 26. Qvit N., Mochly-Rosen D. (2010) Highly specific modulators of protein kinase C localization. Applications to heart failure. Drug Discov. Today Dis. Mech. 7, e87-e93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Colell A., Ricci J. E., Tait S., Milasta S., Maurer U., Bouchier-Hayes L., Fitzgerald P., Guio-Carrion A., Waterhouse N. J., Li C. W., Mari B., Barbry P., Newmeyer D. D., Beere H. M., Green D. R. (2007) GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 129, 983–997 [DOI] [PubMed] [Google Scholar]
- 28. Gottlieb R. A., Carreira R. S. (2010) Autophagy in health and disease. 5. Mitophagy as a way of life. Am. J. Physiol. Cell Physiol. 299, C203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cuervo A. M., Dice J. F. (2000) Regulation of lamp2a levels in the lysosomal membrane. Traffic 1, 570–583 [DOI] [PubMed] [Google Scholar]
- 30. Cuervo A. M., Mann L., Bonten E. J., d'Azzo A., Dice J. F. (2003) Cathepsin A regulates chaperone-mediated autophagy through cleavage of the lysosomal receptor. EMBO J. 22, 47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sahu R., Kaushik S., Clement C. C., Cannizzo E. S., Scharf B., Follenzi A., Potolicchio I., Nieves E., Cuervo A. M., Santambrogio L. (2011) Microautophagy of cytosolic proteins by late endosomes. Dev. Cell 20, 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tristan C., Shahani N., Sedlak T. W., Sawa A. (2011) The diverse functions of GAPDH. Views from different subcellular compartments. Cell. Signal. 23, 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hosokawa N., Hara Y., Mizushima N. (2006) Generation of cell lines with tetracycline-regulated autophagy and a role for autophagy in controlling cell size. FEBS Lett. 580, 2623–2629 [DOI] [PubMed] [Google Scholar]
- 34. Gao Y., Wang H. Y. (2006) Casein kinase 2 Is activated and essential for Wnt/β-catenin signaling. J. Biol. Chem. 281, 18394–18400 [DOI] [PubMed] [Google Scholar]
- 35. Ashmarina L. I., Louzenko S. E., Severin S. E., Jr., Muronetz V. I., Nagradova N. K. (1988) Phosphorylation of d-glyceraldehyde-3-phosphate dehydrogenase by Ca2+/calmodulin-dependent protein kinase II. FEBS Lett. 231, 413–416 [DOI] [PubMed] [Google Scholar]
- 36. Okamoto K., Kondo-Okamoto N., Ohsumi Y. (2009) A landmark protein essential for mitophagy. Atg32 recruits the autophagic machinery to mitochondria. Autophagy 5, 1203–1205 [DOI] [PubMed] [Google Scholar]
- 37. Narendra D., Kane L. A., Hauser D. N., Fearnley I. M., Youle R. J. (2010) p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy. VDAC1 is dispensable for both. Autophagy 6, 1090–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vives-Bauza C., Zhou C., Huang Y., Cui M., de Vries R. L., Kim J., May J., Tocilescu M. A., Liu W., Ko H. S., Magrané J., Moore D. J., Dawson V. L., Grailhe R., Dawson T. M., Li C., Tieu K., Przedborski S. (2010) PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. U.S.A. 107, 378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sun Y., Vashisht A. A., Tchieu J., Wohlschlegel J. A., Dreier L. (2012) Voltage-dependent anion channels (VDACs) recruit Parkin to defective mitochondria to promote mitochondrial autophagy. J. Biol. Chem. 287, 40652–40660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen Y., Dorn G. W., 2nd. (2013) PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340, 471–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schieven G. L., Kirihara J. M., Burg D. L., Geahlen R. L., Ledbetter J. A. (1993) p72syk tyrosine kinase is activated by oxidizing conditions that induce lymphocyte tyrosine phosphorylation and Ca2+ signals. J. Biol. Chem. 268, 16688–16692 [PubMed] [Google Scholar]
- 42. Qin S., Inazu T., Yamamura H. (1995) Activation and tyrosine phosphorylation of p72syk as well as calcium mobilization after hydrogen peroxide stimulation in peripheral blood lymphocytes. Biochem. J. 308, 347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hardwick J. S., Sefton B. M. (1995) Activation of the Lck tyrosine protein kinase by hydrogen peroxide requires the phosphorylation of Tyr-394. Proc. Natl. Acad. Sci. U.S.A. 92, 4527–4531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gopalakrishna R., Anderson W. B. (1989) Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc. Natl. Acad. Sci. U.S.A. 86, 6758–6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Konishi H., Tanaka M., Takemura Y., Matsuzaki H., Ono Y., Kikkawa U., Nishizuka Y. (1997) Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc. Natl. Acad. Sci. U.S.A. 94, 11233–11237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Majumder P. K., Pandey P., Sun X., Cheng K., Datta R., Saxena S., Kharbanda S., Kufe D. (2000) Mitochondrial translocation of protein kinase Cδ in phorbol ester-induced cytochrome c release and apoptosis. J. Biol. Chem. 275, 21793–21796 [DOI] [PubMed] [Google Scholar]
- 47. Hwang N. R., Yim S. H., Kim Y. M., Jeong J., Song E. J., Lee Y., Lee J. H., Choi S., Lee K. J. (2009) Oxidative modifications of glyceraldehyde-3-phosphate dehydrogenase play a key role in its multiple cellular functions. Biochem. J. 423, 253–264 [DOI] [PubMed] [Google Scholar]
- 48. Butterfield D. A., Hardas S. S., Lange M. L. (2010) Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer's disease. Many pathways to neurodegeneration. J. Alzheimers Dis. 20, 369–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Glaser P. E., Gross R. W. (1995) Rapid plasmenylethanolamine-selective fusion of membrane bilayers catalyzed by an isoform of glyceraldehyde-3-phosphate dehydrogenase. Discrimination between glycolytic and fusogenic roles of individual isoforms. Biochemistry 34, 12193–12203 [DOI] [PubMed] [Google Scholar]
- 50. Morero R. D., Viñals A. L., Bloj B., Farías R. N. (1985) Fusion of phospholipid vesicles induced by muscle glyceraldehyde-3-phosphate dehydrogenase in the absence of calcium. Biochemistry 24, 1904–1909 [DOI] [PubMed] [Google Scholar]
- 51. Yeung T., Gilbert G. E., Shi J., Silvius J., Kapus A., Grinstein S. (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319, 210–213 [DOI] [PubMed] [Google Scholar]
- 52. Cuervo A. M., Terlecky S. R., Dice J. F., Knecht E. (1994) Selective binding and uptake of ribonuclease A and glyceraldehyde-3-phosphate dehydrogenase by isolated rat liver lysosomes. J. Biol. Chem. 269, 26374–26380 [PubMed] [Google Scholar]
- 53. Cuervo A. M., Dice J. F. (1996) A receptor for the selective uptake and degradation of proteins by lysosomes. Science 273, 501–503 [DOI] [PubMed] [Google Scholar]
- 54. Sala-Mercado J. A., Wider J., Undyala V. V., Jahania S., Yoo W., Mentzer R. M., Jr., Gottlieb R. A., Przyklenk K. (2010) Profound cardioprotection with chloramphenicol succinate in the swine model of myocardial ischemia-reperfusion injury. Circulation 122, S179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]